Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2258
Peer-review started: December 8, 2016
First decision: January 10, 2017
Revised: January 23, 2017
Accepted: February 16, 2017
Article in press: February 17, 2017
Published online: March 28, 2017
Processing time: 111 Days and 11.5 Hours
Clear-cell sarcoma is a rare, malignant soft tissue tumor that displays melanocytic differentiation with a distinct molecular profile. It is rarely localized in the gastrointestinal tract. Herein we reported a case of multiple synchronous clear-cell sarcomas of the gastrointestinal tract with parotid gland metastasis. A 51-year-old male patient presented with a growing painless mass under the right ear. A preoperative positron emission tomography/computed tomography showed multiple intestinal masses and a mass in the right parotid with increased glucose uptake, and he underwent operative treatment with resection of three tumors in the jejunum and ileum and then received a right parotidectomy. Postoperative pathological examination showed that cells in the intestinal tumor were consistent with clear-cell sarcoma of the gastrointestinal tract, and the malignant cells in the parotid gland were similar to the intestinal tumor. Immunohistochemical studies revealed positive expression of HMB-45, Melan-A, and S-100. EWSR1 gene fusion transcripts were undetectable by fluorescence in situ hybridization.
Core tip: Over the past 13 years, only 53 cases of clear-cell sarcomas of the gastrointestinal tract (CCS-GI) have been reported in the world. Most of the literature on CCS-GI describes a single tumor at diagnosis; our presentation is the third report of simultaneous tumors during the diagnosis to date and is the first case of CCS-GI with metastasis to the parotid gland. We also reviewed the literature on CCS-GI. Because of the high rarity, more cases need to be accumulated for further analysis.
- Citation: Su H, Liu WS, Ren WH, Wang P, Shi L, Zhou HT. Multiple clear-cell sarcomas of small intestine with parotid gland metastasis: A case report. World J Gastroenterol 2017; 23(12): 2258-2265
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2258
Clear-cell sarcoma (CCS) is a rare tumor of unknown origin that was first described by Enzinger[1] in 1965. CCS shows a predilection for the tendons or aponeuroses in the extremities in young adults aged 20-40 years[2]. Ekfors et al[3] described the first clear-cell sarcoma of the gastrointestinal tract (CCS-GI) in 1993, which occurred in the duodenum. Only a few cases[4] of CCS-GI have been reported. CCS-GI has specific histopathological, immunohistochemical, and genetic features. Here, we present a case of three synchronous clear-cell sarcomas in the jejunum and ileum with parotid gland metastasis.
A 51-year-old male presented with a two-year history of a growing painless mass under the right ear, initially with a size of a soybean. The mass grew noticeably in the last six months. There was a one-year history of night sweat and frequent stool (three to four times a day). There was no history of fever, weakness, dysphagia, dyspnea, cough, hoarseness, jaundice, vomiting, melena, hematochezia, abdominal pain, abdominal distension or significant weight loss. The patient had a 5-year medical history of hypertension and he was a hepatitis-B carrier of 30 years and a smoker of 40 pack-years. There was no family history of cancer.
On palpation, a 20 mm × 20 mm relatively well-defined and soft mass with no tenderness was observed along with multiple enlarged cervical nodules. Abdominal examination did not reveal any organomegaly or palpable lumps.
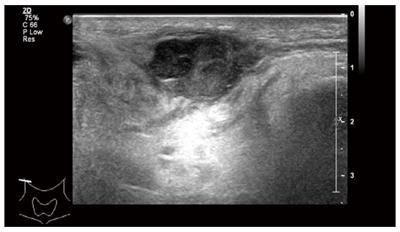
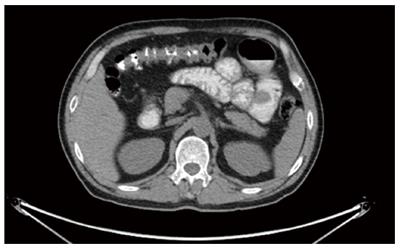
Ultrasonography of the neck two months ago revealed a relatively undefined hypoechoic mass measuring approximately 15 mm × 27 mm in its greatest dimension in the right parotid gland and submandibular gland (Figure 1) along with multiple enlarged right supraclavicular and upper cervical lymph nodes. A needle biopsy of the mass was performed and the pathologic report found malignant tumor cells. The patient was recommended for surgery for the mass in the parotid gland. The preoperative blood routine examination showed that the HGB was 106 g/L. Therefore, the patient underwent positron emission tomography/computed tomography (PET/CT). A 36 mm × 33 mm intestinal mass with increased glucose uptake, and multiple peripheral lymph nodes in the right mid-abdomen were found (Figure 2), and the maximum standard uptake value (SUV) was 6.6. An intestinal lesion with increased glucose uptake in the right hypogastrium was also seen and the SUV was 7.0. The mass in the right parotid and peripheral lymph nodes also showed increased glucose uptake, and the SUV was 10.3. Preoperative tumor makers, such as CA125, CA15-3, CA19-9,CA72-4, AFP, cyfra21-1, NSE,SCC, CEA, and ProGRP, did not show abnormal expression.
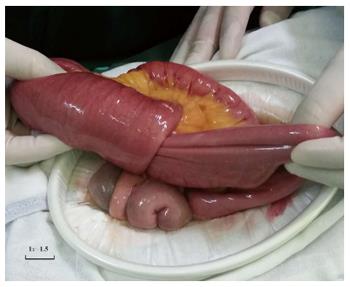
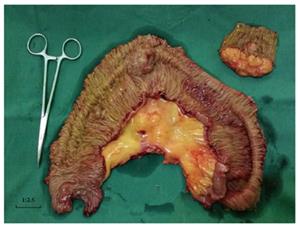
The patient underwent an exploratory laparotomy and the excision of multiple intestinal neoplasms. Operative exploration showed no ascites, pelvic, periaortic, peritoneal, omental deposits, or liver metastasis. No tumors were palpated in the cavity of the stomach, duodenum, colon, rectum, or the mesentery root. Three masses were found at the jejunum and ileum. Intra-operatively, the first tumor was present in the jejunum, located at 80 cm distal to the duodenojejunal junction. Intussusception was observed at the point, and the involved bowels were swollen and expanded (Figure 3). The second tumor was at the end of the intussusception (approximately at the fourth loop of intestine). The third tumor was present in the ileum, located at 80 cm proximal to the ileocecal junction. These three tumors of varying sizes invaded the serosa, and the surface of the serosa had shrunk and was depressed. Multiple enlarged lymph nodes were observed in the intestinal mesentery. Following serial ligation of the mesenteric vessels, resection of the involved bowels, along with the masses and mesentery, was performed, with a proximal margin of 10 cm and a distal margin of 10 cm. The first and second tumors were removed together in one segment of the intestine (Figure 4). Then, a primary anastomosis formed. The patient recovered gradually and then underwent right parotidectomy with retention of the facial nerve, followed by right cervical lymph node dissection 17 d after abdominal surgery because the pathology of the parotid gland neoplasms was undetermined.
Intestinal neoplasms: Upon gross examination, the specimen consisted of two segments of the small intestine: the longer one was approximately 26 cm with attached mesentery, and the other segment was 7.8 cm with attached mesentery. Two tumors were on the longer segment of intestine, one (2.5 cm × 2.2 cm × 1 cm) was at 11 cm from one margin and the other (6.5cm × 5.5cm × 4 cm) was at 19 cm from the same margin. A 2.5 cm × 1.9 cm × 1 cm tumor was on the other segment of the small intestine. The cut surface of the three tumors had hard, obscure borders that were white to tan in appearance.
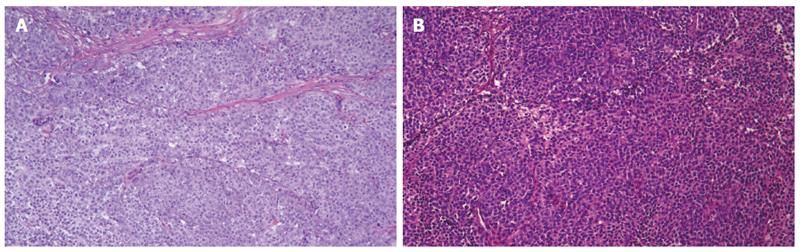
Microscopically, the jejunum and ileum tissues were infiltrated with malignant cells, which was consistent with CCS-GI (a type of gastrointestinal neural ectoderm tumor, GNET) based on morphology and immunohistochemistry (Figure 5A). The tumors had invaded the mucosal and muscular layers. There was no focal necrosis, vessel invasion or nerve invasion. The mitotic index exceeded 20/10 HPFs, and the tumor was grade G3 according to the FNCLL (French Fédération Nationale des Centres de Lutte Contre le Cancer) system.
Lymph node metastases (1/29) without invasion of the outer lymph node capsule: (1) peripheral lymph nodes of the jejunum: 1/26; and (2) peripheral lymph nodes of the ileum, 0/3.
Immunohistochemistry: S100 (3+), Vim (3+), GFAP (-), HMB-45 (2+), Melan-A (2+), Melanomapan (1+), CD56 (2+), Syn (-), CgA (-), AE1/AE3 (-), CD138 (-), CD19 (-), CD20 (-), CD3 (-), CD38 (-), CD79a (-), Ki-67 (+40%), LCA (-), MUM1 (-), CD117 (lesion+), CD34 (-), DOG1 (-), CD10 (-), Calponin (-), P63 (-), EBER (-).
Gene detection: EWSR1 gene fusion transcripts were undetectable by fluorescence in situ hybridization (FISH).
Parotid gland neoplasms: Upon gross examination, a 1-cm diameter nodule was found in a 5.5 cm × 3 cm × 2 cm area of tissue; the cut surface of the nodule had a tough, grey-to-yellow appearance.
Microscopically, the parotid gland tissues were infiltrated with malignant cells, which was consistent with CCS morphology and immunohistochemistry and morphologically similar to the previously assessed intestinal tumor (Figure 5B). Lymph tissues were found in the tumor and at the tumor edge, which may be metastatic lesions.
No lymph node metastases (0/30): (1) right cervical lymph nodes, level II, 0/10; (2) right cervical lymph nodes, level III, 0/12; (3) right cervical lymph nodes, level V, 0/5; (4) peripheral lymph nodes of the superficial lobe of the right parotid gland, 0/2; and (5) peripheral lymph nodes of the caudate lobe of the right parotid gland and tumor, 0/1.
Immunohistochemistry: S100 (3+), Melan-A (3+), Melanomapan (3+), HMB-45 (3+), AE1/AE3 (-), CK18 (-), Calponin (-), P63 (-), SMA (-).
Twenty days after the surgery on the parotid gland, the patient underwent CT imaging of the neck, thorax and abdominopelvic area, and no recurrence or metastasis was observed. He then started with 6 cycles of chemotherapy using an EI regimen (epirubicin 100 mg + ifosfamide 2 g D1-4+mesna 0.4 g 0 h, 4 h, and 8 h after the ifosfamide D1-4). At the time that this article was written, the patient was on the first cycle of the chemotherapy.
CCS-GI is so rare that only 53 cases (including our case) have been reported in the literature to date (Table 1)[3,5-39]. Most of the literature on CCS-GI describes the diagnosis of a single tumor; only two case reports[25,38] have described the diagnosis of two simultaneous tumors to date. CCS-GI often involves the ileum and jejunum, stomach and colon[4-7,9-12,14-35,38,39]. Because of the aggressive clinical course, regional and distant metastases are common in CCS-GI at presentation[5-7,9,10,15,17,21,25,27,29,31,37,39]. The lymph nodes, liver, and mesentery are the most common locations of the metastases at the time of presentation. The patient in our report had three synchronous masses in the jejunum and ileum, with metastasis to the parotid gland, and he attended the hospital mainly due to the swollen parotid gland. The presence of lymph nodes both inside and outside of the parotid gland makes it a common site of metastasis for head and neck neoplasms[40], but it is a very rare metastatic site for gastrointestinal tumors. In the limited literature on CCS-GI, this is the first case of CCS-GI with metastasis to the parotid gland.
| Ref. | Age (yr)/sex | Location | Maximum diameter of tumor(cm) | S-100 | HMB-45 | Melan-A | Genetic findings | Outcome |
| Alpers et al[5] | 26/F | Jejunum | 1.5 | ND | ND | ND | ND | Liver mets |
| Ekfors et al[3] | 38/M | Duodenum | 3.0 | Positive | Positive | ND | ND | Not given |
| Donner et al[6] | 37/M | Ileum | 6.5 | Positive | Negative | ND | t(12;22)(q13;q12-13) | Liver mets at 24 and 36 mo |
| Fukuda et al[7] | 74/M | Colon | 3.0 | Positive | Positive | ND | EWSR1-ATF1 by RT-PCR | Liver mets at 9 mo |
| Hu et al[8] | 10/M | Rectum | 5.0 | Positive | Positive | ND | ND | NA |
| Pauwels et al[9] | 30/M | Stomach | 4.0 | Positive | Negative | ND | t(12;22)(q13;q12) | LN and peritoneal mets at diagnosis |
| Zambrano et al[10] | 15/F | Jejunum | 5.0 | Positive | Negative | Negative | t(12;22)(q13;q12) | DOD 16 mo |
| 21/F | Jejunum | 4.0 | Positive | Negative | Negative | ND | DOD 12 mo | |
| 35/F | Ileum | 3.5 | Positive | Negative | Negative | ND | Liver mets at 12 mo | |
| 37/F | Ileum | 4.5 | Positive | Negative | Negative | ND | NA | |
| 32/M | Ileum | 5.0 | Positive | Negative | Negative | ND | NA | |
| 13/M | Stomach | 6.7 | Positive | Negative | Negative | ND | Local recurrence at 12 mo;2nd Local recurrence at 36 mo | |
| Achten et al[11] | 57/M | Jejunum | 6.5 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NA |
| Venkataraman et al[12] | 21/F | Ileum | 7.0 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NA |
| Covinsky et al[13] | 47/F | Pancreas | NA | Positive | Positive | Positive | EWSR1-ATF1 | NED 24 mo |
| by RT-PCR and | ||||||||
| FISH | ||||||||
| 85/F | Mesentery | NA | Positive | Positive | Positive | EWSR1-ATF1 | DOD 1 mo | |
| by RT-PCR and | ||||||||
| FISH | ||||||||
| Taminelli et al[14] | 35/M | Ileum | 1.8 | Positive | Negative | Positive | EWSR1-ATF1/ by RT-PCR | DOD 15 mo |
| Friedrichs et al[15] | 41/M | Jejunum | 8.7 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | Liver mets at 6 mo |
| Huang et al[16] | 40/M | Stomach | 3.0 | Positive | Negative | Positive | ND | NED 9 mo |
| Antonescu et al[17] | 81/F | Colon | 7.5 | Positive | Negative | Negative | EWSR1-CREB1 by RT-PCR | Mets to liver and |
| peritoneum at | ||||||||
| 60 mo | ||||||||
| 42/F | Ileum | 5.7 | Positive | Negative | Negative | EWSR1-CREB1 by RT-PCR | NA | |
| 42/F | Ileum | 3.5 | Positive | Negative | Negative | EWSR1-CREB1 by RT-PCR | Peritoneal and | |
| liver mets at | ||||||||
| diagnosis | ||||||||
| 51/F | Jejunum | NA | Positive | Negative | Negative | EWSR1 | Peritoneal and | |
| rearrangement | liver mets; AWD | |||||||
| by FISH | ||||||||
| 18/F | Jejunum | NA | Positive | Negative | Negative | EWSR1-ATF1 by RT-PCR | Local recurrence | |
| Granville et al[18] | 16/M | Ileum | 5.0 | Positive | Negative | ND | EWSR1-ATF1 by RT-PCR; t(12;22)(q13;q12) | DOD 15 mo |
| Comin et al[19] | 31/F | Ileum | 2.8 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NA |
| Lyle et al[20] | 46/M | Jejunum | 11.0 | Positive | Positive | Positive | EWSR1 rearrangement by FISH; EWSR1-ATF1 by RT-PCR | NED 7 mo |
| 49/M | Cecum | 10.5 | Positive | Positive | Positive | EWSR1 rearrangement by FISH; EWSR1-ATF1 by RT-PCR | DOD 12 mo | |
| 60/M | Jejunum | 10.0 | Positive | Positive | Positive | EWSR1-ATF1 by RT-PCR | DOD 28 mo | |
| 62/M | Ileum | 4.0 | Positive | Positive | Positive | EWSR1 rearrangement by FISH; EWSR1-ATF1 by RT-PCR | DOD 12 mo | |
| Abdulkader et al[21] | 37/M | Jejunum | 8.2 | Positive | Negative | ND | EWSR1 rearrangement by FISH | Liver mets at 2 mo |
| Lagmay et al[22] | 10/F | Stomach | 7.8 | Positive | Negative | Negative | EWSR1 rearrangement by FISH; EWSR1-ATF1 by RT-PCR | NED 4 mo |
| Joo et al[23] | 60/M | Ileum | 2.4 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NA |
| 46/M | Jejunum | 6.0 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NA | |
| Terazawa et al[24] | Early 20s/F | Ileum | 3.0 | Positive | ND | ND | EWSR1-ATF1 by RT-PCR | NED at 24 mo |
| Shenjere et al[25] | 53/F | Ileum | 5.0 | Positive | Negative | Negative | EWSR1-ATF1 by RT-PCR | Regional LN mets at diagnosis/ NED at 7 mo |
| 26/F | Small and large bowel1 | 13.5/10.1 | Positive | Negative | Negative | EWSR1-CREB1 by RT-PCR | NA | |
| 66/M | Ileum | 2.5 | Positive | Negative | Negative | EWSR1-CREB1 by RT-PCR | Regional LN mets at diagnosis/NED | |
| Balkaransingh et al[26] | 15/M | Ileum | NA | ND | ND | ND | EWSR1 rearrangement by FISH | NA |
| Yang et al[27] | 15/M | Ileum | 4.0 | Positive | ND | ND | EWSR1 rearrangement by FISH | Liver mets at 12 mo |
| Suárez-Vilela et al[28] | 36/F | Jejunum | 1.5 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NA |
| D’Amico et al[29] | 69/F | Ileum | 4.0 | Positive | Negative | ND | EWSR1 rearrangement by FISH | Liver mets at 2 mo |
| Lasithiotakis et al[30] | 49/F | Jejunum | 3.0 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NED 20 mo |
| Huang et al[31] | 45/F | Colon | 4.0 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | Liver mets at 20 mo |
| Mallick et al[32] | 45/M | Jejunum | 4.4 | Positive | Negative | Negative | ND | NA |
| Kong et al[33] | 17/M | Stomach | 6.0 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NED 10 mo |
| Liu et al[34] | 76/M | Jejunum | 2.5 | Positive | Negative | Negative | EWSR1-ATF1 by RT-PCR | NA |
| Thway et al[35] | 36/M | Ileum | 3.0 | Positive | Negative | Negative | EWSR1-CREB1 by RT-PCR | DOD 7 mo |
| Huang [36] | 36/M | Pancreas | 4.0 | Positive | Positive | Positive | EWSR1 rearrangement by FISH | Liver mets at 10 mo |
| Yegen et al[37] | 25/F | Ileum | 3.2 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | Liver mets at diagnosis and at 15 mo. Ovarian mets and peritoneal dissemination at 47 mo |
| Moslim et al[38] | 57/M | Duodenum and Jejunum2 | 5.5/7.5 | Positive | Negative | Positive | EWSR1 rearrangement by FISH | NED 30 mo and then DOD 4 mo later |
| Chen et al[39] | 29/F | Jejunum | 6.0 | Positive | Negative | Negative | EWSR1 rearrangement by FISH | NED 17 mo |
| Our case | 51/M | Duodenum and Jejunum3 | 6.5/2.5/2.5 | Positive | Positive | Positive | EWSR1 rearrangement undetectable by FISH | NED up to date |
CCS-GI shows specific histopathological, immunohistochemical, ultrastructural, and genetic features[2,4]. In 2010, Kosemehmetoglu et al[41] first divided CCS-GI into two subtypes according to its histomorphology: (1) CCS-like gastrointestinal tumor (CCSLGT); and (2) CCS of soft tissue (CCS-ST). However, there has been disagreement about whether these subtypes are two independent entities[31]. In 2003, Zambrano et al[10] reported 6 cases of CCSLGTs. They found that the CCSLGTs were at least focally positive for the S100 protein, but most did not express melanocytic markers such as HMB-45 or Melan-A. Meanwhile, Huang et al[36] found that certain CCS-STs were positive for the S100 protein and most could express melanocytic markers such as HMB-45 or Melan-A. Several reports found that > 90% of cases of CCS were associated with the reciprocal translocation t (12; 22) (q13; q12), resulting in fusion of the EWSR1 gene, located at 22q12, and the ATF1 gene, located at 12q13[2,41-46]. To date, these translocations have never been observed in malignant melanoma[13,22,43-46], which has a very similar histologic appearance to CCS[20]. Immunohistochemical staining of CCS reveals positivity for the S100 protein as well as melanocyte-specific markers, with this combination of staining allowing for CCS to be distinguished from malignant melanoma histologically. In our case, the tumor was consistent with CCS-GI based on morphology, was positive for the S100 protein, and expressed melanocytic markers such as HMB-45 and Melan-A, but EWSR1 gene fusion transcripts were undetectable by FISH.
Currently the most effective treatment for CCS-GI is extensive resection of the tumor and peripheral lymph nodes; chemotherapy and radiotherapy appear to have little effect[31]. The clinical behavior of CCS-GI seems to be highly aggressive, with high rates of local recurrence, lymph node or visceral metastases, and death, generally within < 36 mo[41,46]. In the current report, the patient underwent excision of multiple intestinal neoplasms and right parotidectomy before the first cycle of the chemotherapy and no recurrence or metastasis has been observed during the follow-up to date.
In conclusion, CCS-GI is a highly rare soft-tissue sarcoma with distinct morphological, immunohistochemical, and genetic features. This case demonstrates that the parotid gland is a potential metastatic site for CCS-GI. Prior to developing a routine method to diagnose and treat CCS-GI, more cases need to be accumulated for further analysis.
A 51-year-old male presented with a two-year history of a growing painless mass lesion under the right ear that had grown noticeably over the past six months and a one-year history of night sweat and frequent stool.
A relatively well-defined soft mass with no tenderness was observed along with multiple enlarged cervical nodules.
Small intestinal stromal tumors, lymphoma, head and neck neoplasm, sarcomatoid carcinoma.
The patient’s laboratory test had no remarkable findings.
Positron emission tomography/computed tomography showed an intestinal mass with involvement of multiple peripheral lymph nodes and mass in the right parotid.
The intestinal neoplasms and parotid gland neoplasm were consistent with CCS based on morphology and immunohistochemistry.
The patient underwent curative resection and postoperative chemotherapy.
Only 53 cases of clear-cell sarcomas of the gastrointestinal tract (CCS-GI) have been reported in the literature to date, and CCS-GI shows distinct morphological, immunohistochemical, and genetic features.
CCS-GI is a highly rare soft tissue sarcoma.
The present case report is the third instance of diagnosis of simultaneous multiple CCS-GIs to date and the first case of CCS-GI with metastasis to the parotid gland.
The authors have described a case of multiple clear-cell sarcomas of the small intestine with parotid gland metastasis. The article highlights the morphological, immunohistochemical, and genetic features of the tumors.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Muhammad JS, Mulder KE S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Wang CH
| 1. | Enzinger FM. Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163-1174. [PubMed] |
| 2. | Hocar O, Le Cesne A, Berissi S, Terrier P, Bonvalot S, Vanel D, Auperin A, Le Pechoux C, Bui B, Coindre JM. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012:984096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Ekfors TO, Kujari H, Isomäki M. Clear cell sarcoma of tendons and aponeuroses (malignant melanoma of soft parts) in the duodenum: the first visceral case. Histopathology. 1993;22:255-259. [PubMed] |
| 4. | Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, Adsay V, Chou PM, Amanuel B, Vantuinen P. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2012;36:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Alpers CE, Beckstead JH. Malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells. Enzyme histochemistry distinguishes tumor cells from giant cells. Am J Surg Pathol. 1985;9:57-64. [PubMed] |
| 6. | Donner LR, Trompler RA, Dobin S. Clear cell sarcoma of the ileum: the crucial role of cytogenetics for the diagnosis. Am J Surg Pathol. 1998;22:121-124. [PubMed] |
| 7. | Fukuda T, Kakihara T, Baba K, Yamaki T, Yamaguchi T, Suzuki T. Clear cell sarcoma arising in the transverse colon. Pathol Int. 2000;50:412-416. [PubMed] |
| 8. | Hu XL, Wang WX. Clear cell sarcoma of the rectum: a case report. Zhonghua Bing Li Xue Za Zhi. 2001;30:77. [DOI] [Full Text] |
| 9. | Pauwels P, Debiec-Rychter M, Sciot R, Vlasveld T, den Butter B, Hagemeijer A, Hogendoorn PC. Clear cell sarcoma of the stomach. Histopathology. 2002;41:526-530. [PubMed] |
| 10. | Zambrano E, Reyes-Mugica M, Franchi A, Rosai J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75-81. [PubMed] |
| 11. | Achten R, Debiec-Rychter M, De Wever I, Sciot R. An unusual case of clear cell sarcoma arising in the jejunum highlights the diagnostic value of molecular genetic techniques in establishing a correct diagnosis. Histopathology. 2005;46:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Venkataraman G, Quinn AM, Williams J, Hammadeh R. Clear cell sarcoma of the small bowel: a potential pitfall. Case report. APMIS. 2005;113:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Covinsky M, Gong S, Rajaram V, Perry A, Pfeifer J. EWS-ATF1 fusion transcripts in gastrointestinal tumors previously diagnosed as malignant melanoma. Hum Pathol. 2005;36:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Taminelli L, Zaman K, Gengler C, Peloponissios N, Bouzourene H, Coindre JM, Hostein I, Guillou L. Primary clear cell sarcoma of the ileum: an uncommon and misleading site. Virchows Arch. 2005;447:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Friedrichs N, Testi MA, Moiraghi L, Modena P, Paggen E, Plötner A, Wiechmann V, Mantovani-Löffler L, Merkelbach-Bruse S, Buettner R. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: further evidence for a new tumor entity. Int J Surg Pathol. 2005;13:313-318. [PubMed] |
| 16. | Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, Lu Z, Zhou X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2006;448:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma--association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (36)] |
| 18. | Granville L, Hicks J, Popek E, Dishop M, Tatevian N, Lopez-Terrada D. Visceral clear cell sarcoma of soft tissue with confirmation by EWS-ATF1 fusion detection. Ultrastruct Pathol. 2006;30:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Comin CE, Novelli L, Tornaboni D, Messerini L. Clear cell sarcoma of the ileum: report of a case and review of literature. Virchows Arch. 2007;451:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Lyle PL, Amato CM, Fitzpatrick JE, Robinson WA. Gastrointestinal melanoma or clear cell sarcoma? Molecular evaluation of 7 cases previously diagnosed as malignant melanoma. Am J Surg Pathol. 2008;32:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Abdulkader I, Cameselle-Teijeiro J, de Alava E, Ruiz-Ponte C, Used-Aznar MM, Forteza J. Intestinal clear cell sarcoma with melanocytic differentiation and EWS [corrected] rearrangement: report of a case. Int J Surg Pathol. 2008;16:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Lagmay JP, Ranalli M, Arcila M, Baker P. Clear cell sarcoma of the stomach. Pediatr Blood Cancer. 2009;53:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Joo M, Chang SH, Kim H, Gardner JM, Ro JY. Primary gastrointestinal clear cell sarcoma: report of 2 cases, one case associated with IgG4-related sclerosing disease, and review of literature. Ann Diagn Pathol. 2009;13:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Terazawa K, Otsuka H, Morita N, Yamashita K, Nishitani H. Clear-cell sarcoma of the small intestine detected by FDG-PET/CT during comprehensive examination of an inflammatory reaction. J Med Invest. 2009;56:70-75. [PubMed] |
| 25. | Shenjere P, Salman WD, Singh M, Mangham DC, Williams A, Eyden BP, Howard N, Knight B, Banerjee SS. Intra-abdominal clear-cell sarcoma: a report of 3 cases, including 1 case with unusual morphological features, and review of the literature. Int J Surg Pathol. 2012;20:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Balkaransingh P, Saad SA, Govil SC, Thind PK, Ballance CM, Weiss AR. Clear cell sarcoma of the gastrointestinal tract presenting as a second malignant neoplasm following neuroblastoma in infancy. Pediatr Blood Cancer. 2012;58:481-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Yang JC, Chou AJ, Oeffinger KC, La Quaglia MP, Wolden SL. Clear cell sarcoma of the gastrointestinal tract after very low-dose therapeutic radiation therapy: a case report. J Pediatr Surg. 2012;47:1943-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Suárez-Vilela D, Izquierdo FM, Tojo-Ramallo S, R Riera-Velasco J, Escobar-Stein J. Malignant gastrointestinal neuroectodermal tumor showing overlapped immunophenotype with synovial sarcoma: CD99 and SOX10 antibodies are useful in differential diagnosis. Am J Surg Pathol. 2012;36:1905-198; author reply 1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | D’Amico FE, Ruffolo C, Romeo S, Massani M, Dei Tos AP, Bassi N. Clear cell sarcoma of the ileum: report of a case and review of the literature. Int J Surg Pathol. 2012;20:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Lasithiotakis K, Protonotarios A, Lazarou V, Tzardi M, Chalkiadakis G. Clear cell sarcoma of the jejunum: a case report. World J Surg Oncol. 2013;11:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Huang HF, Liu Q, Hong BU, Chen M, Chen HJ, Lin YY, Zhang HY, Pathology DO, Hospital WC and University S. Clear cell sarcoma of gastrointestinal tract: clinicopathologic analyses and review of literatures. Linchuang Yu Shiyan Binglixue Zazhi. 2014;30:383-388. [DOI] [Full Text] |
| 32. | Mallick S, Singh L, Rajan K, Sharma MC, Bansl V, Dinda AK. Malignant melanoma of soft parts with osteoclast-rich giant cells: A rare tumour of the jejunum. Australas Med J. 2014;7:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Kong J, Nan LI, Shiwu WU, Guo X, Congyou GU and Feng Z. Malignant gastrointestinal neuroectodermal tumor: A case report and review of the literature. Oncol Lett. 2014;8:2687-2690. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Liu C, Ren Y, Li X, Cao Y, Chen Y, Cui X, Li L, Li F. Absence of 19 known hotspot oncogenic mutations in soft tissue clear cell sarcoma: two cases report with review of the literature. Int J Clin Exp Pathol. 2014;7:5242-5249. [PubMed] |
| 35. | Thway K, Judson I, Fisher C. Clear cell sarcoma-like tumor of the gastrointestinal tract, presenting as a second malignancy after childhood hepatoblastoma. Case Rep Med. 2014;2014:984369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Huang J, Luo RK, Du M, Zeng HY, Chen LL, Ji Y. Clear cell sarcoma of the pancreas: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:2171-2175. [PubMed] |
| 37. | Yegen G, Güllüoğlu M, Mete Ö, Önder S, Kapran Y. Clear cell sarcoma-like tumor of the gastrointestinal tract: a case report and review of the literature. Int J Surg Pathol. 2015;23:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Moslim MA, Falk GA, Cruise M, Morris-Stiff G. Simultaneous Clear Cell Sarcomas of the Duodenum and Jejunum. Case Rep Med. 2016;2016:1534029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Chen L, Zhou AP. Small intestinal clear cell sarcoma in gestation period masquerading as an abdominal abscess. Clin Misdiagnosis and Mistherapy. 2016;29:33-34. [DOI] [Full Text] |
| 40. | Park SW, Eade T, Pang L, Wignall A, Veivers D. Role of neck dissection in metastatic squamous cell carcinoma to the parotid gland. J Laryngol Otol. 2016;130 Suppl 4:S54-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Kosemehmetoglu K, Folpe AL. Clear cell sarcoma of tendons and aponeuroses, and osteoclast-rich tumour of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: a review and update. J Clin Pathol. 2010;63:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Coindre JM. New WHO classification of tumours of soft tissue and bone. Ann Pathol. 2012;32:S115-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Wang WL, Mayordomo E, Zhang W, Hernandez VS, Tuvin D, Garcia L, Lev DC, Lazar AJ, López-Terrada D. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts). Mod Pathol. 2009;22:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 44. | Panagopoulos I, Mertens F, Isaksson M, Mandahl N. Absence of mutations of the BRAF gene in malignant melanoma of soft parts (clear cell sarcoma of tendons and aponeuroses). Cancer Genet Cytogenet. 2005;156:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Panagopoulos I, Mertens F, Dêbiec-Rychter M, Isaksson M, Limon J, Kardas I, Domanski HA, Sciot R, Perek D, Crnalic S. Molecular genetic characterization of the EWS/ATF1 fusion gene in clear cell sarcoma of tendons and aponeuroses. Int J Cancer. 2002;99:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Langezaal SM, Graadt van Roggen JF, Cleton-Jansen AM, Baelde JJ, Hogendoorn PC. Malignant melanoma is genetically distinct from clear cell sarcoma of tendons and aponeurosis (malignant melanoma of soft parts). Br J Cancer. 2001;84:535-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |