Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2209
Peer-review started: November 18, 2016
First decision: December 19, 2016
Revised: January 5, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 28, 2017
Processing time: 132 Days and 20 Hours
To examine the role of soluble fibrin monomer complex (SFMC) in the prediction of hypercoagulable state after gastroenterological surgery.
We collected data on the clinical risk factors and fibrin-related makers from patients who underwent gastroenterological surgery at Hiroshima University Hospital between April 1, 2014 and March 31, 2015. We investigated the clinical significance of SFMC, which is known to reflect the early plasmatic activation of coagulation, in the view of these fibrin related markers.
A total of 123 patients were included in the present study. There were no patients with symptomatic VTE. Thirty-five (28%) patients received postoperative anticoagulant therapy. In the multivariate analysis, a high SFMC level on POD 1 was independently associated with D-dimer elevation on POD 7 (OR = 4.31, 95%CI: 1.10-18.30, P = 0.03). The cutoff SFMC level was 3.8 μg/mL (AUC = 0.78, sensitivity, 63%, specificity, 89%). The D-dimer level on POD 7 was significantly reduced in high-SFMC patients who received anticoagulant therapy in comparison to high-SFMC patients who did not.
The SFMC on POD 1 strongly predicted the hypercoagulable state after gastroenterological surgery than the clinical risk factors and the other fibrin related markers.
Core tip: We found that the plasma level of soluble fibrin monomer complex (SFMC) on POD 1 was more strongly associated with D-dimer elevation on POD 7 than were the clinical risk factors or other fibrin-related markers in 123 cases after gastroenterological surgery, suggesting the possible role of SFMC in the prediction of a hypercoagulable state and subsequent venous thromboembolism. The present study also demonstrated the possibility that the plasma levels of SFMC could be used as an indication for anticoagulant therapy in patients who have undergone gastroenterological surgery.
- Citation: Kochi M, Shimomura M, Hinoi T, Egi H, Tanabe K, Ishizaki Y, Adachi T, Tashiro H, Ohdan H. possible role of soluble fibrin monomer complex after gastroenterological surgery. World J Gastroenterol 2017; 23(12): 2209-2216
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2209.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2209
Venous thromboembolism (VTE) remains a significant complication after gastroenterological surgery. Thrombosis is sometimes fatal and can worsen a patient’s quality of life[1]. VTE is an important, potentially preventable condition that has the potential to increase the rates of morbidity and mortality[2,3].
The current American College of Chest Physicians (ACCP 2012) guideline recommends pharmacological prophylaxis with low-molecular weight heparin or low-dose unfractionated heparin in addition to mechanical prophylaxis such as elastic stockings and intermittent pneumatic compression (IPC) for general and abdominal-pelvic surgery patients who are at high risk for VTE (approximately 6.0%)[4,5]. The Caprini score is widely accepted for selecting patients with a high clinical risk for VTE (score ≥ 5); however, the majority of patients who undergo gastroenterological surgery for malignant tumors are considered to be high risk. Although postoperative anticoagulant therapy is regarded as important for preventing VTE, it is not routinely used after gastroenterological surgery, mainly because it is associated with bleeding complications and epidural hematoma after epidural anesthesia.
The risk of VTE varies according to the thrombotic risk factors of individual patients; these include age, sex, obesity, cancer, familial history, infection, heart disease, respiratory disease, hormone treatment and poor functional status[1,4,6]. Thus, in order to confirm a suspected VTE event after gastroenterological surgery, it is important to develop a diagnostic marker with high sensitivity and specificity. The establishment of a marker that can identify patients who are at high risk for VTE will help to minimize the disadvantages associated with anticoagulant therapy and unnecessary radiography.
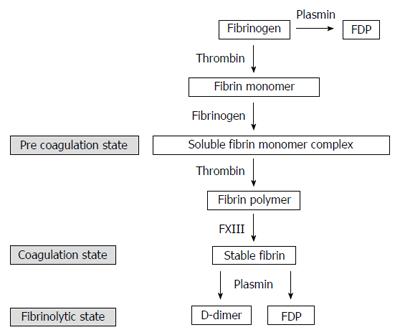
Soluble fibrin monomer complex (SFMC) appears in the bloodstream during the extremely early stage of blood coagulation. Thrombin cleaves fibrinopeptides from a fibrinogen molecule, and yields a fibrin monomer. When fibrin monomers are produced in the presence of fibrinogens, two fibrinogen molecules and one fibrin monomer create a soluble complex known as SFMC (Figure 1). SFMC reflects the plasmatic activation of coagulation and fibrinolysis[7,8]. However, there is little known about the clinical significance of SFMC after gastroenterological surgery.
The aim of the present study was to examine the possible role of the plasma level of SFMC in the prediction of hypercoagulable state and the subsequent VTE after gastroenterological surgery and to assess whether it can be used to indicate postoperative anticoagulant therapy.
We retrospectively collected data related to the clinical risk factors for VTE and fibrin-related makers from 135 consecutive patients who had undergone gastroenterological surgery due to a diagnosed malignance or to treat a general abdominal disorder at Hiroshima University Hospital between April 1, 2014, and March 31, 2015. The levels of D-dimer, fibrin degradation products (FDP), SFMC, and thrombin antithrombin complex (TAT) (fibrin-related markers) were measured at four time points in the perioperative period (before and 1, 3, and 7 d after surgery). Twelve patients were excluded from the study due to missing fibrin-related marker data.
Symptomatic VTE did not occur in this study population. The D-dimer level on POD 7 reflected the hypercoagulable state after surgery and it have previously demonstrated the association of the presence of VTE; therefore, the D-dimer level on POD 7 was used as the main outcome in this study.
Mechanical prophylaxis against VTE, including the postoperative use of elastic stockings (ESs) and IPC was routinely applied in all cases. In the present study, unfractionated heparin (via continuous infusion unfractionated heparin for one week at a dose that maintained the APTT at 1.5 to 2 times the reference value) was administrated for the patients who was preoperatively medicated by anticoagulant therapy. Pharmacological prophylaxis was administered to the patients at high clinical risk of VTE, as determined by the original risk classification based on the Caprini score and the Japanese VTE guidelines. The safety and validity of this risk classification were demonstrated in the previous article[9]. Pharmacological prophylaxis was administrated by low molecular weight heparin: Enoxaparin sodium [via subcutaneous injection, two times a day, with enoxaparin sodium (2000 IU) for one week]. Thus, postoperative anticoagulant therapy was administrated in 35 patients (28%). Unless contraindicated, anticoagulant therapy initiated from 24 h after surgery to one week after surgery.
Post-operative pain control in patients receiving anticoagulant therapy was achieved via intravenous anesthesia (as a substitute for epidural anesthesia).
The levels of D-dimer (LIAS AUTO® D-dimer NEO, Sysmex, Kobe, Japan), SFMC (AUTO LIA® FM, Sysmex, Kobe, Japan), and FDP (LIAS AUTO® P-FDP, Sysmex, Kobe, Japan) were measured by the latex agglutination method using a commercial immunoassay kit (LIAS AUTO® D-dimer NEO, Sysmex, Kobe, Japan). All tests were performed on a Sysmex CS5100 analyzer (Sysmex, Kobe, Japan).
TAT was measured by an enzyme-linked immunosorbent assay (HISCL® TAT, Sysmex, Kobe, Japan). This test was performed on a Sysmex HISCL2000i analyzer (Sysmex, Kobe, Japan). In all analyses, statistical significance was set at a P value less than 0.05. The standard values were as follows: D-dimer, ≤ 1 μg/mL; FDP, ≤ 5 μg/mL; SFMC, ≤ 7 μg/mL; and TAT, < 4 ng/mL.
Pearson’s χ2 test was used to analyze each clinical characteristic, in order to determine the factors associated with postoperative hypercoagulability. These variables were dichotomized in the analysis. Receiver operating characteristic (ROC) curves were created to determine the appropriate cutoff points. Factors with a P value of < 0.05 on the univariate analysis were subjected to a multivariate analysis using a logistic regression model. The results of the multivariate analysis are presented as the odds ratio (OR) and 95% CI with the corresponding P-value. All of the analyses were performed using the JMP software program (version 11, SAS Institute, Cary, NC, United States). The statistical methods of this study were reviewed by Minoru Hattori from Hiroshima University.
The final study population included 123 patients (68 males and 55 females), the median age was 67 years (range, 32 to 89 years), the median operation time was 319 min (range, 74-795), the median bleeding volume was 70 mL (range, 5-4135). The patients’ characteristics and clinical data are summarized in Table 1. There were no patients with symptomatic VTE in this study population. Thirty-five patients (28%) received postoperative anticoagulant therapy. Bleeding complications occurred in 5 (14%) patients who received anticoagulant therapy (Clavien-Dindo Grade 1, n = 4; Grade 2, n = 1).
| Characteristic | n = 123 |
| Age, median (range) | 67 (32-89) |
| Sex, Female | 55 (45) |
| BMI (kg/m2), median (range) | 22.8 (15.2-33.2) |
| Performance status | |
| 0-2 | 119 (97) |
| 3-4 | 4 (3) |
| Surgical procedure | |
| Gastrectomy | 32 (26) |
| Small bowel resection | 5 (4) |
| Colectomy | 45 (36) |
| Proctectomy | 32 (26) |
| Stoma closure | 2 (2) |
| Others | 7 (6) |
| Surgical technique | |
| Laparoscopic surgery | 86 (70) |
| Operative time (min), median (range) | 319 (74-795) |
| Bleeding volume (ml), median (range) | 70 (5-4135) |
| Clinical risk factors for VTE | |
| Malignancy | 118 (96) |
| Metastatic disease | 16 (13) |
| Diabetes mellitus | 16 (13) |
| Varicose vein | 1 (0.8) |
| Hormone therapy | 4 (3) |
| CV catheter | 4 (3) |
| Preoperative infection | 7 (6) |
| Cardiovascular disease | 6 (5) |
| Antiplatelet therapy | 10 (8) |
| Pelvic surgery | 22 (18) |
| Previous history of VTE | 0 |
We analyzed the correlation between D-dimer elevation on POD 7 and the clinical risk factors for VTE among the 88 patients who did not receive anticoagulant therapy. The median cutoff level for D-dimer on POD 7 was 6.45. In the univariate analysis the group with a higher D-dimer level (≥ 6.45 μg/mL) on POD 7, included a greater number of patients of ≥ 75 years of age, required a longer surgical time (≥ 321 min), and had a higher levels of D-dimer, FDP, TAT, and SFMC on POD 1 than the group of patients with lower D-dimer levels (< 6.45 μg/mL) on POD 7. According to a multivariate analysis, the SFMC on POD 1 (OR = 4.31, 95%CI: 1.10-18.30, P = 0.03) was an independent risk factor for D-dimer elevation on POD7 (Table 2). Their cutoff points with sensitivities and specificities were determined by a ROC analysis. The cutoff point of SFMC was 3.8 μg/mL, with an area under the curve (AUC) of 0.78, a sensitivity of 63% and a specificity of 89%.
| Clinical risk factors for VTE | D-dimer (POD 7) | Univariate | Multivariate | |||
| Low(< 6.45) | High(≥6.45) | P value | OR | 95%CI | P value | |
| Age | ||||||
| < 75 | 37 | 26 | < 0.01 | 2.48 | 0.70-9.21 | 0.15 |
| ≥ 75 | 7 | 18 | ||||
| Sex | ||||||
| Male | 20 | 21 | 0.83 | |||
| Female | 24 | 23 | ||||
| Performance status | ||||||
| 0-2 | 43 | 43 | 1.00 | |||
| 3-4 | 1 | 1 | ||||
| Operative time (min) | ||||||
| < 321 | 32 | 19 | < 0.01 | 2.09 | 0.64-6.92 | 0.21 |
| ≥ 321 | 12 | 25 | ||||
| Bleeding volume (mL) | ||||||
| < 113 | 35 | 28 | 0.09 | |||
| ≥ 113 | 9 | 16 | ||||
| Laparoscopic surgery | ||||||
| No | 11 | 16 | 0.24 | |||
| Yes | 33 | 28 | ||||
| Malignancy | ||||||
| Absence | 2 | 3 | 0.64 | |||
| Presence | 42 | 41 | ||||
| Metastatic disease | ||||||
| Absence | 38 | 39 | 0.74 | |||
| Presence | 6 | 5 | ||||
| Diabetes mellitus | ||||||
| Absence | 39 | 39 | 1.00 | |||
| Presence | 5 | 5 | ||||
| Hormone therapy | ||||||
| Absence | 43 | 43 | 1.00 | |||
| Presence | 1 | 1 | ||||
| CV catheter | ||||||
| Absence | 42 | 43 | 0.55 | |||
| Presence | 2 | 1 | ||||
| Preoperative infection | ||||||
| Absence | 42 | 41 | 0.64 | |||
| Presence | 2 | 3 | ||||
| Antiplatelet therapy | ||||||
| Absence | 41 | 40 | 0.69 | |||
| Presence | 3 | 4 | ||||
| Pelvic surgery | ||||||
| Absence | 41 | 41 | 1.00 | |||
| Presence | 3 | 3 | ||||
| Caprini score | ||||||
| < 7 | 24 | 15 | 0.05 | |||
| ≥ 7 | 20 | 29 | ||||
| Fibrin-related markers | ||||||
| D-dimer (μg/mL) < 0.6 | 17 | 12 | 0.11 | |||
| Preoperative ≥ 0.6 | 13 | 21 | ||||
| D-dimer (μg/mL) < 3.8 | 33 | 11 | < 0.01 | 2.88 | 0.56-14.82 | 0.19 |
| POD1 ≥ 3.8 | 11 | 33 | ||||
| FDP (μg/mL) < 10.1 | 38 | 18 | < 0.01 | 1.42 | 0.25-7.65 | 0.68 |
| POD1 ≥ 10.1 | 6 | 26 | ||||
| TAT (ng/mL) POD1 | ||||||
| < 8.3 | 34 | 12 | < 0.01 | 1.83 | 0.44-7.27 | 0.39 |
| ≥ 8.3 | 10 | 32 | ||||
| SFMC (μg/mL) < 3.8 | 39 | 16 | < 0.01 | 4.31 | 1.10-18.30 | 0.03 |
| POD1 ≥ 3.8 | 5 | 28 | ||||
We analyzed the correlation between SFMC elevation on POD 1 and the clinical risk factors and surgical factors. In the univariate analysis, there were significant differences in age, the operative time, and the administration of antiplatelet therapy. Subsequently, in the multivariate analysis, age and operative time were found to be independent risk factors for SFMC elevation on POD 1 (Table 3).
| Clinical risk factors for VTE | SFMC (POD 1) | Univariate | Multivariate | |||
| Low(< 3.8) | High(≥ 3.8) | P value | OR | 95%CI | P value | |
| Age | ||||||
| < 75 | 59 | 27 | 0.01 | 2.44 | 1.07-5.66 | 0.03 |
| ≥ 75 | 16 | 21 | ||||
| Sex | ||||||
| Male | 45 | 23 | 0.18 | |||
| Female | 30 | 25 | ||||
| BMI (kg/m2) | ||||||
| < 27 | 71 | 47 | 0.37 | |||
| ≥ 27 | 4 | 1 | ||||
| Performance status | ||||||
| 0-2 | 73 | 46 | 0.64 | |||
| 3-4 | 2 | 2 | ||||
| Operative time (min) | ||||||
| < 321 | 45 | 18 | 0.01 | 2.33 | 1.08-5.12 | 0.02 |
| ≥ 321 | 30 | 30 | ||||
| Bleeding volume (mL) | ||||||
| < 113 | 47 | 28 | 0.63 | |||
| ≥ 113 | 28 | 20 | ||||
| Laparoscopic surgery | ||||||
| No | 25 | 12 | 0.32 | |||
| Yes | 50 | 36 | ||||
| Malignancy | ||||||
| Absence | 5 | 0 | 0.06 | |||
| Presence | 70 | 48 | ||||
| Metastatic disease | ||||||
| Absence | 65 | 42 | 0.89 | |||
| Presence | 10 | 6 | ||||
| Diabetes mellitus | ||||||
| Absence | 67 | 40 | 0.33 | |||
| Presence | 8 | 8 | ||||
| Varicose vein | ||||||
| Absence | 75 | 47 | 0.2 | |||
| Presence | 0 | 1 | ||||
| Hormone therapy | ||||||
| Absence | 72 | 47 | 0.55 | |||
| Presence | 3 | 1 | ||||
| CV catheter | ||||||
| Absence | 72 | 47 | 0.55 | |||
| Presence | 3 | 1 | ||||
| Preoperative infection | ||||||
| Absence | 71 | 45 | 0.83 | |||
| Presence | 4 | 3 | ||||
| Cardiovascular disease | ||||||
| Absence | 73 | 44 | 0.15 | |||
| Presence | 2 | 4 | ||||
| Antiplatelet therapy | ||||||
| Absence | 72 | 41 | 0.03 | 3.04 | 0.74-15.30 | 0.12 |
| Presence | 3 | 7 | ||||
| Pelvic surgery | ||||||
| Absence | 62 | 39 | 0.84 | |||
| Presence | 13 | 9 | ||||
| Caprini score | ||||||
| < 7 | 36 | 17 | 0.16 | |||
| ≥ 7 | 39 | 31 | ||||
| Fibrin-related markers | ||||||
| D-dimer (μg/mL) < 0.6 | 26 | 11 | 0.12 | |||
| Preoperative ≥ 0.6 | 30 | 25 | ||||
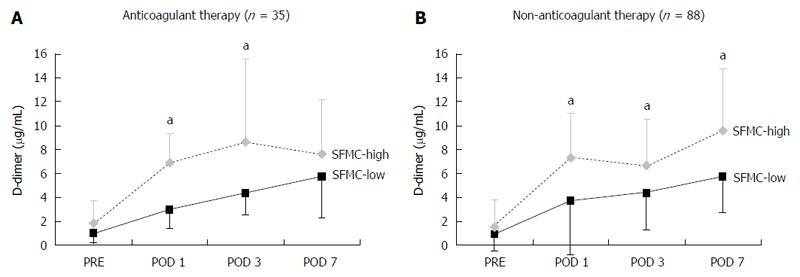
The anticoagulant therapy group and the no anticoagulant therapy group (n = 88) were divided into two subgroups [the SFMC-high group (POD 1 SFMC ≥ 3.8 μg/mL) and the SFMC-low group (POD 1 SFMC < 3.8 μg/mL)], and the D-dimer levels on PODs 1, 3, and 7 were examined to confirm the patients’ hypercoagulability. In the no anticoagulant therapy group, the D-dimer levels were significantly higher at every point of measurement than they were in the SFMC-low group. In the anticoagulant therapy group, however, there was no significant difference in the D-dimer levels on POD 7 (P = 0.14). Among the SFMC-High group, the D-dimer level on POD 7 was significantly reduced in patients who underwent anticoagulant therapy in comparison to patients who did not. This suggests the possibility that anticoagulant therapy might be indicated based on the SFMC level.
In the current study, we demonstrated the SFMC predicted the postoperative hypercoagulable state more strongly than other clinical risk factors, including the Caprini score and the levels of other fibrin related markers on POD 1.
A hypercoagulable state is a precursor condition of VTE, which is a significant complication that is associated with a poor prognosis, increased morbidity and a longer hospital stay[3]. Since it is well known that most cases of VTE are asymptomatic, perioperative patients who do not receive pharmacological prophylaxis should be carefully monitored to allow for the early detection of VTE[10]. If we could detect the hypercoagulable state and presence of VTE using a simple blood test, we could expect a dramatic reduction in unnecessary imaging examinations, which would reduce both radiation exposure and the use of contrast agents that are needed for computed tomographic pulmonary angiography (CTPA)[11,12]. The aim of a marker that identifies patients with a high risk of developing VTE will help to minimize the disadvantages of anticoagulant therapy and unnecessary radiographic examinations.
SFMC, which reflects acute intravascular fibrin formation, has been recognized as an independent marker for predicting VTE after orthopedic surgery, due to the substantial elevation of SFMC levels in patients who develop VTE[3,10,11,13]. Although SFMC is a cost-effective and safe diagnostic method, little is known about the changes in SFMC levels after gastroenterological surgery. No studies have evaluated SFMC levels or the cutoff SFMC level for the diagnosis of thrombosis after gastroenterological surgery. Since most VTE events occur during the first week after surgery, we evaluated the risk factors based on the characteristics of patients, surgical factors, and blood tests on POD 1 with the aim of detecting suspected cases of VTE during the early postoperative phase[3,7,10,11,13].
In the current study, we demonstrated that among 88 patients without anticoagulant therapy, the SFMC level on POD 1 was an independent risk factor for D-dimer elevation on POD 7. There were no significant differences in the other clinical risk factors or fibrin-related markers. With a cutoff point of 3.8 μg/mL, the diagnostic sensitivity, specificity and odds ratio of the SFMC on POD 1 were 63%, 89% and 4.31, respectively. In previous studies in which SFMC was used to predict VTE (cutoff points: 7.05-19.8 μg/mL) the sensitivity and specificity were 88% and 62 to 90%, respectively[10,13]. This difference in the cutoff points is considered to be due to the clinical endpoint (D-dimer elevation or the occurrence of VTE). An ROC analysis showed moderate accuracy in the prediction of D-dimer elevation on POD 7 using a cutoff point of 6.45 μg/mL (AUC: 0.78). Given that some reports used postoperative D-dimer cutoff values of 6.1 to 7.5 μg/mL for predicting the VTE, we consider this clinical endpoint to be reasonable[11,14].
Although, patients who are considered to have a high clinical risk for VTE based on the presence of risk factors such as pelvic surgery, obesity, and a previous history of thrombosis tend to receive appropriate perioperative anticoagulant therapy, the administration of perioperative anticoagulant therapy to patients who are deemed to have a low clinical risk of VTE is controversial[15]. Surgeons may withhold perioperative anticoagulant therapy due to the risk of bleeding complications. Major bleeding is reported to occur in 2.9% to 9.4% of patients during the period of pharmacological prophylaxis[16,17]. In the current study, post-operative bleeding complications, including subcutaneous bleeding, conjunctival bleeding, melena, and intraabdominal hemorrhage, occurred in 11.4% (5 of 35) of the patients. However, the incidence of major bleeding that necessitated a blood transfusion was 2% (1 of 35). The bleeding complications were classified as Grade 1, n = 4; Grade 2, n = 1 (Clavien-Dindo classification). We were therefore able to administer chemoprophylaxis without serious bleeding complications. Although this study did not use epidural anesthesia to avoid the risk of spinal epidural hematoma, previous studies have reported spinal epidural hematoma due to anticoagulant therapy to be extremely rare[18]. Thus, the use of epidural anesthesia during anticoagulant therapy should be the subject of future studies.
In the current study, elderly patients and a longer duration of surgery had an impact on the occurrence of SFMC elevation on POD 1 (Table 3), and anticoagulant therapy inhibited D-dimer elevation on POD 7 in the SFMC-high group (Figure 2). These results suggest that the selective administration of anticoagulant therapy to the patients of the SFMC-high group, especially patients who had these two risk factors, might be effective for preventing the development of VTE.
The relationship between the preoperative SFMC and D-dimer levels and the development of VTE after surgery is important; however, the preoperative SFMC and D-dimer levels could not predict postoperative VTE in previous studies[10,13]. These studies indicate that the preoperative SFMC level was not increased in patients who developed postoperative VTE.
This study is associated with several limitations. First, because there were no cases of symptomatic VTE was found, the D-dimer level on POD 7, which is well known to have high sensitivity (79%-95%) and a negative predictive value of nearly 100%, was used for the clinical endpoint, rather than the occurrence of VTE[10,19,20]. However, D-dimer elevation can also represent infection, malignancy, heart failure, chronic renal disease and liver disease[19]. Thus, it might not reflect true VTE. It would be therefore be better to consider the inclusion of asymptomatic VTE and to confirm the cutoff points in further clinical studies. Second, the further diagnostic work-up of patients with asymptomatic VTE, such as ultrasound, CT and CTPA, was performed at the discretion of the surgical team.
In conclusion, the plasma level of SFMC on POD 1 strongly associated with D-dimer elevation on POD7 than the clinical risk factors and the other fibrin related markers, which indicated the possible role of SFMC in the prediction of hypercoagulable state and subsequent VTE. The present study also demonstrated the possibility that the plasma levels of SFMC could be used as an indication for anticoagulant therapy, and the selective administration of anticoagulant therapy to the patients of the SFMC-high group would be effective for preventing the development of VTE. We are planning to perform another prospective study to examine the protective effects against VTE that are achieved by administering anticoagulant therapy based on the plasma levels of SFMC on POD 1.
We thank Minoru Hattori for statistical support.
Venous thromboembolism (VTE) remains a significant complication after gastroenterological surgery. Therefore, a diagnostic marker with high sensitivity and specificity that can be used to identify patients at high risk for a hypercoagulable state and subsequent VTE will help to minimize the disadvantages associated with anticoagulant therapy and unnecessary radiography.
The risk of VTE varies according to the thrombotic risk factors of individual patients; these include age, sex, obesity, cancer, family history, infection, heart disease, respiratory disease, hormone treatment and poor functional status. However, there is no simple maker that detects the hypercoagulable state and the presence of VTE after gastroenterological surgery.
This paper reports that the soluble fibrin monomer complex (SFMC) on POD 1 was more strongly associated with D-dimer elevation on POD 7 than were the clinical risk factors or other fibrin-related markers after gastroenterological surgery.
SFMC was able to be used as a marker to predict a postoperative hypercoagulable state and subsequent VTE after gastroenterological surgery. The present study also demonstrated the possibility that the plasma levels of SFMC could be used as an indication for anticoagulant therapy for patients who have undergone gastroenterological surgery.
SFMC appears in the bloodstream during the extremely early stage of blood coagulation. It reflects the plasmatic activation of coagulation and fibrinolysis.
The authors examined the role of SFMC in the prediction of hypercoagulable state after gastroenterological surgery, and they concluded that the SFMC on POD 1 strongly predicted the hypercoagulable state after gastroenterological surgery than the clinical risk factors and the other fibrin related markers. VTE is serious problem after surgery. This article is thought to be significant for prediction of hypercoagulable state on early phase after gastroenterological surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aoyagi K S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Yamashita Y, Wada H, Nomura H, Mizuno T, Saito K, Yamada N, Asanuma K, Usui M, Kamimoto Y, Matsumoto T. Elevated fibrin-related markers in patients with malignant diseases frequently associated with disseminated intravascular coagulation and venous thromboembolism. Intern Med. 2014;53:413-419. [PubMed] |
| 2. | Hamidi S, Riazi M. Cutoff values of plasma d-dimer level in patients with diagnosis of the venous thromboembolism after elective spinal surgery. Asian Spine J. 2015;9:232-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Nguyen NT, Stamos MJ. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg. 2014;18:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S-e277S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1470] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 5. | Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:7S-47S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1182] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 6. | Erem HH, Kiran RP, Remzi FH, Vogel JD. Venous thromboembolism in colorectal surgery: skip SCIP or comply? Tech Coloproctol. 2014;18:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Galster H, Kolb G, Kohsytorz A, Seidlmayer C, Paal V. The pre-, peri-, and postsurgical activation of coagulation and the thromboembolic risk for different risk groups. Thromb Res. 2000;100:381-388. [PubMed] |
| 8. | Ieko M, Nakabayashi T, Tarumi T, Naito S, Yoshida M, Kanazawa K, Mizukami K, Koike T. Soluble fibrin monomer degradation products as a potentially useful marker for hypercoagulable states with accelerated fibrinolysis. Clin Chim Acta. 2007;386:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Shimomura M, Kochi M, Hinoi T, Egi H, Adachi T, Kobayashi T, Tashiro T, Ohdan H. Clinical significance of Pharmacological Prophylaxis based on the Original Classification of Venous Thromboembolism after Lower Abdominal Surgery. Hiroshima J Med Sci. 2016;65:53-59. |
| 10. | Yukizawa Y, Inaba Y, Watanabe S, Yajima S, Kobayashi N, Ishida T, Iwamoto N, Choe H, Saito T. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthop. 2012;83:14-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Watanabe H, Madoiwa S, Sekiya H, Nagahama Y, Matsuura S, Kariya Y, Ohmori T, Mimuro J, Hoshino Y, Hayasaka S. Predictive blood coagulation markers for early diagnosis of venous thromboembolism after total knee joint replacement. Thromb Res. 2011;128:e137-e143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, Kovacs G, Ward J, Kovacs MJ. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98-107. [PubMed] |
| 13. | Wada H, Kobayashi T, Abe Y, Hatada T, Yamada N, Sudo A, Uchida A, Nobori T. Elevated levels of soluble fibrin or D-dimer indicate high risk of thrombosis. J Thromb Haemost. 2006;4:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Jiang Y, Li J, Liu Y, Li YC, Zhang WG. Risk factors for deep vein thrombosis after orthopedic surgery and the diagnostic value of D-dimer. Ann Vasc Surg. 2015;29:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Nelson DW, Simianu VV, Bastawrous AL, Billingham RP, Fichera A, Florence MG, Johnson EK, Johnson MG, Thirlby RC, Flum DR. Thromboembolic Complications and Prophylaxis Patterns in Colorectal Surgery. JAMA Surg. 2015;150:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Simonneau G, Laporte S, Mismetti P, Derlon A, Samii K, Samama CM, Bergman JF. A randomized study comparing the efficacy and safety of nadroparin 2850 IU (0.3 mL) vs. enoxaparin 4000 IU (40 mg) in the prevention of venous thromboembolism after colorectal surgery for cancer. J Thromb Haemost. 2006;4:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92:1212-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 213] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Singelyn FJ, Verheyen CC, Piovella F, Van Aken HK, Rosencher N. The safety and efficacy of extended thromboprophylaxis with fondaparinux after major orthopedic surgery of the lower limb with or without a neuraxial or deep peripheral nerve catheter: the EXPERT Study. Anesth Analg. 2007;105:1540-157, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Kim YJ, Im S, Jang YJ, Park SY, Sohn DG, Park GY. Diagnostic Value of Elevated D-Dimer Level in Venous Thromboembolism in Patients With Acute or Subacute Brain Lesions. Ann Rehabil Med. 2015;39:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009;29:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |