Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2414
Peer-review started: November 18, 2016
First decision: February 9, 2017
Revised: February 27, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 7, 2017
Processing time: 139 Days and 19.6 Hours
To define clinical criteria to differentiate eosinophilic gastrointestinal disorder (EoGD) in the esophagus.
Our criteria were defined based on the analyses of the clinical presentation of eosinophilic esophagitis (EoE), subepithelial eosinophilic esophagitis (sEoE) and eosinophilic esophageal myositis (EoEM), identified by endoscopy, manometry and serum immunoglobulin E levels (s-IgE), in combination with histological and polymerase chain reaction analyses on esophageal tissue samples.
In five patients with EoE, endoscopy revealed longitudinal furrows and white plaques in all, and fixed rings in two. In one patient with sEoE and four with EoEM, endoscopy showed luminal compression only. Using manometry, failed peristalsis was observed in patients with EoE and sEoE with some variation, while EoEM was associated with hypercontractile or hypertensive peristalsis, with elevated s-IgE. Histology revealed the following eosinophils per high-power field values. EoE = 41.4 ± 7.9 in the epithelium and 2.3 ± 1.5 in the subepithelium; sEoE = 3 in the epithelium and 35 in the subepithelium (conventional biopsy); EoEM = none in the epithelium, 10.7 ± 11.7 in the subepithelium (conventional biopsy or endoscopic mucosal resection) and 46.8 ± 16.5 in the muscularis propria (peroral esophageal muscle biopsy). Presence of dilated epithelial intercellular space and downward papillae elongation were specific to EoE. Eotaxin-3, IL-5 and IL-13 were overexpressed in EoE.
Based on clinical and histological data, we identified criteria, which differentiated between EoE, sEoE and EoEM, and reflected a different pathogenesis between these esophageal EoGDs.
Core tip: Eosinophilic esophagitis has long been considered as the only eosinophilic gastrointestinal disorder (EoGD) in the esophagus. However, eosinophilic esophageal myositis, characterized by esophageal symptoms and eosinophilic infiltration in the esophageal muscle layer, has been identified using peroral esophageal muscle biopsy. Combining clinical and histological data, we have defined clinical criteria to differentiate EoGDs in the esophagus.
- Citation: Sato H, Nakajima N, Takahashi K, Hasegawa G, Mizuno KI, Hashimoto S, Ikarashi S, Hayashi K, Honda Y, Yokoyama J, Sato Y, Terai S. Proposed criteria to differentiate heterogeneous eosinophilic gastrointestinal disorders of the esophagus, including eosinophilic esophageal myositis. World J Gastroenterol 2017; 23(13): 2414-2423
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2414.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2414
Eosinophilic esophagitis (EoE) is an allergic disorder characterized by esophageal dysfunction and histological “esophageal eosinophilia”, where eosinophilia is defined by a peak number of eosinophils per high-power field (eos/hpf) ≥ 15 in tissue samples obtained by conventional biopsy[1]. “Esophageal eosinophilia” is used to describe the histological finding of increased “epithelial” eosinophil infiltration, meaning that EoE is an epithelial eosinophilic disease. In their study of full-thickness EoE specimens, Rieder et al[2] confirmed the highest density in EoE to be in the epithelium, but with additional distribution of EoE in the submucosa, muscle layer and adventitia. Clinically, proton pump inhibitor-responsive esophageal eosinophilia (PPI-REE) and secondary causes of eosinophilia, such as parasitic infection or gastro-esophageal reflux disease, are excluded from the definition of EoE[3]. However, a subtype of EoE, with esophageal symptoms and subepithelial eosinophilia (SE) observed in the lamina propria and muscularis mucosa in esophageal samples obtained by conventional biopsy, has also been reported recently[4], and termed “subepithelial eosinophilic esophagitis (sEoE)”. Using peroral esophageal muscle biopsy (POEM-b), we have also previously reported an eosinophilic infiltration in the esophageal muscle layer[5-7]. However, as this eosinophilic infiltration of the esophageal muscle layer was not identifiable using conventional biopsy, it cannot be defined as EoE. The term “eosinophilic esophageal myositis (EoEM)” has been introduced to distinguish this eosinophilic infiltration of the esophageal muscle layer from EoE and sEoE. Therefore, although EoE had previously been considered as a single eosinophilic gastrointestinal disorder (EoGD) of the esophagus, heterogeneity in the depth of eosinophil involvement has been suggested as an important clinical variable for diagnosis. However, clinical criteria for differentiating between EoE, sEoE and EoEM have not yet been established. Therefore, the aim of our study was to perform a detailed analysis of clinical data from endoscopy, manometry, laboratory tests, histological examination, and gene expression analyses to identify etiological differences between EoE, sEoE and EoEM as to establish clinical criteria to differentiate between these disorders.
Our study was conducted as part a larger study registered with the UMIN Clinical Trials Registry (UMIN 000018685). The data were obtained from patients evaluated at the Niigata University Medical and Dental Hospital, which is a tertiary referral center in Japan. The present study was approved by our Institutional Review Board (No. 2416) and carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to the start of the study.
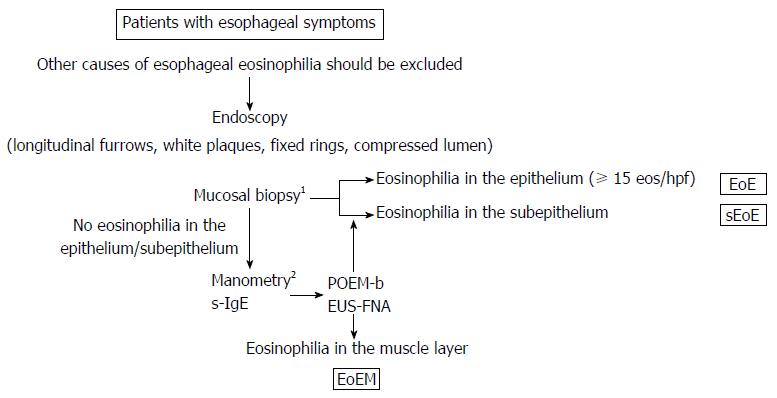
Patients with symptomatic esophageal eosinophilia within any layer of the esophagus (epithelium, subepithelium, from the lamina propria to the submucosa, or muscularis propria) were recruited. A PPI trial was first performed for all patients and, subsequently, patients with PPI-REE were excluded from the study. The diagnosis of EoE was based on the American College of Gastroenterology (ACG) clinical guideline of a peak eos/hpf value ≥ 15 in epithelium obtained by mucosal biopsy[1] Applying the ACG guidelines, we also used a peak eos/hpf value ≥ 15 to define subepithelial and muscle-layer eosinophil inflammation. SE (eosinophilia extending from the lamina propria to the submucosa) was diagnosed by conventional biopsy, with esophageal symptoms classified as sEoE, in contrast to EoE, which was identified by eosinophilia principally in the epithelium. Symptomatic eosinophilia in the muscle-layer of the esophagus was defined as EoEM (Figure 1 and Table 1).
| Diagnosis2 | EoE | sEoE | EoEM | |||||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
| Epithelium/sub-3 | 44/2 | 40/3 | 50/4 | 27/- | 46/0 | 3/35 | 0/0 | 0/- | 0/5 (EMR) | 0/27 (EMR) |
| Muscle layer4 | 36 | 30 | 73 | 48 | ||||||
| Age | 31 | 54 | 54 | 35 | 35 | 75 | 43 | 31 | 75 | 67 |
| M/F | M | F | M | M | M | M | M | M | M | M |
| Allergy history | House dust | Milk | No | No | No | No | No | No | No | Rabbit |
| Symptoms5 | D,F | C,D,F | C,D | D | D | D | C,D | C,D | D | C,D |
| Endoscopic findings6 | +/+/+/- | +/+/-/- | +/+/+/- | +/+/-/- | +/+/-/- | -/-/-/+ | -/-/-/+ | -/-/-/+ | -/-/-/+ | -/-/-/+ |
| Manometry7 | FP | FP | FP | FP | Normal | FP | JE | NE | JE | JE |
| Serum IgE (IU/mL) | 314.0 | 33.5 | 33.3 | 39.0 | 39.0 | 678.0 | 194.0 | 368.0 | 192.0 | 545.0 |
| Histological findings8 | +/+/- | +/+/- | +/+/- | +/+/- | +/+/- | -/-/+ | -/-/+ | -/-/+ | -/-/+ | -/-/+ |
Endoscopy was performed using a digital high-vision endoscope (H260Z, Olympus, Tokyo, Japan). Longitudinal furrows, white plaques, fixed rings, and compression of the lumen in the esophagus were assessed. Longitudinal furrows, white plaques and fixed rings have previously been reported as typical endoscopic findings of EoE[8,9], with luminal compression being the only previously reported endoscopic finding of EoEM[5,6].
Manometry was also performed in all patients using high-resolution manometry (HRM; Star Medical Co., Pte., Ltd., Tokyo, Japan), with patients in the supine position, performing 10 consecutive swallows of 5-mL of water. HRM results were evaluated using the Chicago classification criteria, version 3.0[10]. A jackhammer esophagus (JE) was defined by hypercontractile peristalsis, with a distal contractile integral (DCI) ≥ 8000 mmHg/s·cm. Failed peristalsis was diagnosed by a DCI < 100 mmHg/s·cm. It is important to note that the diagnosis of a nutcracker esophagus (NE) has been eliminated from version 3.0 of the Chicago classification criteria as the significance of using a DCI of 5000 to 8000 mmHg/s·cm to specifically differentiate NE was questioned. However, we maintained NE as a possible diagnosis, based on the Chicago classification criteria published in 2011[11] as patients with NE in our study had symptomatic esophageal eosinophilia.
Six conventional esophageal mucosal biopsies were performed in each case to increase the detection rate of mucosal eosinophilia. Large biopsy forceps (Radial Jaw 4 Biopsy Forceps, Boston Scientific, Massachusetts, US) were used to obtain a sufficient amount of epithelium with subepithelium.
Cases 7 through 10 had no visible eosinophils on conventional biopsy, but a NE/JE was observed by HRM. POEM was determined as the best therapeutic option to resolve the hypertensive/hypercontractile peristalsis[12], and muscle specimens were obtained by POEM-b. For cases 9 and 10, although JE was visible, no eosinophils were identified in any of the six esophageal mucosal biopsies obtained, and a mucosal entry site for POEM/POEM-b was created using cap-fitted endoscopic mucosal resection (cEMR) to allow the full-layer of the mucosa, along with the submucosa, for analysis (Figure 1). Therefore, our histological analysis included mucosal specimens obtained by conventional biopsies for cases 1-10 and by cEMR for cases 9-10, and muscle specimens obtained by POEM-b for cases 7-10.
The maximum number of eosinophils (eos/hpf) was counted separately in the epithelium, subepithelium and muscle layer for each of the 10 cases. The mucosal histology was also assessed to identify: dilated intercellular spaces, downward papillae elongation and basal cell layer destruction. Dilated intercellular spaces and downward papillae elongation have previously been reported in cases of EoE[13,14]. Upward papillae elongation, which can often occur along with the presence of balloon cells in cases of reflux esophagitis and is considered a non-specific histological finding, was excluded from our analysis[15].
POEM-b/cEMR specimens obtained from 5 patients with achalasia (4 males; mean age 44.2 ± 9.6 years) were used as controls for eosinophil counts in our histological assessments and mRNA expression analyses (see below).
In a previous study of EoE, a genome-wide association study was used to identify the significant locus at 2p23 susceptible of encoding Calpain14 (CAPN14) and chr5q22, which mapped to a single LD block encompassing the thymic stromal lymphopoietin (TSLP) and WDR36 genes[16,17]. CAPN14 is specifically induced in the esophageal epithelium after IL-13 treatment and leads to increasingly dilated intracellular spaces in the epithelium[18]. CAPN14 also disrupts the expression of desmoglein-1 (DSG1: barrier molecule), which triggers the entry of antigens into the esophageal epithelium. TSLP is a protein of the cytokine family and is known to promote allergic inflammation by activating dendritic cells, inducing Th2 cell responses, supporting immunoglobulin E (IgE) production, and increasing the population of phenotypically and functionally distinct basophils[19]. A set of candidate genes for eosinophil chemotaxis, including eotaxin-3 and DSG1 has also been identified by transcriptome analysis[20]. C-C chemokine receptor type-3 (CCR3), which is expressed on the surface of eosinophils, mast cells and basophils, is the chemokine receptor for eotaxin[21-23], with an elevated expression of exotoxin-3 having been reported in EoE[24]. Moreover, Th2 cells are thought to be central regulators of the hallmark features of eosinophilic diseases via their influence over Th2 cytokines, such as IL-5 and IL-13[25-28].
Based on the above, real-time quantitative reverse transcription polymerase chain reaction (real-time qRT-PCR) analyses were performed on the samples obtained by conventional biopsy, cEMR and POEM-b. Total RNA was extracted using Trizol (Invitrogen, California, CA, United States), according to the standard protocol. Thereafter, cDNA was amplified using the ABI 7700 sequence-detector system (Applied Biosystems, Foster City), with a set of primers and probes corresponding to CAPN14, TSLP, Eotaxin-3, DSG1, CCR3, IL-5, IL13, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The levels of mRNA expression were normalized to a housekeeping gene, such as GAPDH: CAPN14/GAPDH, TSLP/GAPDH, and Eotaxin-3/GAPDH. Finally, the ratio of the mRNA expression relative to mRNA expression in the control tissues was calculated, with the median value of control converted to 1.
Relevant demographic patient variables and histological findings (continuous variables) were expressed by their mean ± SD values. Levels of mRNA were expressed as the mean of EoE and EoEM, respectively, relative to the control. Levels of mRNA within the range of the control samples were deemed to be within normal limits. Abnormal increases in mRNA expression of CAPN14, TSLP, Eotaxin-3, CCR3, IL-5, and IL-13 were defined by a relative ratio exceeding the maximum value of the control samples, while a decrease in the expression of DSG1 was defined by a relative ratio lower than the minimum value of the control.
Our analyses included the data from 10 patients who underwent assessment for esophageal EoGDs over our study period, from July 2014 through September 2016. Among these 10 patients, 5 were diagnosed with EoE, 1 with sEoE and 4 with EoEM (Table 1 and Figure 1).
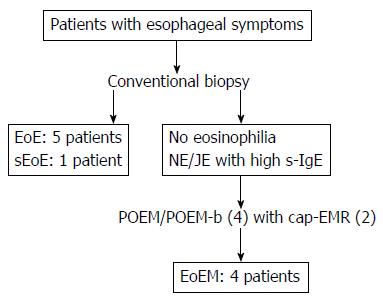
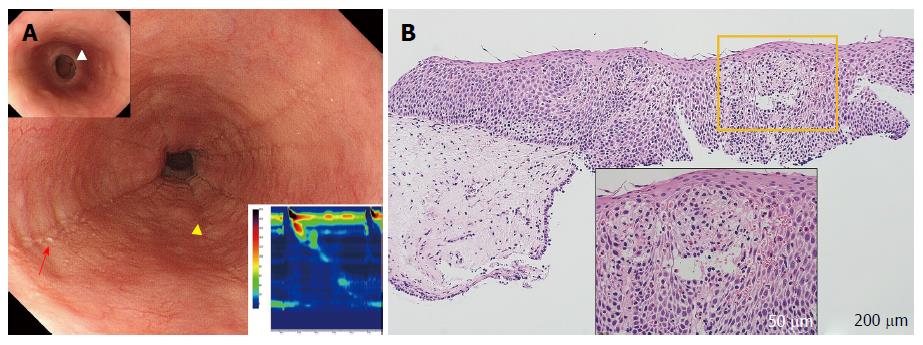
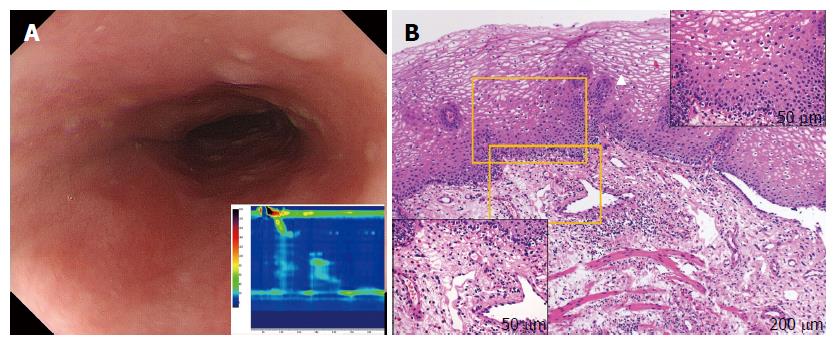
Longitudinal furrows and white plaques were identified in all patients with EoE (cases 1-5), with a fixed ring visible in 2 of these 5 cases (Figure 2A). For patients with sEoE and EoEM (cases 5-10), only a luminal compression was observed, with no visible evidence of longitudinal furrows, white plaques or fixed rings (Figure 3A and Figure 4A).
HRM results in patients with EoE (cases 1-5) were variable, but with failed peristalsis observed in 4 of these 5 cases (Figure 2A, insert), with findings being within normal limits for the remaining case. The patient with sEoE (case 6) presented with failed peristalsis (Figure 3A, insert) and elevated s-IgE level (678.0 IU/mL, normal range ≤ 173 IU/mL). It is important to note that levels of s-IgE did vary, overall, among cases of EoE. Patients with EoEM (cases 7-10) demonstrated either hypercontractile or hypertensive contractions in the esophagus (JE: 3; Figure 4A, insert; NE: 1), with elevated s-IgE levels (324.8 ± 145.9 IU/mL).
The eos/hpf ratio values were as follows: EoE (cases 1-5), 41.4 ± 7.9 in the epithelium and 2.3 ± 1.5 in the subepithelium, identified by conventional biopsy, noting that the data for the subepithelium in case 4 were excluded because the subepithelial specimens were insufficient; sEoE (case 6), 35 in the subepithelium and 3 in the epithelium, identified by conventional biopsy; and EoEM (cases 7-10), no visible eosinophils in the esophageal epithelium and 10.7 ± 11.7 in the subepithelium (cases 7, 9 and 10), noting that the data in the subepithelium for case 8 were excluded because the subepithelium specimen obtained using conventional biopsy was insufficient. An eos/hpf ratio of 46.8 ± 16.5 was identified in tissue samples obtained from the esophageal muscle layer by POEM-b (cases 7-10). Tissue samples from control subjects were essentially devoid of eosinophils in the epithelium and muscle layer, with a few eosinophils visible in the subepithelium (4.0 ± 2.5).
Dilated intercellular spaces and downward papillae elongation were identified in the mucosal samples from all EoE patients (cases 1-5; Figure 2B). Basal cell layer destruction was visible in the case of sEoE (Figure 3B) and in all EoEM cases (cases 7-10; Figure 4B). Dilated intercellular spaces and downward papillae elongation were not visible in any cases of sEoE and EoEM.
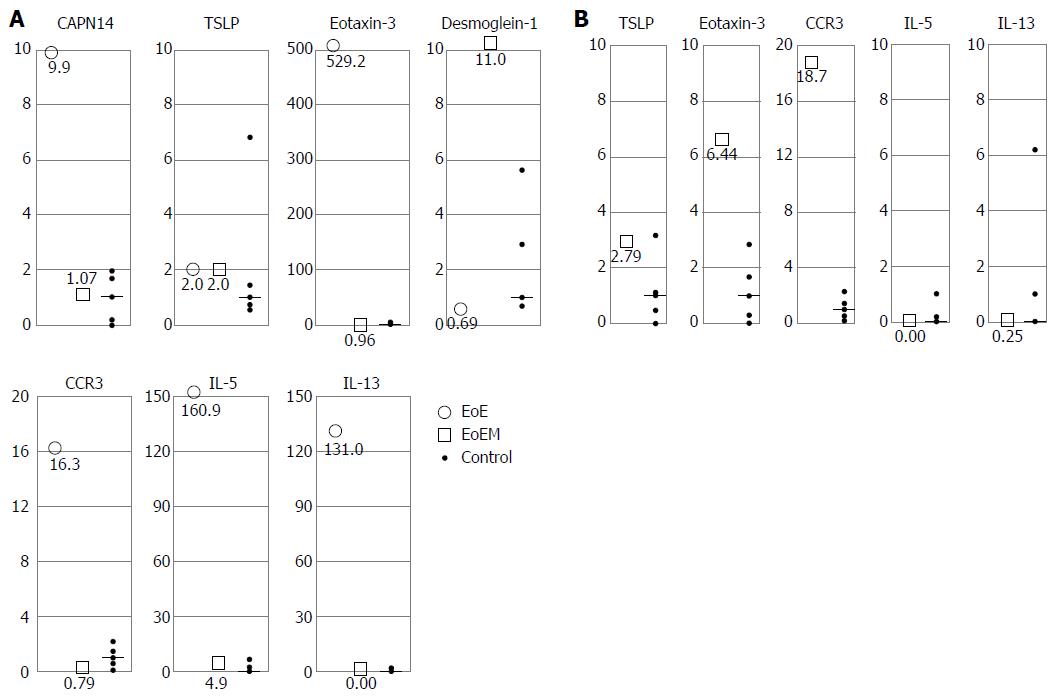
The esophageal mucosal biopsy samples from EoE cases (cases 1, 3, 4 and 5), in addition to the cEMR samples from EoEM cases (cases 9 and 10, both of which included rich subepithelium tissue), were sent for mRNA expression analyses. EoE was associated with the following fold-increase in level of expression: CAPN14, 9.9-fold; eotaxin-3, 529.2-fold; CCR3, 16.3-fold; IL-5, 160.9-fold; and IL-13, 131.0-fold. No increase in the expression of TSLP was identified in these cases (2.0-fold higher values compared to the control), while there was a decrease in the expression of DSG1 (0.69-fold). In contrast, in EoEM, the expression levels of CAPN14, TSLP, eotaxin-3, CCR3, IL-5, and IL-13 were equal to those in controls (1.07, 2.0, 0.96, 0.79, 4.93, and 0.00-fold increases, respectively), and the expression of DSG1 was highly preserved (DSG1: 11.0-fold) (Figure 5A).
Tissue samples of the esophageal muscle-layer obtained by POEM-b in patients with EoEM were also analyzed for mRNA expression, with the following increases noted: eotaxin-3, 6.44-fold; and CCR3, 18.7-fold. Levels of TSLP, IL-5 and IL-13 (2.79, 0.00, and 0.25-fold, respectively) were within control values (Figure 5B).
In this study, we performed histological and gene expression analyses on a case series of EoEM, and compared those with cases of EoE and sEoE. Eosinophilic gastroenteritis (EoGE) is an EoGD characterized by eosinophilia in the stomach, small intestine or large colon, and is sometimes complicated with EoE. Heterogeneity in the depth of eosinophil involvement of the different layers of the gastrointestinal tract, including the mucosal, muscle and serous layers, has been reported in patients with EoGE[29]. In the esophagus, this heterogeneity in the depth of involvement, however, had not previously been characterized due to the difficulty in obtaining tissues samples with sufficient subepithelium, together with epithelium using conventional biopsy, due to the thickness of the stratified squamous epithelium. Furthermore, histological analyses of the esophageal muscle layer are technically difficult and invasive. In contrast, muscle layer and serous-type of EoGE, show ascites that allow for diagnosis by computed tomography and ascites puncture. To our knowledge, our study is the first to demonstrate heterogeneity in the depth of eosinophil involvement in the esophagus using a combination of conventional mucosal biopsy, cEMR, and POEM-b.
In all 5 cases of EoE, longitudinal furrows and white plaques were visible, with fixed rings observable in 2 of these 5 cases. These endoscopic findings are characteristic of EoE, although they are not highly sensitive for diagnosing the disease[9]. These distinctive endoscopic features of EoE are reflected as dilated epithelial intercellular spaces and/or downward papillae elongation histologically. In EoE, epithelial inflammation and subsequent fibrosis lead to peristalsis disturbances[30]. Our HRM results in fact did identify failed peristalsis as the main finding in EoE. However, there was variability in this finding with one patient identified in whom peristalsis was deemed to be within normal limits. In patients with EoE, an allergic response to the allergen stimulated esophageal epithelial cells to produce eotaxin-3, which recruits eosinophils via the CCR3 receptor. In these patients, Th2 cytokines as IL-5 and IL-13 are also overexpressed and induce the loss of barrier integrity in epithelial cells. This process is mediated, in part, by a reduction in the expression of DSG1 and an increase in the expression of epithelial CAPN14, which leads to increasingly dilated intercellular spaces. The results of our mRNA analysis correspond to a previously reported hypothesis on this matter[26].
In our one case of sEoE (case 6), conventional endoscopy did not reveal any of the characteristic findings of EoE, including longitudinal furrows, white plaques, and fixed rings. In this case, elevated levels of s-IgE and failed peristalsis, as confirmed by HRM, suggested that other esophageal EoGDs could be involved and that conventional biopsy, targeting SE, could be used to diagnose sEoE. Epithelial histology did not reveal any dilation of the intercellular spaces or downward papillae elongation in this case. Luminal compression observed on endoscopy was likely caused by subepithelial inflammation secondary to eosinophil infiltration. Subepithelial inflammation may also trigger the destruction of the basal cell layer and lead to upward papillae elongation, but not a downward papillae elongation. Heterogeneity in endoscopic and histological findings has previously been reported in cases of EoE[31]. In fact, cases with “non-EoE-like endoscopic and histological findings” are more likely to represent sEoE than EoE. A lower degree of epithelial eosinophilia, but with a similar clinical course to EoE has also been reported[32]. In our case series, the pathological mechanism of sEoE in case 6 was suspected to be somewhat different from that of EoE based on the endoscopic and histological findings.
In cases of EoEM, a luminal compression was only identified in cases of sEoE, in contrast to the findings reported for EoE. EoGD in the esophagus was also suspected by an elevated s-IgE level and JE/NE on HRM. Histological assessment of the muscle layer by POEM-b, rather than by conventional biopsy alone, was capable of diagnosing EoEM. Mucosal histology in cases of EoEM revealed basal cell layer destruction, along with non-specific upward papilla elongation, reflecting subepithelial and muscle layer inflammation. JE or NE on HRM was potentially caused by localized muscle-layer eosinophilia, accompanied by mastocytosis[33]. mRNA analysis of the mucosa in cases of EoEM did not reveal the characteristic findings of EoE, indicating that EoEM is caused by a pathogenic process that does not extend through the epithelium like EoE. mRNA analysis of the muscle layer revealed elevated levels of CCR3 and eotaxin-3 expression. We suspected the vascular and myocyte manifestations of eotaxin-3 in EoEM, such as the recruitment of eosinophils[34], to be responsible for observed changes in the esophageal muscle layer, although the eotaxin-3 mRNA titer in the muscle layer was actually lower than values obtained in the epithelium of EoE cases. Moreover, the Th2 cytokine profiles of EoE and EoEM are completely different, which further suggests that these two disorders are unrelated. Of note, a positive history of allergy was identified in only one case of EoEM and in two cases of EoE in our series.
Our results confirm that EoE, sEoE and EoEM can be clinically distinguished using the combination of endoscopy, manometry and laboratory tests outlined in Figure 6. Prior to proceeding to distinguish between these EoGDs of the esophagus, all other causes of esophageal symptoms should be excluded. As examples, gastroparesis or chronic intestinal pseudo-obstruction trigger abdominal symptoms that often include the esophageal symptoms and abnormal endoscopic and manometric findings in the esophagus. Luminal compression on endoscopy and failed peristalsis, as well as JE or NE on HRM, are not specific findings for sEoE or EoEM. Moreover, a past or present history of other allergy disorders can result in elevation of s-IgE and, therefore, a comprehensive clinical decision-making process is needed in such cases[35]. Based on the premise outlined above, histological assessment by conventional biopsy is necessary to assess the full esophageal mucosal layer in patients with suspected EoGDs. Although we used large biopsy forceps for conventional biopsies in our study to obtain a sufficient volume of subepithelium tissue together with the epithelium, in some cases sufficient subepithelium still could not be obtained (cases 4 and 8). As well, several biopsies should be performed due to the patchy distribution of eosinophils in cases of EoE. Re-endoscopy with re-biopsy should also be considered if only a few epithelial eosinophils are identified in an insufficient volume of subepithelium. Diagnostic cEMR may be somewhat invasive for obtaining sufficient subepithelial tissue, and therefore, it was only performed in combination with POEM/POEM-b in our study. Endoscopic ultrasound-guided fine needle aspiration may be a good option for cases in which sEoE and EoEM are suspected[36].
There are several limitations in our study, which need to be acknowledged. Foremost, other disorders such as reflux esophagitis and achalasia are associated with low-grade SE, as shown in the tissue samples from patients in our control group. Therefore, a reliable cut-off number of eosinophils for the diagnosis of sEoE will need to be determined in future studies. mRNA analyses for cases of symptomatic achalasia were used as a control for two reasons. the first, tissue samples are obtained using the same POEM-b method. Second, tissue samples in achalasia do not show eosinophilia in the esophageal muscle layer[37]. Non-symptomatic individuals without any known esophageal disorders would provide a more appropriate control, although this would pose a difficult ethical problem. This was a small-size pilot study and further studies, including larger sample sizes, are needed to confirm our findings. In fact, we are continuing to collect data using our procedure outlined in Figure 6 with the aim of supplementing our case series in future reports. Future research should also specifically aim to include a larger number of patients with sEoE patients.
In conclusion, we propose clinical criteria for differentiating EoE, sEoE and EoEM, taking into account the histological heterogeneity in the depth of eosinophil involvement was observed among these disorders. Our findings predict a difference in the pathogenesis of these disorders, and further research will be required to fully elucidate these differences.
Eosinophilic esophagitis (EoE) is an allergy disorder, defined by a histologically severe eosinophil infiltration in the esophageal epithelium. EoE has long been considered to be the only eosinophilic gastrointestinal disorder (EoGD) of the esophagus.
Peroral endoscopic myotomy (POEM) was developed to provide a less invasive technique to perform transoral esophageal muscle layer biopsy (peroral esophageal muscle biopsy: POEM-b). Using POEM-b, a new disorder with an eosinophilic infiltration in the esophageal muscle layer was detected, and a new name “Eosinophilic esophageal myositis: EoEM” was given.
This is the first study to have addressed the clinical differentiation of EoGDs of the esophagus. Pathogenesis of EoEM was also analyzed by real-time qRT-PCR analyses of the esophageal samples.
EoEM need to be differentiated in cases of symptomatic esophageal motility disorders.
EoEM is defined as esophageal symptoms and histologically severe eosinophil infiltration in esophageal muscle layer. EoEM has no epithelial eosinophilia as EoE.
The authors presented a study which determined the criteria to differentiate heterogeneous eosinophilic esophagus. The study reflected a different pathogenesis between EoE, sEoE, and EoEM. The study is interesting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Jurcic P, Yu CH S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679-692; quiz 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 844] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 2. | Rieder F, Nonevski I, Ma J, Ouyang Z, West G, Protheroe C, DePetris G, Schirbel A, Lapinski J, Goldblum J. T-helper 2 cytokines, transforming growth factor β1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology. 2014;146:1266-1277.e1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016;14:13-22.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 4. | Yamabe A, Irisawa A, Shibukawa G, Abe Y, Saito A, Imbe K, Hoshi K, Igarashi R. Clinical effects of eosinophilic esophagitis observed using endoscopic ultrasound. Clin J Gastroenterol. 2014;7:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Sato H, Takeuchi M, Takahashi K. Eosinophilic infiltration of the muscularis propria in a patient with jackhammer esophagus treated with per-oral endoscopic myotomy. Clin Gastroenterol Hepatol. 2015;13:e33-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Takahashi K, Sato H, Sato Y. An unusual case of an esophageal functional disorder. Gastroenterology. 2015;149:e15-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Sato H, Takeuchi M, Takahashi K, Sato Y, Hashimoto S, Mizuno K, Suzuki K, Kobayashi M, Honma T, Inoue H. Nutcracker and jackhammer esophagus treatment: a three-case survey, including two novel cases of eosinophilic infiltration into the muscularis propria. Endoscopy. 2015;47:855-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147:1238-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Kinoshita Y, Furuta K, Ishimaura N, Ishihara S, Sato S, Maruyama R, Ohara S, Matsumoto T, Sakamoto C, Matsui T. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1452] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 11. | Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24 Suppl 1:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 12. | Khashab MA, Messallam AA, Onimaru M, Teitelbaum EN, Ujiki MB, Gitelis ME, Modayil RJ, Hungness ES, Stavropoulos SN, El Zein MH. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc. 2015;81:1170-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, Smout AJ, Bredenoord AJ. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol. 2015;110:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Zentilin P, Savarino V, Mastracci L, Spaggiari P, Dulbecco P, Ceppa P, Savarino E, Parodi A, Mansi C, Fiocca R. Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol. 2005;100:2299-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 16. | Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, Bredenoord AJ, Furuta GT, Spergel JM, Hakonarson H. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, Gober L, Kim C, Glessner J, Frackelton E. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 18. | Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, Travers J, Kottyan LC, Rothenberg ME. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1:e86355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, Benitez AJ, Ruymann KR, Muir AB, Hill DA. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol. 2015;135:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 679] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 22. | Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Collington SJ, Westwick J, Williams TJ, Weller CL. The function of CCR3 on mouse bone marrow-derived mast cells in vitro. Immunology. 2010;129:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Zafra MP, Cancelliere N, Rodríguez del Río P, Ruiz-García M, Estévez L, Andregnette V, Sánchez-García S, Fiandor A, Collantes E, Sastre J. Misregulation of suppressors of cytokine signaling in eosinophilic esophagitis. J Gastroenterol. 2013;48:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, Collins MH, Putnam PE, Wells SI, Rothenberg ME. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 362] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 26. | Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, Alexander ES, Butz BK, Jameson SC, Kaul A. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208-217, 217.e1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:281-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Straumann A, Aceves SS, Blanchard C, Collins MH, Furuta GT, Hirano I, Schoepfer AM, Simon D, Simon HU. Pediatric and adult eosinophilic esophagitis: similarities and differences. Allergy. 2012;67:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 29. | Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54-58. [PubMed] |
| 30. | van Rhijn BD, Oors JM, Smout AJ, Bredenoord AJ. Prevalence of esophageal motility abnormalities increases with longer disease duration in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil. 2014;26:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Jiao D, Ishimura N, Maruyama R, Ishikawa N, Nagase M, Oshima N, Aimi M, Okimoto E, Mikami H, Izumi D. Similarities and differences among eosinophilic esophagitis, proton-pump inhibitor-responsive esophageal eosinophilia, and reflux esophagitis: comparisons of clinical, endoscopic, and histopathological findings in Japanese patients. J Gastroenterol. 2017;52:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Ravi K, Talley NJ, Smyrk TC, Katzka DA, Kryzer L, Romero Y, Arora AS, Alexander JA. Low grade esophageal eosinophilia in adults: an unrecognized part of the spectrum of eosinophilic esophagitis? Dig Dis Sci. 2011;56:1981-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198-1204.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Sato H, Nakajima N, Hasegawa G, Kawata Y, Sato Y, Suzuki K, Honma T, Terai S. Immunohistochemical differentiation of eosinophilic esophageal myositis from eosinophilic esophagitis. J Gastroenterol Hepatol. 2017;32:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Ishimura N, Furuta K, Sato S, Ishihara S, Kinoshita Y. Limited role of allergy testing in patients with eosinophilic gastrointestinal disorders. J Gastroenterol Hepatol. 2013;28:1306-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Igarashi R, Irisawa A, Shibukawa G, Yamabe A, Fujisawa M, Sato A, Maki T, Arakawa N, Yoshida Y, Yamamoto S. Eosinophilic esophageal myositis diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy: a case report. Clin J Gastroenterol. 2016;9:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Nakajima N, Sato H, Takahashi K, Hasegawa G, Mizuno K, Hashimoto S, Sato Y, Terai S. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |