Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2194
Peer-review started: October 25, 2016
First decision: December 1, 2016
Revised: December 16, 2016
Accepted: February 16, 2017
Article in press: February 17, 2017
Published online: March 28, 2017
Processing time: 154 Days and 10.1 Hours
To observe the natural course of 1-3 cm gastric submucosal tumors originating from the muscularis propria (SMTMPs).
By reviewing the computerized medical records over a period of 14 years (2000-2013), patients with 1-3 cm gastric SMTMPs who underwent at least two endoscopic ultrasound (EUS) examinations were enrolled. Tumor progression was defined as a ≥ 1.2 times enlargement in tumor diameter observed during EUS surveillance. All patients were divided into stationary and progressive subgroups and further analyzed. We also reviewed the patients in the progressive subgroup again in 2016.
A total of 88 patients were studied, including 25 in the progressive subgroup. The mean time of EUS surveillance was 24.6 mo in the stationary subgroup and 30.7 mo in the progressive subgroup. Risk factors for tumor progression included larger tumor size and irregular border. Initial tumor size > 14.0 mm may be considered a cut-off size for predicting tumor progression. Seventeen patients underwent surgery, of whom 13 had gastrointestinal stromal tumors (GISTs) and 4 had leiomyomas. Tumor progression was found only in patients with GISTs. All of the tumors exhibited benign behaviors without metastasis until 2016.
Most 1-3 cm gastric SMTMPs (71.6%) are indolent. Tumor progression was found only in GISTs, and it is a good predictor for differentiating GISTs from leiomyomas. Predictors of tumor progression include larger tumor size (> 14.0 mm) and irregular border.
Core tip: Most gastric submucosal tumors originating from muscularis proprias (SMTMPs) are gastrointestinal stromal tumors (GISTs) or leiomyomas. GISTs have a malignant potential but leiomyomas are benign. We enrolled patients with 1-3 cm gastric SMTMPs and under endoscopic ultrasound surveillance over a period of 14 years between 2000 and 2013 to observe the natural behaviors of such tumors. We also reviewed the patients with progressive tumors again in 2016.
- Citation: Hu ML, Wu KL, Changchien CS, Chuah SK, Chiu YC. Endosonographic surveillance of 1-3 cm gastric submucosal tumors originating from muscularis propria. World J Gastroenterol 2017; 23(12): 2194-2200
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2194.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2194
Due to advances in endoscopy and its widespread use, detection of submucosal tumors (SMTs) of the gastrointestinal (GI) tract is not uncommon. In the evaluation of SMTs of the GI tract, endoscopic ultrasound (EUS) is a useful tool for identifying the tumor’s layer of origin, measuring its size, providing the details of tumor echotexture, and differentiating it from external compression[1]. Among SMTs in the stomach, gastrointestinal stromal tumors (GISTs) are the most common[2]. When EUS reveals a hypoechoic submucosal tumor originating from the muscularis propria (SMTMP) in the stomach, GIST is considered first followed by leiomyoma[3-9]. Because all GISTs have a malignant potential and leiomyomas have a benign nature, tissue acquisition is often recommended for such tumors. At present, EUS-guided fine needle aspiration (EUS-FNA) is a feasible method. However, the diagnostic rate may be limited when the tumor is smaller or the tumor location is difficult to approach[10-12].
Based on the National Institute of Health Consensus, tumor size and mitotic activity are the two most important factors for predicting malignant potential of a GIST[13]. Obviously, tissue obtained by EUS-FNA can demonstrate GISTs only but cannot provide further information regarding mitotic activity. EUS features suggestive of a malignant GIST include larger tumor size, heterogeneous hypoechotexure, irregular tumor border, and internal cystic or calcified changes[8,14,15]. At present, a GIST > 3 cm is considered to have higher malignant potential and is recommended for surgical resection[16]. As for GISTs < 1 cm, they are frequently considered to harbor a low risk of malignancy and tissue acquisition in these cases is controversial[17]. Notably, GISTs in the stomach are often indolent and rapid progression is uncommon. It should be considered whether all the myogenic submucosal tumors in the stomach are necessary for pathologic demonstration to differentiate GISTs from leiomyomas, especially in 1-3 cm tumors. Until now, associated discussions regarding the natural course and management of 1-3 cm gastric SMTMPs are limited. Here, we reviewed computerized medical records over a period of 14 years from our institution to study the natural behaviors of such tumors.
All the patients who underwent at least two EUS examinations to follow gastric SMTMP during a period of 14 years between January 2000 and December 2013 were retrospectively reviewed using the computerized medical record system of Kaohsiung Chang Gung Memorial Hospital, a tertiary medical center in Kaohsiung City in Taiwan.
In all patients, EUS was performed using a miniprobe with a 12 MHz radial scan (Olympus UM-2R, Tokyo, Japan). When EUS showed a myogenic tumor with hypoechoic echotexture originating from the muscularis propria in the stomach, it was regarded as a gastric GIST first or leiomyoma. We used the maximal tumor diameter as tumor size. The intervals of EUS follow-up were not defined, mainly depending upon the clinician’s discretion.
If the tumor size exceeded 3 cm, we recommended FNA or surgical resection. When a tumor was < 1 cm, we considered it to be benign. Therefore, we excluded the patients with an initial tumor size larger than 3 cm or persistently smaller than 1 cm. We also excluded the patients who underwent EUS only once without subsequent follow-up. We also enrolled the patients whose small tumors subsequently grew to 1 cm or more during surveillance. Therefore, only the patients with 1-3 cm myogenic tumors under EUS surveillance were enrolled in this study.
If a patient underwent surgery to remove a GIST, the pathology of GIST was classified into “very low risk”, “low risk”, “intermediate risk”, or “high risk” using tumor size and mitotic count based on the National Institute of Health consensus[13].
We defined a ratio of follow-up tumor size to initial tumor size ≥ 1.2 as tumor progression based on the Response Evaluation Criteria in Solid Tumor (RECIST)[18]. Patients were then divided into a progressive subgroup and a stationary subgroup. Baseline characteristics of each subgroup, initial tumor size, echotexture, border and location of myogenic tumors, the number of surveillance procedures, and the interval and duration of EUS were recorded and further analyzed.
We followed the patients in the progressive subgroup again in 2016 by medical record review and phone call contact.
Continuous variables were analyzed using the Mann Whitney U test and categorical variables analyzed using the Pearson χ2 test. The sensitivity and specificity of various tumor sizes were analyzed using a receiver operating characteristic (ROC) curve, and the optimal cutoff value was determined. All statistical analyses were performed using SPSS statistical software (SPSS for Windows, version 13; SPSS Inc., IL). A P-value < 0.05 was considered statistically significant.
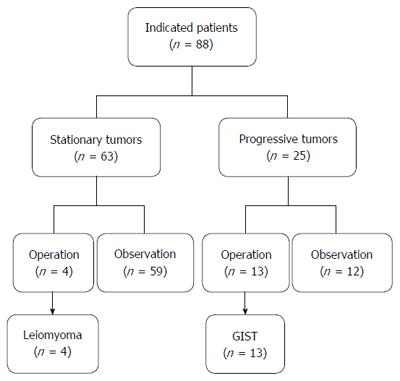
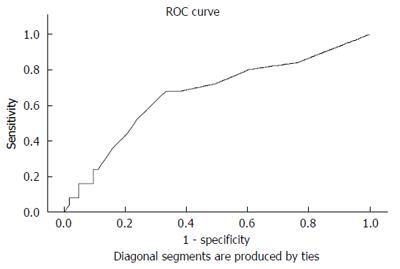
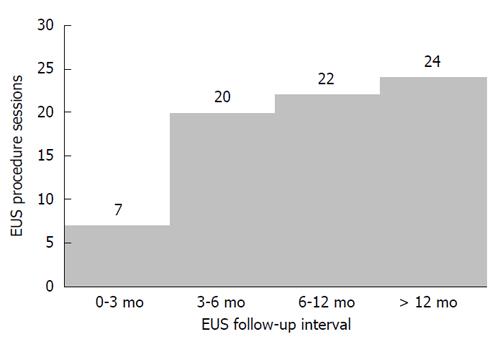
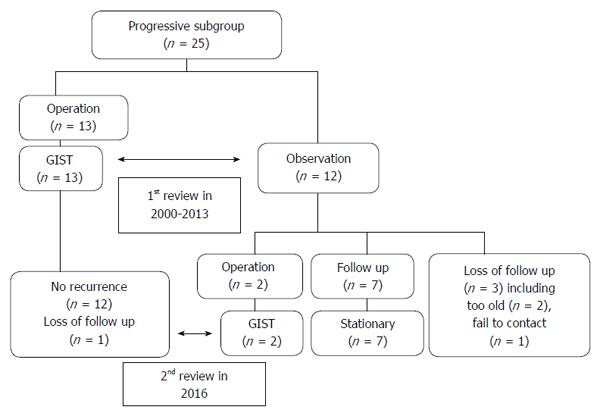
During the 14 years between 2000 and 2013, 6755 EUS procedures were performed by four endosonographers. Of these, 1725 EUS results were associated with gastric SMTMPs. Based on the inclusion and exclusion criteria, 88 patients (44 males and 44 females) were identified and enrolled in the study. The initial patient age was 57.1 ± 11.0 years (mean ± SD) and the initial tumor size was 14.7 ± 4.9 mm. Both the duration and interval of EUS surveillance ranged from 1.1 mo to 144.9 mo. The number of EUS surveillance procedures ranged from 2 to 9. Of the 88 patients, 25 (28.4%) were in the progressive subgroup and 63 (71.6%) in the stationary subgroup (Figure 1). The basic characteristics and EUS findings in each subgroup are shown in Table 1. By comparing the progressive and stationary subgroups, initially larger tumor size and irregular tumor border were identified to be predictors of tumor progression. Regarding initial tumor size, we performed an ROC curve analysis to determine the optimal cut-off size for predicting potential tumor progression. We found 1.4 cm to be the optimal cut-off tumor size associated with tumor progression, with a sensitivity of 68.0%, a specificity of 66.7%, and an accuracy of 67.0 % (Figure 2). The interval of EUS surveillance in the progressive subgroup is shown in Figure 3. The interval of most EUS examinations was ≥ 3 mo (66/73 = 90.4%). A total of 17 patients underwent surgery. Of these, 13 patients from the progressive subgroup were confirmed to have GISTs and 4 patients from the stationary subgroup were confirmed to have leiomyomas. Basic characteristics and EUS findings for patients with confirmed GISTs and leiomyomas are shown in Tables 2, 3 and 4. CD117 was positive in all 13 patients with confirmed GISTs (100%), whereas CD34 was positive in 11 (84.6%). Pathology results for confirmed cases suggested 4 GISTs with a very low malignant potential, 6 with a low potential, 2 with an intermediate potential, and 1 with a high potential. No patient was found to have malignant transformation or distant metastasis during surveillance. Notably, tumor progression (tumor enlargement ≥ 1.2 times) was only shown in the cases with GISTs. Among another 12 patients in the progressive subgroup, we followed them until 2016. Two patients eventually underwent surgery due to gradually enlarged tumors and were confirmed to have GISTs with a low malignant potential. Two patients refused EUS surveillance due to old age (> 80 years). Seven patients who took regular follow-ups remained condition stable without tumor metastasis. One patient was lost to follow-up. The flow chart of these 12 patients in the progressive subgroup is shown in Figure 4.
| Basic characteristic or EUS finding | Stationary group n = 63 | Progressive group n = 25 | P value |
| Age (mean ± SD, yr) | 57.4 ± 10.6 | 56.4 ± 12.4 | 0.690 |
| Sex (M/F) | 35/28 | 9/16 | 0.100 |
| Location | 0.650 | ||
| Cardia | 16 | 5 | |
| Fundus | 16 | 8 | |
| Body | 24 | 11 | |
| Antrum | 7 | 1 | |
| EUS tumor size and echotexture | |||
| Initial tumor size (mean ± SD, mm) | 13.9 ± 4.5 | 16.6 ± 5.5 | 0.020 |
| Homogeneous/ heterogeneous hypoechoicity | 44/19 | 12/13 | 0.060 |
| Smooth/irregular tumor border | 56/7 | 15/10 | 0.002 |
| With/without internal cystic change or calcification | 8/55 | 4/21 | 0.680 |
| EUS surveillance | |||
| Surveillance duration (mean ± SD, mo) | 24.6 ± 20.3 | 30.7 ± 21.7 | 0.220 |
| Case | Age (yr)/sex | Location | Heterogeneous hypoechoic echotexture | Irregular border | Internal cystic change or calcification | Initial size (I, mm) | Final size (F, mm) | Tumor progression (F/I≥ 1.2) | Surveillance procedures | Surveillance duration (mo) | Malignant potential |
| 1 | 41/F | Body | + | - | - | 15 | 23 | + | 4 | 82.1 | Very low |
| 2 | 67/F | Fundus | + | - | + | 15 | 23 | + | 5 | 66.5 | Very low |
| 3 | 50/F | Cardia | - | + | - | 16 | 20 | + | 4 | 22.8 | Very low |
| 4 | 70/M | Body | - | - | - | 15 | 20 | + | 8 | 37.9 | Very low |
| 5 | 57/F | Cardia | + | + | - | 28 | 50 | + | 3 | 19.3 | Low |
| 6 | 46/M | Fundus | + | + | - | 30 | 35 | + | 2 | 3.4 | Low |
| 7 | 55/F | Antrum | - | - | - | 18 | 23 | + | 2 | 63.0 | Low |
| 8 | 69/F | Body | - | - | - | 21 | 28 | + | 2 | 3.7 | Low |
| 9 | 49/M | Body | + | + | - | 24 | 30 | + | 3 | 47.9 | Low |
| 10 | 61/F | Fundus | + | + | - | 24 | 33 | + | 6 | 41.9 | Low |
| 11 | 54/M | Body | + | + | - | 21 | 28 | + | 5 | 32.1 | Intermediate |
| 12 | 59/F | Body | + | + | - | 18 | 23 | + | 2 | 5.5 | Intermediate |
| 13 | 60/F | Fundus | + | + | - | 30 | 51 | + | 2 | 31.3 | High |
| Case | Age (yr)/sex | Location | Heterogeneous hypoechoic echotexture | Irregular border | Internal cystic change or calcification | Initial size (I, mm) | Final size (F, mm) | Tumor progression (F/I≥ 1.2) | Surveillance procedures | Surveillance duration(mo) |
| 1 | 69/F | Body | - | - | - | 10 | 10 | - | 2 | 3.5 |
| 2 | 52/M | Fundus | - | - | - | 10 | 9 | - | 2 | 3.7 |
| 3 | 64/F | Antrum | + | - | - | 13 | 13 | - | 3 | 21.3 |
| 4 | 50/M | Cardia | + | + | + | 18 | 20 | - | 2 | 3.0 |
| Basic characteristic or EUS finding | GIST n = 13 | Leiomyoma n = 4 | P value |
| Age (median, range, yr) | 57 (41-70) | 58 (50-69) | 0.785 |
| Sex (M/F) | 4/9 | 2/2 | 0.482 |
| Location | 0.868 | ||
| Cardia | 2 | 1 | |
| Fundus | 4 | 1 | |
| Body | 6 | 1 | |
| Antrum | 1 | 1 | |
| EUS tumor size and echotexture | |||
| Initial tumor size (median, mm) | 21 | 11.5 | 0.015 |
| Final tumor size (median, mm ) | 28 | 11.5 | 0.003 |
| Homogeneous/heterogeneous hypoechoicity | 4/9 | 2/2 | 0.482 |
| Smooth/ irregular tumor border | 5/8 | 0/4 | 0.682 |
| With/without internal cystic change or calcification | 1/12 | 0/4 | 0.567 |
| EUS surveillance | |||
| Surveillance duration (median, range, mo) | 31.3 (3.1-81.0) | 3.6 (3.0-21.4) | 0.023 |
| Surveillance procedure (median, range, times) | 3 (2-8) | 2 (2-3) | 0.163 |
| Tumor progression | 13 | 0 | < 0.001 |
GISTs are the most common mesenchymal tumors in the GI tract. Pathologically, most GISTs are composed of spindle cells and epithelioid cells which are derived from interstitial cells of Cajal[19-21]. Most GISTs (approximately 65%) occur in the stomach, followed by 30%-35% in the small intestine and 5%-10% in the colon. About 95% of GISTs are characterized by the positive expression of c-kit receptor tyrosine kinase (CD117), whereas approximately 60%-70% of the tumors are positive for CD34[22-24]. Most gastric GISTs are asymptomatic and are detected incidentally as submucosal tumors during endoscopy. Therefore, the real incidence of GISTs in the stomach remains unclear. EUS is the most common modality for the evaluation of submucosal tumors. A suspected GIST is a hypoechoic and myogenic tumor originating mostly from the muscularis propria and occasionally from the muscularis mucosae. Similar to GISTs in terms of EUS findings, leiomyomas are also tumors of muscular origin. Unlike GISTs, leiomyomas are negative for CD117 and CD34, but positive for smooth muscle actin (SMA) and desmin on immunohistochemical staining. Moreover, leiomyomas are completely benign.
Recent studies have demonstrated that all GISTs have a malignant potential. Therefore, suspected GISTs should be confirmed histologically and managed accordingly. However, GISTs often behave differently at different locations. A GIST in the stomach is often more indolent than a GIST with a similar size and mitotic count located in another GI tract site[25]. Therefore, EUS surveillance alone is feasible for a small suspected GIST in the stomach that does not require immediate tissue proof or resection[2,26].
Most GISTs < 1 cm harbor a very low malignant potential, while GISTs ≥ 3 cm with irregular tumor borders, heterogeneous hypoechogenicity, and internal cystic or calcified changes suggest a higher malignant potential. All leiomyomas are benign. Therefore, we were interested in the natural course of 1-3 cm SMTMPs in the stomach. To evaluate tumor growth, we calculated the ratio of follow-up tumor size to initial tumor size on EUS and defined the ratio of ≥ 1.20 as tumor progression based on RECIST. Among 88 patients with 1-3 cm gastric myogenic tumors, we found that most tumors were indolent and tumor progression was detected in 25 (28.4%) patients. No patients suffered from major complications such as tumor bleeding, obstruction, perforation or malignant transformation during surveillance. A total of 19 (17 + 2) patients underwent surgery. Of these, 15 patients had GISTs and 4 patients had leiomyomas. Notably, tumor progression (tumor enlargement ≥ 1.2 times) was found only in GISTs but not in leiomyomas. Therefore, tumor progression may be a good predictor for differentiating GISTs from leiomyomas. Moreover, we found that larger tumors with irregular margins showed a tendency toward progressive change and should be monitored more closely. From the ROC curve analysis, we found 1.4 cm to be the optimal cut-off tumor size associated with tumor progression. The same 1.4 cm cut-off size was reported by Fang et al[27] in their study, which is similar to that reported by Lachter et al[28] who found tumor size larger than 1.7cm to be indicative of tumor progression. Tumors with heterogeneous hypoechotexture showed no statistical significance for predicting tumor progression (P = 0.06) in our study, but the finding is limited by our small number of cases and requires clarification in a larger study. Regarding the appropriate interval of EUS surveillance, it is difficult to conclude how often a suspected gastric GIST should be followed since malignant GISTs were not detected during surveillance in our study. Although an evidences-based optimal EUS surveillance policy remains lacking for small GISTs, yearly EUS follow-up for small sized GISTs (< 3 cm) should be considered from a study of Prachayakul et al[26] in 2012. At present, a guideline from European society of medical oncology recommended that an interval of 3 mo in the first follow-up and then annual EUS surveillance may be optimal for small suspected GISTs if no tumor growth occurs during surveillance[29]. In this review of 1725 EUS surveillances for gastric submucosal tumors from the 14 years of medical records, we found that most 1-3 cm SMTMPs in the stomach were indolent with only 28.4% of patients experiencing tumor progression (tumor enlargement ≥ 1.2 times). EUS surveillance is optimal for small gastric myogenic submucosal tumors without immediately obtaining tissue. Tumor progression is a good predictor for differentiating GISTs from leiomyomas. Risk factors for tumor progression include larger tumor and irregular borders. Initial tumor size > 14.0 mm may be considered a cut-off size for predicting tumor progression.
The authors thank the staff for contributing to the study accomplishment including data collection, writing recommendation and statistical assistance. The study was accomplished without any potential conflicts of interests, and without any support of grants or funding.
Most gastric submucosal tumors originating from muscularis propria (SMTMPs) are gastrointestinal stromal tumors (GISTs) and leiomyomas. Leiomyoma is benign but GIST has a malignant potential. Surgery is recommended if GISTs larger than 3 cm. Endoscopic ultrasound (EUS) fine needle aspiration is helpful to differentiate between GISTs and leiomyomas, but sometimes it is difficult to obtain tissue and cannot provide mitotic activity of GISTs.
Because studies regarding the natural behaviors of 1-3 cm gastric SMTMPs are limited, the authors made a retrospective study by reviewing the past 14 years of computerized medical records in a tertiary medical center between 2000 and 2013.
Most gastric SMTMPs are indolent from our study. Risk factors for tumor progression include larger tumor size and irregular border.
Initial tumor size > 14.0 mm may be considered a cut-off size for predicting tumor progression. Therefore, a gastric SMTMP with irregular border or ≥ 14.0 mm in size should be observed closely and treated accordingly.
GISTs are the common submucosal tumors arising from the muscularis propria in the stomach and have a malignant potential though the behavior of most tumors is indolent. EUS is a useful tool to detect submucosal tumors of the gastrointestinal tract.
This study provides important information (long term surveillance, EUS surveillance interval, a cut-off value of tumor size of > 14.0 mm) in the management of gastric small SMTMPs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bordas JM, Kobara H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Boyce GA, Sivak MV, Rösch T, Classen M, Fleischer DE, Boyce HW, Lightdale CJ, Botet JF, Hawes RH, Lehman GA. Evaluation of submucosal upper gastrointestinal tract lesions by endoscopic ultrasound. Gastrointest Endosc. 1991;37:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54:3-24. [PubMed] |
| 3. | Yasuda K, Nakajima M, Yoshida S, Kiyota K, Kawai K. The diagnosis of submucosal tumors of the stomach by endoscopic ultrasonography. Gastrointest Endosc. 1989;35:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Caletti G, Zani L, Bolondi L, Brocchi E, Rollo V, Barbara L. Endoscopic ultrasonography in the diagnosis of gastric submucosal tumor. Gastrointest Endosc. 1989;35:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Yasuda K, Cho E, Nakajima M, Kawai K. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S17-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Buscarini E, Stasi MD, Rossi S, Silva M, Giangregorio F, Adriano Z, Buscarini L. Endosonographic diagnosis of submucosal upper gastrointestinal tract lesions and large fold gastropathies by catheter ultrasound probe. Gastrointest Endosc. 1999;49:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856-862. [PubMed] |
| 9. | Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 14. | Chak A, Canto MI, Rösch T, Dittler HJ, Hawes RH, Tio TL, Lightdale CJ, Boyce HW, Scheiman J, Carpenter SL. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | American Gastroenterological Association Institute. American Gastroenterological Association Institute medical position statement on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2215-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Bennett JJ, Rubino MS. Gastrointestinal stromal tumors of the stomach. Surg Oncol Clin N Am. 2012;21:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 19. | Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728-734. [PubMed] |
| 21. | Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 357] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1-29; quiz S30. [PubMed] |
| 23. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1178] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 24. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Tashiro T, Hasegawa T, Omatsu M, Sekine S, Shimoda T, Katai H. Gastrointestinal stromal tumour of the stomach showing lymph node metastases. Histopathology. 2005;47:438-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Prachayakul V, Aswakul P, Pongprasobchai S, Pausawasdi N, Akaraviputh T, Sriprayoon T, Methasate A, Kachintorn U. Clinical characteristics, endosonographic findings and etiologies of gastroduodenal subepithelial lesions: a Thai referral single center study. J Med Assoc Thai. 2012;95 Suppl 2:S61-S67. [PubMed] |
| 27. | Fang YJ, Cheng TY, Sun MS, Yang CS, Chen JH, Liao WC, Wang HP. Suggested cutoff tumor size for management of small EUS-suspected gastric gastrointestinal stromal tumors. J Formos Med Assoc. 2012;111:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Lachter J, Bishara N, Rahimi E, Shiller M, Cohen H, Reshef R. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008;55:1653-1656. [PubMed] |
| 29. | Puchalski CM. Spirituality in the cancer trajectory. Ann Oncol. 2012;23 Suppl 3:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |