Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2223
Peer-review started: June 29, 2016
First decision: August 29, 2016
Revised: October 10, 2016
Accepted: November 15, 2016
Article in press: November 15, 2016
Published online: March 28, 2017
Processing time: 276 Days and 12.4 Hours
To determine the placebo response rate associated with different types of placebo interventions used in psychological intervention studies for irritable bowel syndrome.
Randomized controlled trials comparing psychological interventions (stress management/relaxation therapy (cognitive) behavioral therapy, short-term psychodynamic therapy, and hypnotherapy) for the treatment of adult patients with irritable bowel syndrome (IBS) diagnosed with the Manning or Rome criteria with an adequate placebo control treatment and reporting data on IBS symptom severity were identified by searching PubMed, Embase, the Cochrane Library, CINAHL and PsycINFO databases. Full-text articles that were written in English and published between 1966 and February 2016 in peer-reviewed journals were selected for the present review. Placebo interventions were considered to be adequate if the number of sessions and the amount of time spent with the therapist were the same as in the active treatment. The placebo response rate (PRR) was computed for IBS symptom severity (primary outcome measure) as well as for anxiety, depression and quality of life (secondary outcome measures).
Six studies, with a total of 555 patients met the inclusion criteria. Four studies used an educational intervention, whereas two studies used a form of supportive therapy as the placebo intervention. The PRR for IBS symptom severity ranged from 25% to 59%, with a pooled mean of 41.4%. The relative PRR for the secondary outcome measures ranged from 0% to 267% for anxiety, 6% to 52% for depression 20% to 125% for quality of life. The PRR associated with pharmacological treatments, treatment with dietary bran and complementary medicine ranged from 37.5% to 47%. Contrary to our expectations, the PRR in studies on psychological interventions was comparable to that in studies on pharmacological, dietary and alternative medical interventions.
The PRR is probably determined to a larger extent by patient-related factors, such as expectations and desire for the treatment to be effective, than the content of the placebo intervention.
Core tip: This study highlights the fact that providing patients with realistic, but positive information about the expected effect of the treatment for irritable bowel syndrome is important to optimize the placebo response.
- Citation: Flik CE, Bakker L, Laan W, van Rood YR, Smout AJPM, de Wit NJ. Systematic review: The placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol 2017; 23(12): 2223-2233
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2223.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2223
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by recurrent episodes of abdominal pain, discomfort, and altered bowel habits that are not explained by structural or biochemical abnormalities[1]. Several pathophysiological mechanisms underlying IBS have been proposed. According to the bio-psycho-social model of IBS, a disturbance in intestinal motility and enhanced visceral sensitivity interact with other factors, such as environmental influences, parent-child interactions and disturbed stress responses[2].
Because of the limited effect of pharmacotherapy[3,4], there has been increasing interest in psychological treatments for IBS. Two Cochrane reviews provided evidence for the effectiveness of cognitive behavioral therapy (CBT), interpersonal psychotherapy (IPT)[5] and hypnotherapy[6]. Another review[3] concluded that CBT, IPT and hypnotherapy, not relaxation therapy, were more effective than typical care in relieving IBS symptoms. In 2014, a systematic review showed that relaxation therapy was effective in reducing IBS symptoms[7].
In the research on psychological treatment methods, it is possible that the treatment effect is the result of increased attention and time investment on the patient rather than the therapy itself. In randomized controlled trials, a placebo group should be used to control for this effect. The placebo group is defined as a “matched control group participating in an activity regarded therapeutically inert from the theoretical perspective of the therapy under study”[8].
Although a placebo control is different in pharmacological studies than in psychological studies, they are equally important in both cases for achieving a methodologically valid comparison. In pharmacological research the placebo response rate (PRR) is variable and may be affected by the type, dosage, size, color, frequency, and route of administration of the placebo medication[9]. In psychological interventions, the PRR may result from the consultation itself and the relationship with the physician/therapist[10]. IBS patients experienced greater benefits from augmented, positive interaction with a practitioner than from limited or no interaction at all (i.e., being put on a waiting list)[10]. They also benefitted more from an increased number of office visits and a longer duration of treatment[11,12], suggesting that supportive and empathic interaction with a practitioner might influence clinical outcomes. Placebo effects can be defined as “the beneficial effects that are attributable to the responses of the patient to the context in which the treatment is delivered, rather than to specific actions of the treatment”[13]. In RCTs in which psychological interventions are studied, a control intervention with an equal number and length of sessions, using an individual or a group format and with comparably trained of therapists[8] should be used to control for these effects. Currently, researchers who examine psychological interventions debate whether and to what degree the effects of psychotherapy are based on placebo effects or therapeutic factors[8,14,15].
From a methodological perspective, the PRR is viewed as an effect that needs to be corrected for. However, from a clinical perspective, a high PRR and a good treatment response are considered to be equally positive outcomes. From this perspective, when the PRR associated with psychological interventions is larger than associated with pharmacological interventions, the psychological placebo treatment may be of greater clinical relevance. The positive relationship with the therapist can be used as an additional beneficial factor.
We presumed that the placebo response would be greater in psychological interventions than in drug trials. So far, studies on the PRR in IBS have focused primarily on pharmacological treatments, treatment with dietary bran and complementary medicine. PRR rates in these studies ranged from 37.5% to 47%[11,16-18].
One systematic review of alternative therapies for irritable bowel syndrome included a meta-analysis of psychological therapies[19]. A separate evaluation of the results of four of the 17 included studies that used a “true placebo group” was reported. The PRR of these four studies was 30.4%.
This study searched the MEDLINE database for articles published through 2001, sample sizes were low and the IBS criteria for the inclusion of studies were not defined. Since then, results of a number of new studies have been published. The present study aims to review systematically the PRR associated with different types of placebo control interventions in studies on psychological interventions in IBS and compare them to the PRR of placebo control interventions of drug trials.
Types of studies: Randomized controlled trials comparing psychological interventions for the treatment of IBS with a placebo control treatment that were written in English and published as a full text in a peer-reviewed journal, were eligible for inclusion. Cross-over studies were excluded, as were studies comparing two types of psychological therapeutic interventions without a placebo control.
Types of participants: Studies including male or female patients over the age of 18 years with IBS diagnosed according to Manning or Rome I, II or III criteria were included in the analysis.
Types of interventions: In accordance with earlier Cochrane reviews[5,6], the following psychological interventions for the treatment of IBS were considered: stress management/relaxation therapy (cognitive) behavioral therapy, short-term psychodynamic therapy, and hypnotherapy.
Types of placebo treatments: Because of the potential impact of the format of the placebo intervention on the outcome[8], only studies with placebo-controlled interventions using the same number of sessions and therapeutic time as the active treatment were considered to be eligible for inclusion (For Baskin’s other criteria, see Table 1). Studies using a waiting list, usual care, symptom monitoring and therapeutic contact by phone or internet, were excluded.
| Ref. | Year | Country | n | Mean age (yr) | Female sex | Recruitment | Years of illness | Criteria | Therapy | Control | Format | Ses-sions | Trained therapist intervention/placebo | Protocol inter-vention/placebo |
| Craske et al[23] 2011 | 2011 | United States | 110 | 39.4 | 74 | Community advertisement; university clinic | RII | Cognitive-behavior-al therapy | Psycho-educatio-nal support | Individual | 10 | Unclear/unclear | Yes/yes | |
| Drossman et al[24] 2003 | 2003 | United States and Canada | 215 | 37.3 | 100 | Community and hospital advertisement; physician referral in community or university-based practices | R1 | Cognitive-behavior-al therapy | Psycho-educational support | Unclear | 12 | Yes/yes | Yes/yes | |
| Gaylord et al[25] 2011 | 2011 | United States | 75 | 42.7 | 100 | Local advertisement; physician care | RII | Mindful-ness | Support | Group | 9 | Yes/yes | Yes/yes | |
| Moser et al[26] 2013 | 2013 | Austria | 100 | 45.5 | 79 | Primary, secondary care and university clinic | 4/10.5 | RIII | Hypno-therapy | Support | Group | 10 | Yes/yes | Yes/no |
| Payne et al[27] 1995 | 1995 | United States | 34 | 40.1 | 85 | Personal physician | 16 | RI | Cognitive therapy | Psycho-educatio-nal support | Therapy: individual; Control: group | 10 | Yes/unclear | Yes/yes |
| Shinozaki[28] 2010 | 2010 | Japan | 21 | 31.6 | 52 | University clinic | Relax | Psycho-educatio-nal support | Individual | 8 | Yes/unclear | Yes/yes |
Types of outcome measures: Studies were eligible for inclusion if they reported improvement in IBS symptoms and/or abdominal pain (measured with a validated IBS questionnaire) and/or adequate relief of abdominal pain and discomfort or satisfactory relief of IBS symptoms as recommended by the Rome III classification system for the design of IBS treatment trials[20].
Studies were excluded if no information on the effectiveness of the psychological interventions was available or if the proportion of patients in each group with overall symptom improvement after therapy was not reported.
Electronic searches: We performed a systematic search of RCTs published from 1966 to February 2016 that were available in PubMed, Embase, the Cochrane Library, CINAHL and PsychINFO databases. The following search terms were used: “irritable bowel syndrome” [MeSH] OR “colonic diseases, functional” [MeSH: NoExp] OR “irritable bowel syndrome” [tiab] OR “irritable bowel syndromes” [tiab] OR “irritable colon” [tiab] OR “mucous colitis” [tiab] OR “ibs” [tiab] OR “functional colonic disease” [tiab] OR “functional colonic diseases” [tiab] OR “spastic colon” [tiab];
Combined with: ((cognitive[tiab] OR psychological[tiab] OR psychologic[tiab] OR psychodynamic[tiab] OR psychoanalytic[tiab] OR “psycho analytic”[tiab] OR stress[tiab] OR relaxation[tiab] OR conditioning[tiab] OR “problem solving”[tiab] OR interpersonal[tiab] OR “hypno analytic”[tiab] OR behavioral[tiab] OR behavioural[tiab] OR behavior[tiab] OR behaviour[tiab]) AND (therapy[tiab] OR therapies[tiab] OR treatment[tiab] OR treatments[tiab] OR intervention[tiab] OR interventions[tiab] OR management[tiab])) OR (psychotherapy[tiab] OR psychotherapies[tiab] OR psychoeducation[tiab] OR “psycho education”[tiab] OR psychoeducational[tiab] OR psychotherapy[tiab] OR hypnotherapy[tiab] OR hypnosis[tiab] OR hypnoses[tiab] OR hypnotism[tiab] OR hypnoanalysis[tiab] OR mesmerism[tiab] OR “hypno analysis”[tiab] OR autohypnosis[tiab] OR “auto hypnosis”[tiab] OR psychoanalyses[tiab] OR psychoanalysis[tiab] OR “psycho analysis”[tiab] OR biofeedback[tiab]) OR (“Behavior Therapy”[MeSH] OR “Psychoanalysis”[MeSH] OR “Psychoanalytic Therapy”[MeSH]). No filters or limits were used.
Study selection: Two authors (CF and LB) reviewed the title and abstract of each identified article to determine the extent to which it met eligibility criteria, such as type of study, participants, interventions, placebo treatments and outcome measures, as described previously. A manual search of the references listed in the articles retrieved from the online search was performed to identify additional studies. The full texts of the selected articles were then reviewed by the same authors to assess eligibility based on the same criteria. Discrepancies between the selections made by CF and LB were resolved by a third author (NdW).
Data extraction: From the resulting selection of papers, information on the number of patients, patient characteristics (gender, mean age, and mean duration of illness), criteria for diagnosis (Rome I, Rome II, Rome III or Manning), treatment setting, intervention (type, group or individual delivery format, number of sessions, training of therapists and use of treatment/placebo manual), placebo control (type, group or individual delivery format, number of sessions, training of therapists and use of treatment/placebo manual), duration of treatment, duration of the follow-up period, and results relating to the primary and secondary outcome measures were extracted.
Assessment of risk of bias: The risk of bias assessment tool developed by the Cochrane Collaboration for RCTs[21] was used. The following sources of bias can be assessed with high, low or unclear bias ratings: adequate generation of the allocation sequence; concealment of allocation to conditions; blinding of participants and personnel; handling of incomplete outcome data; and selective outcome reporting. The percentage of patients who dropped out of the intervention and placebo control group as well as the results of the intention-to treat (ITT) analysis (when provided) were added.
In this review, the post-treatment IBS symptom severity scores was the primary outcome measure. Most studies presented the results of the ITT analysis, although only one study included the results of the per protocol (PP) analyses. Secondary outcome measures were improvement of symptoms of anxiety and depression as well as quality of life. Quality of life was recommended as an outcome measure by the Rome III committee, whereas anxiety and depression were chosen as secondary outcome measures due to their high rates of co-morbidity[22].
The response rate of the primary outcome measures was calculated by dividing the percentage of patients who responded according to the study criteria by the number of patients in the ITT analysis or who completed treatment. Relative placebo responses (Rel-PR) with 95% confidence intervals (95%CI) were calculated as the ratio of placebo response to active treatment response. Additionally, the mean Rel-PR across all studies was calculated.
The weighted average PRR was calculated by adding up the PRR per study multiplied by the number of patients in the placebo control group of that study and dividing the product by the total number of control patients in all of the studies.
Criteria for response evaluation were not available for the secondary measures; therefore, PRRs for the secondary outcome measures of anxiety, depression and quality of life were calculated by setting the response rate for these measures in the active arm at 100% and computing the response rate in the placebo arm as a relative percentage of the active arm. A relative response rate > 100% indicated that the placebo intervention was more effective than the treatment intervention. To allow for comparison of the PRR between the primary and secondary outcome measures, we recalculated the rates for the primary outcome measures in this way.
For the secondary outcome measures, the PRR for the different types of placebo interventions were calculated by adding up the PRR per study multiplied with the number of patients in the placebo control group of that study and dividing the product by the total number of control patients in all of the studies.
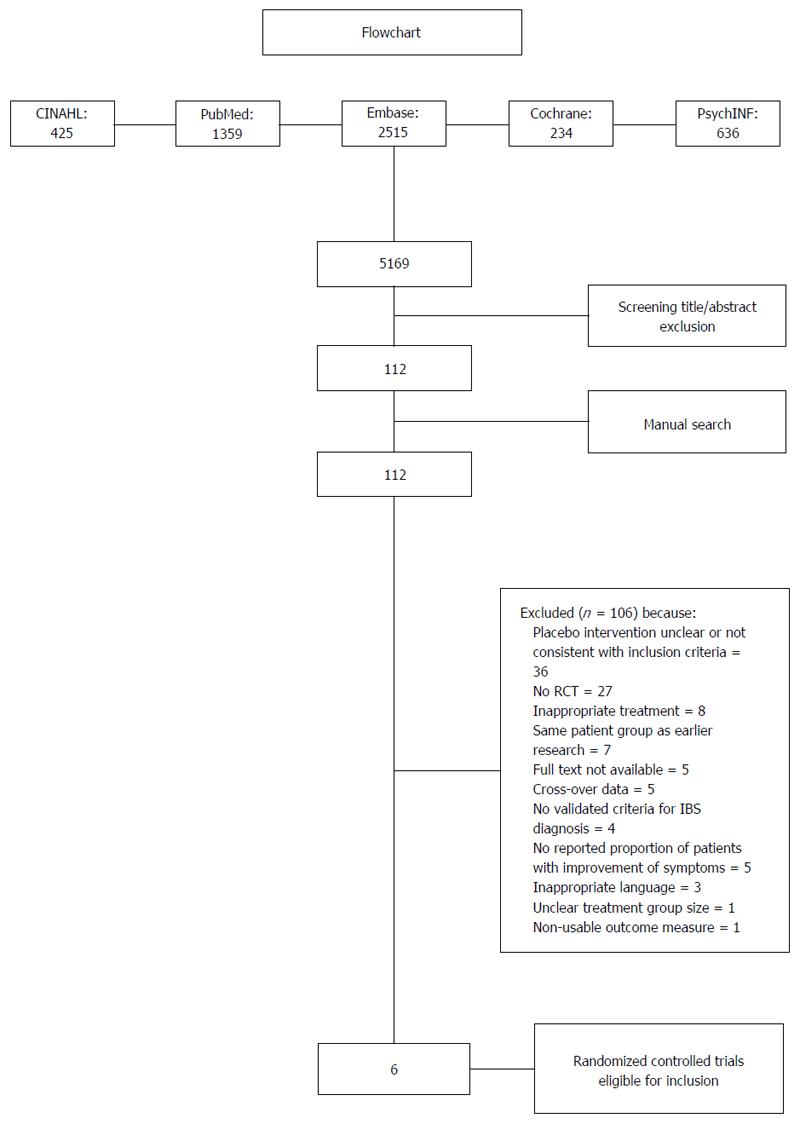
The literature search resulted in the identification of 5169 studies. After screening the titles and abstracts, 112 studies were potentially eligible (see the flowchart in Figure 1). The manual search yielded no additional studies (Figure 1).
After reviewing the full manuscripts of these studies, 106 studies were excluded for various reasons (see the flowchart in Figure 1), leaving six eligible trials[23-28] that were included in the analysis. The characteristics of the included studies are shown in Table 1. Sample sizes ranged from 21[21] to 215[24]. Patients were recruited from primary, secondary and tertiary care institutions, although they were also partially recruited through advertisements in three studies[23-25]. The treatment setting was unclear in two of the selected studies[25,27] (Table 1).
The mean age of the study populations ranged from 31.6 to 45.5 years. The proportion of female participants ranged from 52.4% to 100%. Only one study reported the duration of IBS[26]: a median of 4 years for the intervention group and 10.5 years for the placebo group. The duration of treatment and the placebo intervention ranged from 8 wk to 3 mo. The duration of the follow-up period ranged from 3 mo to 12 mo.
Four of the six studies fulfilled almost all quality criteria (Table 2).
| Ref. | Year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Dropout treatment/placebo control (%) | ITT or PP |
| Craske et al[23] 2011 | 2011 | Low | Low | Low | Low | Low | Low | Interoceptive Exposure:34; Stress Management: 36/16 | ITT |
| Drossman et al[24] 2003 | 2003 | Low | Low | Low | Low | Low | Low | 13/24 | ITT |
| Gaylord et al[25] 2011 | 2011 | Low | Low | Low | Low | Low | Low | 6/18 | ITT |
| Moser et al[26] 2013 | 2013 | Low | Low | Low | Low | Low | Low | 0/2 | PP |
| Payne et al[27] 1995 | 1995 | Unclear | Unclear | Low | Unclear | Low | Low | 0/0 | ITT |
| Shinozaki[28] 2010 | 2010 | Unclear | Unclear | High | High | Low | Low | 0/0 | ITT |
Four studies used an educational program as the placebo intervention[23,24,27,28]. In these studies, educational materials were provided and discussed with a therapist. In the study by Payne and Blanchard[27], individual cognitive therapy was compared to an educational placebo intervention delivered in a group format. The other studies compared individual CBT (with interoceptive exposure to visceral sensations) or stress management[23], individually delivered CBT[24] and autogenic training[28] to an individual educational placebo intervention.
Two studies on mindfulness and hypnotherapy delivered in a group format used support therapy as the placebo intervention[25,26]. In the study by Gaylord et al[25] the placebo intervention sessions were facilitated by social workers who served as group leaders, focussing on specific predesigned topics and promoting open group discussions. The placebo intervention in the study by Moser et al[26] consisted of doctor’s visits of the same duration as the treatment.
Primary outcome measure: One of the six studies investigated the effects of two separate psychological interventions and compared them with the effect of one placebo intervention[23], which brings the total number of outcomes to seven (see Table 3). All studies reported a significant reduction in IBS symptoms for at least one of the treatment interventions. For the response rate for the primary outcome measure of the placebo and active intervention arms, see Table 3. We performed the calculations using post-treatment figures. However, for the study by Craske et al[23], we used the figures at three-month follow-up because only they were reported.
| Ref. | Placebo treatment | Primary outcome measure | Duration of treatment1 | Follow-up | Placebo response | Treatment response |
| Craske et al[23] 2011 | Psycho-educational support | BSS index | 10 wk | 3 mo | 59% (13/22) | 62% (29/47)1 |
| 54% (22/41)2 | ||||||
| Drossman et al[24] 2003 | Psycho-educational support | Composite score3 | 12 wk | 37.3% (19/51) | 70% (77/110) | |
| Gaylord et al[25] 2011 | Support group | IBS-SSS | 8 wk | 3 mo | 45.2% (17.6/39) | 68.8% (27.4/36) |
| 53.1% (20.7/39) | 75% (27/36) | |||||
| Moser et al[26] 2013 | Supportive talks | IBS-IS | 12 wk | 12 mo | 40.9%( 18/44) | 60.8%(28/46) |
| 25% (11/44) | 54.3% (25/46) | |||||
| Payne et al[27] 1995 | Psycho-educational support | CPSR | 8 wk | 3 mo | 25% (3/12) | 75% (9/12) |
| 18% (2/12) | 83% (10/12) | |||||
| Shinozaki[28] 2010 | Psycho-educational support | AR | 8 wk | 30% (3/10) | 81.8% (9/11) |
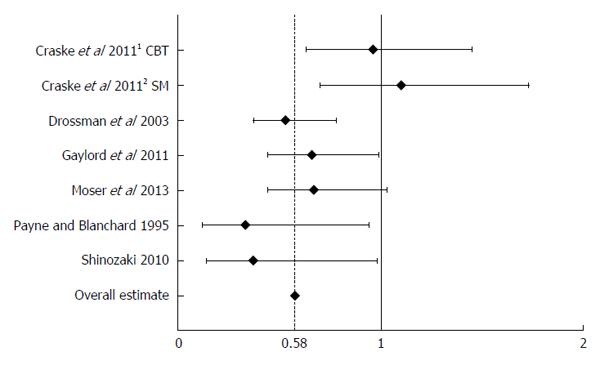
Rel-PRs ranged from 0.33 (95%CI: 0.12–0.94) in the study by Payne and Blanchard[27] to 1.1 (95%CI: 0.7-1.73) in the study by Craske[23]. For details on the Rel-PRs, see Figure 2.
After adjusting for study sample size, the weighted average PRR for all studies was 41.4%. In subgroup analysis, after adjusting for study sample size, the pooled PRR was 39.5% for the educational programs and 42.9% for the supportive interventions, including doctor’s visits[26].
Secondary outcome measures: Data on anxiety were presented in five studies[23,25-28], whereas data on depression were provided in three studies[25,27,28]. Five studies assessed quality of life using the IBS-QOL or SF-36 as the outcome measure[23-26,28]. The relative PRR for anxiety ranged from 0%[23,27] to 267%[28]. The relative PRR for depression ranged from 6%[27,28] to 52%[25]. For quality of life, it ranged from 20%[26] to 125%[23]. The relative placebo responses are presented in Table 4.
| Author | Symptoms | Anxiety | Depression | Quality of life |
| Craske CBT-IE | Bowel Symptom Severity (BSS) | VSI | - | IBS-QOL (FA and IF) |
| 56%/89% = 63% | 0%/44% = 0% | - | FA: 31/25 = 125% | |
| IF: 9/10 = 84% | ||||
| Craske SM | BSS | VSI | - | IBS-QOL (FA and IF) |
| 56%/82% = 68% | 0%/23% = 0% | - | FA: 31/17 = 184% | |
| IF: 9/14 = 64% | ||||
| Drossmann | Mc Gill pain Questionnaire | - | - | IBS-QOL |
| 2.77/4.58 = 60% | - | - | 4.8/9.35 = 51% | |
| Gaylord | IBS-Symptom Severity Score | VSI | Brief state inventory-depression | IBS-QOL |
| (IBS-SSS) | Brief State Inventory-anxiety | |||
| 42.2/68.8 = 61% | 1.16/5.78 = 20% | 0.78/1.49 = 52% | 3.7/0.19 = 36% | |
| 1.64/3.86 = 42% | ||||
| Moser | IBS-Impact Scale (IBS-IS) | HADS | - | SF-36 |
| Hospital Anxiety and Depression Scale | ||||
| 40.9/60.8 = 67% | 0.5/3.7 = 14% | - | 24/117.9 = 20% | |
| Payne and Blanchard | Composite Primary Symptom Reduction (CPSR) | STAI (state) | BDI | - |
| STAI (trait) | ||||
| 25%/75% = 33% | FALSE | 0.4/6.3 = 6% | - | |
| FALSE | ||||
| Shinozaki | Adequate Relief | State Trait Anxiety Inventory (state) | Self rating depression scale | SF-36 |
| Self Reported IBS Questionnaire (SIBSQ) | STAI (trait) | (SDS) | ||
| 30/81.8 = 37% | 3.2/2.8 = 114% | 0.1/1.8 = 6% | 15.5/58.2 = 27% | |
| 19.6/3.2 = 612% | 4/1.5 = 267% |
With regard to the different types of placebo interventions, after adjusting for sample size, the weighted average sizes for the educational placebo interventions were 27.8% for state anxiety, 65.1% for trait anxiety, 6% for depression and 72.7% for quality of life. For the supportive interventions, they were 27.2% for anxiety, 52% for depression and 20.8% for quality of life.
Our results showed that the PRR in six studies investigating the effect of psychological treatment on IBS for the primary outcome varied from 25.0% to 59.0%. The pooled adjusted mean PRR was 41.4%, which is comparable to the PRR reported in studies on pharmacological therapy (37.5%)[16]; medication and dietary fibre (47%)[18], medication and alternative medicine (40.7%)[17] and complementary medicine (42.6%)[11]. Our presumption that the response to placebo interventions in studies on psychological treatment for IBS would be greater than that to pharmacological interventions, was not confirmed by our results.
Compared to the placebo medication used in the pharmacological studies, the placebo interventions used in the psychological studies involved extensive patient-professional contact. It has been proposed[10,29] that the personality of and empathy exhibited by the therapist during the placebo intervention are responsible for the placebo effect. Furthermore, the more time that the therapist spends with a patient, the greater the placebo response. Hence, one would expect that the PRR in psychological studies would be higher. The fact that we found comparable PRR to those reported in pharmacological studies is obviously inconsistent with this hypothesis. Other factors may need to be considered. Vase et al[30] showed that the combination of expected pain relief and desire for pain relief accounted for up to 81% of the variance in the effect of active treatment. They concluded that “adding a verbal suggestion for pain relief in drug treatment can increase the magnitude of placebo analgesia to that of an active agent.” Kirsch[14] also argued that the placebo effect is generally dependent on the activation of response expectancy in the patient. From this perspective, the PRR is determined by the expectation of and desire for symptom relief of the patient, which is influenced by the way that the therapy is introduced and executed by the nurse, doctor or therapist. A positive interpersonal encounter with affective communication and adequate information from the health professional can positively influence the patient’s expectations and result in an improvement in health status[31]. Therefore, the words that a general practitioner uses to create expectations within the patient are important, in both pharmacotherapy and psychological interventions[32]. The fact that we did not find a difference in placebo response in our study supports the idea that contextual factors and cognitive and emotional changes, such as expectancy, desire and memory play a role in the development of the placebo response[33].
An important strength of the present study is the use of strict inclusion criteria to define IBS, psychological treatment[5,6] and placebo control conditions. Although this approach also resulted in a small number of studies and a relatively low number of patients, we consider the comparability of the format of psychological and placebo intervention to be essential for a valid assessment of the “true” placebo effect.
After adjusting for sample size, the pooled PR in the previous systematic review by Spanier et al[19] was 30.4%. Three of the four studies included in that analysis were excluded in this study, which involved different inclusion and exclusion criteria. Specifically, Blanchard et al[34] had no strict diagnostic criteria for IBS and Shaw et al[35] used usual care as the control intervention, which was not an appropriate control group according to our definition.
In a recent meta-analysis by Ford et al[36], 31 studies were included. Five of them were also included in our review, but we excluded the remaining 26 studies for the following reasons: the IBS criteria were not clear (2 studies) or Latimers criteria were used (1 study); it was not a randomized controlled trial (1 study); the intervention used was inappropriate according to our criteria [self/management by a nurse (1 study), not by a therapist (2 studies), by e-mail (1 study)]; or the control group did not fulfill the Baskin criteria [symptom monitoring (7 studies), care as usual (6 studies), waiting list (1 study), medication (1 study) or not having the same number of therapeutic sessions (3 studies)].
It would be interesting to compare the PRR of the psychological interventions for irritable bowel syndrome to that in studies on psychological interventions for other diseases. In the systematic review entitled “Psychological Interventions for treatment of inflammatory bowel disease” located in the Cochrane database and published in 2011[37], none of the control groups in the included studies met our criteria for control groups. In a study by Keefer et al[38] on gut-directed hypnotherapy for ulcerative colitis published in 2013, a control group that met our criteria was used. The placebo rate was 40%, which was comparable to the placebo rate found in our research. In a systematic review published in 2005, Enck and Klosterhalfen[12] compared the PRRs for functional bowel disorders with those of non-intestinal diseases and other organic gastrointestinal diseases. Most of the studies focused on drug treatment. The authors stated that the placebo effects in functional bowel disorders were similar to those in non-intestinal diseases (depression, pain and Parkinson’s disease) and not too dissimilar to those in other gastrointestinal diseases (duodenal ulcer, inflammatory bowel disease).
The placebo effect on the secondary outcome measures differed considerably across studies. However, the overall trends showed the greatest effects on symptom scores and the smallest effects on quality of life, anxiety and depression, which is aligned with the findings reported by Vase et al[30]. Pain is the main complaint of IBS patients, and almost invariably these patients possess the hope and desire that treatment will bring relief of their IBS-related pain. The combination of expected pain relief and desire for pain relief generates the largest placebo effect, and consequently, the effect on symptom scores is likely to be the greatest.
The relatively high PRR for anxiety in the study of Shinozaki et al[28] (267%) may have been caused by the content of the educational program, which was completely focused on dietary education. Most IBS patients have considerable anxiety surrounding the potential for dietary substances to act as complaint-inducing agents. A program with this content is apparently helpful in reducing this anxiety. In the study by Craske et al[23], the educational program had a positive impact on the patients’ food avoidance. Additionally, the effect on the Food Avoidance scale of the IBS-QOL scale was greater than the effects in the two treatment arms (125% and 184%). The results of these studies suggest that it may be worthwhile to include an educational module in IBS treatments.
In the study by Shinozaki et al[28], the PRR > 100% of the Self-Reported IBS Questionnaire (SIBSQ) indicated that the placebo intervention was more effective than the treatment intervention. It is not clear why this study found a significant positive treatment effect of autogenic training on the primary outcome measure of “adequate relief” and a significant positive effect of the placebo intervention on the primary symptom measure SIBSQ.
In conclusion, despite the more extensive patient-professional contact, the PRR in the placebo arm of RCTs with psychological treatment interventions is comparable to that of RCTs on drug interventions. This finding does not support the hypothesis that the personality and empathy of the professional are the main determinants of the placebo effect. Most likely, the PR is determined to a greater extent by patient- than doctor-related factors. Particularly important is the combination of expectations about and desire for symptom relief, both of which are influenced by the way that the therapy is introduced and executed. Thus, for optimal control group comparison in studies investigating psychological treatment for IBS, patients in the control group should have similar expectations from the control intervention as patients in the active intervention arm. Therefore, future RCT’s should map the expectations of patients in both RCT arms before starting the intervention.
In clinical practice, the placebo response can be used optimally by enhancing the expectations of the patient through the provision of realistic but positive information about the expected effect of the treatment. The preference of patients for a certain treatment might be related to the expected benefit, although it could also be the result of other contextual factors, such as the way in which the treatment is delivered (group versus individually). Future research should investigate the effect of patients’ preference for a certain treatment arm on the treatment outcome.
The search was conducted by Mrs. C.P.M. Sloof, MSc and Mrs M.B.A. Wilhelm, MSc, both clinical librarian at St Antonius Hospital, Nieuwegein, the Netherlands.
Irritable bowel syndrome (IBS) is a common chronic functional gastrointestinal disorder characterized by recurrent episodes of abdominal pain, discomfort, and altered bowel habits that cannot be explained by structural or biochemical abnormalities. Because of the limited effect of pharmacotherapy, there has been increasing interest in psychological treatments for IBS. As in pharmacological treatments, placebo effects play a role in psychological therapies. In psychological interventions, the placebo response may result from the consultation itself and the relationship with the physician/therapist. The authors presumed that the placebo response would be greater in psychological interventions than in drug trials. Therefore, the authors compared the placebo response rate in studies on psychological interventions for IBS with the placebo response rates in pharmacological studies.
Awareness that proper assessment of the effect of psychological interventions for IBS requires comparison with a placebo treatment is growing. A number of randomized placebo-controlled trials on the effect of psychological interventions on IBS have been published.
In previous meta-analyses on the placebo effect associated with psychological interventions for IBS, the criteria used for inclusion in the analyses have been liberal. For instance, some studies included patients who did not fulfill Rome criteria for IBS, whereas others used usual care as the control intervention. For our systematic review, the authors chose to include only randomized controlled trials (RCTs) that included a placebo intervention that met the strict prerequisites formulated by Baskin et al (2003).
Contrary to our expectations, in our study we found that, despite the more extensive patient-professional contact, the response rate in the placebo arm of RCTs with psychological treatment interventions was comparable to that of RCTs with drug interventions. Thus, it seems that the personality and empathy of the professional are not the main determinants of the placebo effect. Instead, it appears that the placebo response is more determined by patient- than doctor-related factors. For optimal control group comparison in studies investigating psychological treatment for IBS, patients in the control group should have similar expectations from the control intervention as patients in the active intervention arm. Therefore, future RCTs should map the expectations of patients in both RCT arms before starting the intervention. Future research should also explore the effect of patients’ preference for a certain treatment arm on the treatment outcome.
The diagnosis of irritable bowel syndrome is made using consensus-based criteria, the most recent of which are the Rome criteria, recurrent abdominal pain associated with two or more of the following: (1) related to defecation; (2) associated with a change in the frequency of stool; and (3) associated with a change in the form (appearance) of stool. These criteria must have been fulfilled for the last 3 mo, with symptom onset occurring at least 6 mo prior to the diagnosis. A placebo is defined as an activity regarded therapeutically inert from the theoretical perspective of the therapy under examination.
Good, well-conducted study. Suggested to add that a supportive doctor-patient relationship with empathy and listening is likely to maximize the placebo response to pharmacological treatment, not only in IBS but also in other disease states.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Costanian C, Gazouli M, Kaplan A, Tandon RK S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Guthrie E, Thompson D. Abdominal pain and functional gastrointestinal disorders. BMJ. 2002;325:701-703. [PubMed] |
| 2. | Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770-1798. [PubMed] |
| 3. | Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 4. | Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;CD003460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev. 2009;CD006442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Webb AN, Kukuruzovic RH, Catto-Smith AG, Sawyer SM. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2007;CD005110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Park SH, Han KS, Kang CB. Relaxation Therapy for Irritable Bowel Syndrome: A Systematic Review. Asian Nursing Research. 2014;8:182-192. [DOI] [Full Text] |
| 8. | Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: a meta-analysis of structural equivalence of placebo controls. J Consult Clin Psychol. 2003;71:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Thompson WG. Placebos: a review of the placebo response. Am J Gastroenterol. 2000;95:1637-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 821] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 11. | Dorn SD, Kaptchuk TJ, Park JB, Nguyen LT, Canenguez K, Nam BH, Woods KB, Conboy LA, Stason WB, Lembo AJ. A meta-analysis of the placebo response in complementary and alternative medicine trials of irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Enck P, Klosterhalfen S. The placebo response in functional bowel disorders: perspectives and putative mechanisms. Neurogastroenterol Motil. 2005;17:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 526] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 14. | Kirsch I. Placebo psychotherapy: synonym or oxymoron? J Clin Psychol. 2005;61:791-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Wampold BE, Budge SL, Laska KM, Del Re AC, Baardseth TP, Fluckiger C, Minami T, Kivlighan DM, Gunn W. Evidence-based treatments for depression and anxiety versus treatment-as-usual: a meta-analysis of direct comparisons. Clin Psychol Rev. 2011;31:1304-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Spiller RC. Impact of dietary fiber on absorption from the small intestine. Curr Opin Gastroenterol. 1999;15:100-102. [PubMed] |
| 19. | Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med. 2003;163:265-274. [PubMed] |
| 20. | Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Higgins JPT, Altman DG. Assessing risk of bias in included studies. Higgins J, Green S, editors. The Cochrane Collaboration: Cochrane handbook for systematic reviews of interventions 2008; . |
| 22. | Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140-1156. [PubMed] |
| 23. | Craske MG, Wolitzky-Taylor KB, Labus J, Wu S, Frese M, Mayer EA, Naliboff BD. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther. 2011;49:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 24. | Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 411] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, Frey W, Leniek K, Whitehead WE. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106:1678-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (2)] |
| 26. | Moser G, Trägner S, Gajowniczek EE, Mikulits A, Michalski M, Kazemi-Shirazi L, Kulnigg-Dabsch S, Führer M, Ponocny-Seliger E, Dejaco C. Long-term success of GUT-directed group hypnosis for patients with refractory irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2013;108:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. J Consult Clin Psychol. 1995;63:779-786. [PubMed] |
| 28. | Shinozaki M, Kanazawa M, Kano M, Endo Y, Nakaya N, Hongo M, Fukudo S. Effect of autogenic training on general improvement in patients with irritable bowel syndrome: a randomized controlled trial. Appl Psychophysiol Biofeedback. 2010;35:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med. 2009;71:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17-25. [PubMed] |
| 31. | Van Dulmen AM, Bensing JM. Health promoting effects of the physician- patient encounter. Psychology, Health & Medicine. 2002;7:3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Wampold BE, Imel ZE, Minami T. The story of placebo effects in medicine: evidence in context. J Clin Psychol. 2007;63:379-390; discussion 405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (1)] |
| 34. | Blanchard EB, Schwarz SP, Suls JM, Gerardi MA, Scharff L, Greene B, Taylor AE, Berreman C, Malamood HS. Two controlled evaluations of multicomponent psychological treatment of irritable bowel syndrome. Behav Res Ther. 1992;30:175-189. [PubMed] |
| 35. | Shaw G, Srivastava ED, Sadlier M, Swann P, James JY, Rhodes J. Stress management for irritable bowel syndrome: a controlled trial. Digestion. 1991;50:36-42. [PubMed] |
| 36. | Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350-1365; quiz 1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 37. | Timmer A, Preiss JC, Motschall E, Rücker G, Jantschek G, Moser G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2011;CD006913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 38. | Keefer L, Taft TH, Kiebles JL, Martinovich Z, Barrett TA, Palsson OS. Gut-directed hypnotherapy significantly augments clinical remission in quiescent ulcerative colitis. Aliment Pharmacol Ther. 2013;38:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |