Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2095
Peer-review started: August 19, 2016
First decision: September 20, 2016
Revised: January 30, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 28, 2017
Processing time: 221 Days and 4.7 Hours
Liver transplant for hepatitis B virus (HBV) currently yields excellent outcomes: it allows to rescue patients with an HBV-related advanced liver disease, resulting in a demographical modification of the waiting list for liver transplant. In an age of patient-tailored treatments, in liver transplantation as well the aim is to offer the best suitable graft to the patient who can benefit from it, also expanding the criteria for organ acceptance and allocation. With the intent of developing strategies to increase the donor pool, we set-up a multicenter study involving 3 Liver Transplant Centers in Italy: patients undergoing liver transplantation between March 03, 2004, and May 21, 2010, were retrospectively evaluated. 1408 patients underwent liver transplantation during the study period, 28 (2%) received the graft from hepatitis B surface antigen positive (HBsAg)-positive deceased donors. The average follow-up after liver transplantation was 63.7 mo [range: 0.1-119.4; SD ± 35.8]. None Primary non-function, re-liver transplantation, early or late hepatic artery thrombosis occurred. The 1-, 3- and 5-year graft and patient survival resulted of 85.7%, 82.1%, 78.4%. Our results suggest that the use of HBsAg-positive donors liver grafts is feasible, since HBV can be controlled without affecting graft stability. However, the selection of grafts and the postoperative antiviral therapy should be managed appropriately.
Core tip: With the intent of developing strategies to increase the donor pool, we set-up a multicenter study involving 3 Liver Transplant Centers in Italy between March 2004 and May 2010. 1408 patients underwent liver transplantation during the study period, and 28 received the graft from hepatitis B surface antigen positive (HBsAg)-positive deceased donors. None primary non-function, re-liver transplantation, early or late hepatic artery thrombosis occurred. Our results show that transplantation of grafts from deceased HBsAg positive donors is feasible and this represents a way to expand the donor pool, especially in the high-endemic areas where a large proportion of patients are highly viremic and HBeAg positive.
- Citation: Ballarin R, Cucchetti A, Russo FP, Magistri P, Cescon M, Cillo U, Burra P, Pinna AD, Di Benedetto F. Long term follow-up and outcome of liver transplantation from hepatitis B surface antigen positive donors. World J Gastroenterol 2017; 23(12): 2095-2105
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2095
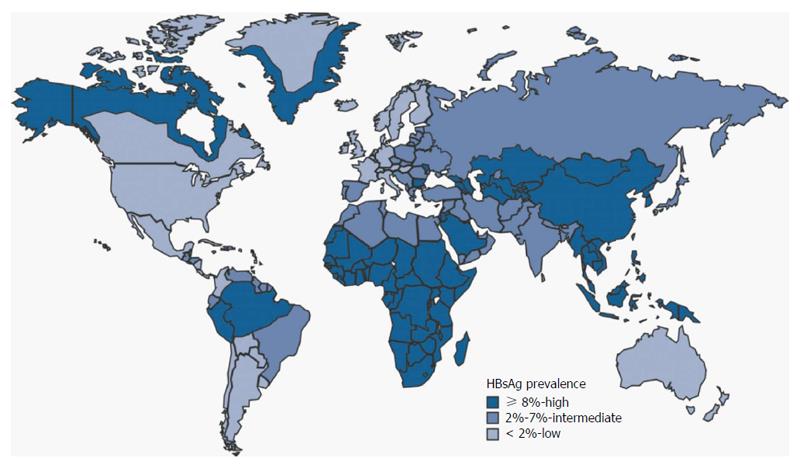
Hepatitis B virus (HBV) prevalence is different from a geographical region to another (Figure 1): currently, in Northern Europe, United States, Canada and Australia it ranges from 0.1% to 2%, while in central and Eastern Europe, as well as in Mid East, India, Central and Southern America, it is between 3% and 7%. Finally, the highest incidence, ranging from 10% to 20%, is registered in Africa and Easter Countries.
Notably, the incidence of hepatocellular carcinoma (HCC) in the same regions mirrors the prevalence of HBV. In Europe, Japan and North America HBV is responsible for 10%-15% of HCC cases, while conversely, in Asia and Africa, HBV is associated to 70% of cases. According to several studies, the relative risk of developing a tumor is close to 100-fold in HBV carriers vs non-carriers[1].
Liver transplant for HBV currently yields excellent outcomes, but in 1983, before the introduction of HBV immune globulin (HBIg) and antiviral therapy, a United States National Institute of Health consensus conference recommended against transplant for HBV because of the poor outcomes from severe recurrent liver disease. The first studies showed HBIg and HBIg plus lamivudine to improve graft and patient survival[2]. Subsequently, successful suppression of HBV DNA before transplant by Adefovir resulted in improved pre- and posttransplant survival[3]. More recently, the use of the more potent antiviral agent, entecavir, entirely prevented post-transplant recurrence, even in some patients with prior lamivudine resistance[4]. Whereas the original protocols utilized a lifetime administration of HBIg to maintain a blood titer high enough to prevent reinfection, and this was supplemented with lamivudine and now more potent antiviral agents, newer protocols have reduced the time of administration of the HBIg to 1 year with continued antiviral administration indefinitely after, or even use Entecavir or Tenofovir as a single agent to achieve an undetectable pretransplant viral load and maintain this indefinitely afterward[3].
Liver transplant for hepatitis B virus (HBV) currently yields excellent outcomes: it allows to rescue patients with an HBV-related advanced liver disease, resulting in a demographical modification of the waiting list for liver transplant. In a review of the Scientific Registry of Transplant Recipients (SRTR) database of registrants to the liver transplant list in the United States from 1985 to 2006, the overall number of registrants for HBV began declining after 1998 when oral antiviral therapy was first introduced[5]. Of the main indications for transplant owing to HBV (advanced liver disease, acute liver failure, and HCC), only HCC was increasing in number; registrants for advanced liver disease was declining most rapidly. This trend should continue; the data suggest that those with an early response to antiviral treatment with Tenofovir for acute severe reactivation of HBV have improved non-transplant survival (57% vs 13% for placebo-treated patients)[6].
However, antiviral therapy did not influence survival for those with acute liver failure owing to de novo HBV infection in a North American cohort of patients with acute liver failure[7]. It will likely be at least another decade until the incidence of HCC owing to HBV-induced liver disease begins to significantly decline, and this in part will be owing to treatment of HBV (as well as immunization of populations that began in the early 1990s). Eventually the choice of treatment to prevent HBV reinfection must take into account treatment efficacy, patient adherence, and cost.
The unmatched demand and supply rate between organs for transplantation is well known. As a matter of fact, we observed during the last decade a similar annual rate of donors in Europe and United States, while an increase of the “demand” for liver transplantation has been reported, in terms of new patients added in the waiting lists, longer mean waiting time and drop-out rate. Moreover, the lack of organs led to the exclusion from the waiting list of many patients who can benefit from a transplant[8,9].
In an age of patient-tailored treatments, in liver transplantation as well the aim is to offer the best suitable graft to the patient who can benefit from it. In Europe and in the United States is estimated that almost 10% to 30% of patients listed for liver transplant dies before organ availability[8]. In the United States status I patients i.e., patients entering in the waiting list at the highest medical urgency, reported a 12 folds increased risk of death while on the list compared with those entering at the two lowest categories of urgency[10]. Data from Scandinavia between 1990 and 2001 show that the mortality rate among patients waiting for liver transplant was 16%, while 27% of patients listed for a highly urgent liver transplantation failed to get the graft[11].
For many patients with a severe clinical status needing urgent transplant, the so-called marginal organ donor can provide a chance of cure. Patients that never obtained a transplant due to their clinical characteristics may as well benefit from a marginal donor, overcoming the problem of organ shortage.
The terms extended donor or expanded donor (ECD) mean changes in donor acceptability criteria, which not justifies the negative connotations of these terms. Although criteria to select organs for donation were revised and modified over years, this evolution did not affect neither patients’ nor organs’ survival. Characteristics of donor and recipient, together with allocation scheme, organ procurement and transplant procedure define the “ideal organ”. Moreover, marginal donors can allow to obtain comparable survival rates when an appropriate allocation is ruled out.
In 2001 a national commettee of experts nominated by the Italian National Transplant Centre (Centro Nazionale Trapianti-CNT) released a document for all personnel involved in the evaluation process of potential organ donor. The Commettee was made up of infectious disease experts, immunologists, clinical experts, surgeons, coordinators, anatomopathologists, medical examiners and oncologists. During the preparation phase, which lasted one year, the text underwent a series of changes and supplements, resulting in a final version shared with the scientific community and approved by the Italian National Transplant Centre as technical annex (guidelines) to the Ministry Decree of August 2, 2002[12].
These Guidelines focus on two main aspects: (1) The definition of acceptable/unacceptable risks for donor suitability or single organ utilization; and (2) the establishment of practical steps for the risk evaluation process.
The first aim was to identify the different risk levels and as a result five risk levels have been defined: (1) unacceptable risk; (2) increased but acceptable risk; (3) calculated risk; (4) not assessable risk; and (5) standard risk.
Unacceptable risk: The donor classified under this category should be excluded from donation and no organ can be used for transplantation. For example, HIV1 or 2 positive donors fall into this category, as well as HBsAg and HDV contemporaneous seropositivity. Neoplastic diseases represents an unacceptable risk with the following exceptions: carcinoma in situ, basal cell carcinoma, cutaneous squamous cell carcinoma without metastases, carcinoma in situ of the cervix, carcinoma in situ of vocal cords, urothelial papillary carcinoma (T0 according to the TNM classification). Eventually, systemic infections caused by agents for which treatments are not feasible and documented prior disease must also be considered as exclusion criteria.
Increased but acceptable risk: This category includes organs that can be used in case of urgency or particular clinical conditions of recipients. In these cases, even when the evaluation process shows the presence of pathogens or transmissible disease, organ utilization is allowed in the light of a risk benefit assessment. Patients struck by fulminant hepatitis, or retransplants for liver primary non function, or patients who underwent hepatectomy for trauma with complete organ function loss are included in this category.
Calculated risk: Includes all cases where the presence of a specific pathogen or a serological status of the donor (HBsAg+, or anti-HCV+ or HBcAb+) is compatible with transplantation recipients with the same disease or serological status, independently from recipient’s health conditions.
Not assessable risk: Includes cases for which the evaluation process does not allow an appropriate risk assessment for transmittable diseases for lack of one or more assessment elements (e.g., failure to collect an accurate medical history for lack of relatives, unavailability of microbiology data despite a well-grounded suspicion of infectious pathology).
Standard risk: Includes cases for which the evaluation process did not identify any risk factor for transmittable disease. It is the most frequent condition in the assessment of donors and grafts.
The national guidelines also identify some special conditions that concern two main aspects, namely neoplastic and infectious risks.
About infections, special attention should be paid to the following cases: donor with HCV infection; donor with HBV infection (HBsAg positivity); donors with anticore IgG antibodies against B virus (HBcAb). In such cases the guidelines impose the adoption of the following procedures.
HBsAg positive donor: If a donor turns out to be HBsAg positive, transplantation is allowed in a HBsAg positive recipient, after informed consent, provided that the following conditions are met: (1) the donor has a negative HDV antigen, negative IgM anti HDV antibodies, negative IgG anti HDV antibodies or with a titre < 1:100 or below the significant level according to the assay used; the absence of IgM anti HDV does not exclude delta virus chronic infection; (2) the liver recipient is not co-infected by delta virus; and (3) the patient follow-up can be monitored on the basis of a common national protocol established by the National Transplant Centre and to record data on a National Registry.
HBsAg negative donor: If the recipient is HBsAg negative, he has no anti-HBV antibodies or has a protective anti-HBsAg titre (≥ 10 mUI/mL), transplantation can be performed, after informed consent, when the following conditions are met: (1) the donor has a negative HDV antigen, negative IgM anti HDV antibodies, negative IgG anti HDV antibodies or with a titre < 1:100 or below the significant level according to the used assay; and (2) the patient follow-up can be monitored on the basis of a common national protocol established by the National Transplant Centre and to record data on a National Registry.
As a supplement to these measures, the Italian National Transplant Centre has deemed as proper to support further transplant network health workers, through adhoc developed information tools and an expert task force (second opinion) for evaluation of doubtful cases.
With the intent of developing strategies to increase the donor pool, we set-up a multicenter study involving 3 Liver Transplant Centers in Italy: the Universities of Modena, Bologna and Padova. The study was approved by the institutional review boards at each center. Patients undergoing liver transplantation between March 2004, and May 2010, were retrospectively evaluated. Among 1408 patients who underwent liver transplanation during the study period, 28 (2%) received the graft from HBsAg-positive deceased donors. All subjects were informed of the possible risks, consented to enter the study and signed a written form. For each HBsAg case we collected general clinical features and data regarding the transplantation, including MELD score and ischemia time. Then we retrospectively analyzed post-operative data, namely immunosuppressive therapy, histological evidence of HBV recurrence and antiviral therapy, and episodes of acute rejection.
The Italian regulations issued by the CNT allow HBsAg positive HDV negative recipients, HBcAb positive HDV negative patients, and HBV negative subjects with severe end-stage liver disease and a low life expectancy, to receive grafts from HBsAg positive HDV negative donors. Liver biopsy during organ procurement drives the evaluation on graft status, together with the serovirological complete assessment of HBV and HCV status, including HBV DNA. Moreover, Ishak score ≤ 1 and low inflammation, together HDV negative test in both donor and recipient, are required. HBV viral load, liver function test and age are not considered as exclusion criteria.
We performed liver biopsies routinely pre- and postperfusion in all cases. All the centers performed a liver biopsy protocol at months 6 and 12. However, all centers performed liver biopsies whenever biochemical or clinical signs of liver dysfunction became evident.
There was agreement on the definition of HBV recurrence as the contemporary presence of serum HBV-DNA and graft histology with evidence of lymphocytic infiltrates suggestive of recurrent HBV infection. An experienced pathologist is required for this evaluation, in order to avoid confusion with acute cellular rejection signs, like absence of endothelitis and cholangitis. Ishak score and the Knodell modified HAI were used to stage the disease, giving to each biopsy a HAI inflammatory grade (scale of 0-18), a fibrosis stage (scale of 0-6), and a total score combining the previous 2. Steatosis score was recorded as none (0%), mild (1%-30%), moderate (31%-60%), or severe (61%-100%), according to the degree of steatosis noted in the biopsy.
We performed a standard antiviral prophylaxis in all patients, independently from serovirological profile.
All HBsAg-positive recipients were on antiviral treatment with nucleos(t)ide analogues before liver transplantation and continued the same antiviral therapy with the addition of HBV-specific immunoglobulins (HBIg) after liver transplantation. The HBsAg-negative recipients began a similar combined treatment after LT, with lamivudine (LMV) and HBIg.
HBIg administration consisted of 10000 IU during the anhepatic phase, then 5000 IU every day for the first month, subsequently 5000 IU weekly for the second month and finally 5000 IU every 3-4 wk to maintain an anti-HBs titre above 250 IU/mL. This is the standard regimen of the transplant centers and it is applied even to HBV patients receiving an HBsAg-negative graft. Tacrolimus administration in the post-operative setting was adjusted to maintain a plasma concentration between 5 and 12 ng/mL. Steroids were started at a dose of 20 mg daily, then tapered down and discontinued within 6 mo.
We reported continuous data as mean ± SD, and then compared those data by using the 2-side Student’s t test. The χ2 test with Yates’ correction, or Fisher’s exact test when appropriate, was used to compare groups for categorial variables. Survival of grafts and patients were evaluated using the Kaplan-Meier method and compared with the log-rank test. The statistical significance was accepted for P < 0.05. All the statistical analysis were performed using SPSS© 19.0.
Four out of 28 recipients were female (median age at liver transplantation: 57.6 years, range: 26-67). Data were collected from liver transplantation until the last follow-up visit and the average follow-up after liver transplantation was 63.7 mo (range: 0.1-119.4; SD ± 35.8). Recipient characteristics were reported on Tables 1 and 2.
| Case | Age | Gender | AB0 | BMI | Indication | Real MELD | MELD correct | UNOS | Wating List(d) | Year LT | HCC criteria | Downstaging type (No.) |
| 1 | 62 | M | 0 | 27 | HCC/HBV | 26 | 36 | 2A | 37 | 2007 | MILAN IN | LOC(1)1 |
| 2 | 65 | M | B | 21 | HCC/HBV | 16 | 39 | 3 | 522 | 2007 | MILAN IN | LOC(1) + SUR(1) |
| 3 | 54 | M | A | 22 | HCC/HBV | 10 | 33 | 3 | 513 | 2007 | MILAN IN | LOC(1) |
| 4 | 45 | M | A | 24 | HCC/HBV | 14 | 34 | 2B | 419 | 2007 | MILAN OUT | LOC (3) |
| 5 | 62 | M | 0 | 29 | HCC/HBV | 12 | 33 | 3 | 445 | 2008 | MILAN OUT | LOC (2) |
| 6 | 53 | M | 0 | 28 | HCC/HBV | 12 | 35 | 2B | 515 | 2008 | MILAN IN | LOC (1) |
| 7 | 45 | M | A | 23 | HCV/HBV | 24 | 24 | 2A | 101 | 2005 | ||
| 8 | 64 | M | B | 27 | HBV/HCV | 33 | 33 | 2A | 478 | 2005 | ||
| 9 | 26 | M | A | 23 | HBV | 23 | 23 | 2B | 742 | 2005 | ||
| 10 | 64 | M | A | 22 | HCC/HBV | 11 | 23 | 2B | 144 | 2005 | MILAN IN | LOC (2) |
| 11 | 65 | F | B | 25 | HCC/HBV | 12 | 24 | 2A | 195 | 2006 | MILAN IN | LOC (2) |
| 12 | 56 | M | A | 24 | HCC/HBV | 10 | 24 | 2B | 217 | 2006 | MILAN OUT | LOC (5) |
| 13 | 61 | F | 0 | 23 | HCC/HBV | 14 | 30 | 2B | 352 | 2006 | MILAN IN | LOC (2) |
| 14 | 59 | M | A | 24 | HBV | 30 | 30 | 2B | 118 | 2007 | ||
| 15 | 48 | F | A | 27 | CBS | 21 | 33 | 2A | 82 | 2007 | ||
| 16 | 65 | M | A | 24 | HCC/HBV | 8 | 25 | 2B | 358 | 2009 | MILAN IN | LOC (2) |
| 17 | 57 | M | A | 30 | HCC/HBV | 14 | 39 | 2B | 576 | 2009 | MILAN OUT | LOC (7) |
| 18 | 55 | M | B | 28 | HCC/HBV | 18 | 26 | 2B | 38 | 2009 | MILAN IN | LOC (3) |
| 19 | 65 | M | A | 24 | HCC/HBV | 7 | 25 | 2B | 371 | 2009 | MILAN IN | LOC (3) |
| 20 | 63 | M | A | 28 | HCC/HBV | 16 | 25 | 2B | 1962 | 2010 | MILAN IN | LOC (2) |
| 21 | 60 | M | A | 34 | HCC/HBV | 10 | 27 | 2B | 330 | 2010 | MILAN OUT | LOC (1) + SUR (1) |
| 22 | 61 | M | A | 24 | HBV | 11 | 11 | 3 | 855 | 2010 | ||
| 23 | 67 | M | 0 | 19 | HBV | 16 | 16 | 2B | 742 | 2004 | ||
| 24 | 55 | M | A | 24 | HBV | 17 | 17 | 3 | 127 | 2004 | ||
| 25 | 60 | F | 0 | 29 | HBV/HCV | 17 | 17 | 2B | 961 | 2004 | ||
| 26 | 55 | M | A | 26 | HCC/HBV | 10 | 20 | 3 | 748 | 2005 | MILAN IN | LOC (1) |
| 27 | 54 | M | A | 27 | HCC/HBV | 13 | 19 | 3 | 134 | 2006 | MILAN OUT | LOC (2) |
| 28 | 67 | M | 0 | 22 | HCC/HBV | 12 | 29 | 3 | 575 | 2009 | MILAN IN | LOC (1) + SUR (1) |
| Recipient variables | n = 28 |
| Transplant center (No. of patients) | |
| Modena | 6 (21.4) |
| Bologna | 16 (57.1) |
| Padova | 6 (21.4) |
| Gender | |
| Male | 24 (85.7) |
| Female | 4 (14.3) |
| AB0 blood group | |
| Isogroup | 28 (100) |
| 0 | 7 (25) |
| A | 17 (60.7) |
| B | 4 (14.3) |
| Age (yr), mean (range, SD) | 57.6 (26-67, ± 8.7) |
| Body mass index, mean (range, SD) | 25.3 (19-34, ± 3.1) |
| Real MELD score, mean (range, SD) | 15.6 (7-33, ± 6.5) |
| Correct MELD score, mean (range, SD) | 26.7 (11-39, ± 7.2) |
| UNOS status | |
| 2A | 5 (17.5) |
| 2B | 15 (53.6) |
| 3 | 8 (28.6) |
| Time waiting list (d), mean (range, SD) | 452 (37-1962, ± 393.5) |
| Associated hepatocellular carcinoma | 19 (69.7) |
| Meeting Milan criteria | 13 (46.4) |
| Meeting UCSF criteria | 6 (21.4) |
| HBsAg status | |
| Positive | 23 (82.1) |
| Negative | 5 (17.9) |
| HBV DNA positive at LT | 12 (52.1) |
| HCV co-infection | 4 (14.3) |
| HDV co-infection | 0 |
HBV related cirrhosis, with or without HCC, was the indication for liver transplantation in 27 patients (Table 1), while 1 patient was transplanted due to secondary biliary cirrhosis.
The five HBsAg-negative patients showed serological evidence of past HBV infection. The MELD score (Model of End Stage Liver Disease) was applied to stage their liver disease status. In case of HCC, an extra score based on HCC stage was added, according to the centre (or regional) allocation policy.
Patients were transplanted after an average of 452 d on waiting list (range: 37-1962; SD ± 394) and at the time of liver transplantation presented an average MELD biochemical score of 15.6 (range: 7-33; SD ± 6.5) and an average MELD score correction (depending from other clinical variables) of 26.8 (range 11-39; SD ± 7.2).
The median body mass index (BMI) at the time LT was 25.3 (range: 19-34; SD ± 3.2).
Nineteen patients had hepatocellular carcinoma (67.9%) with 13 cases (68.4%) resulting within the Milan criteria, whereas 6 patients (31.6%) were outside Milan and inside UCSF criteria.
Table 1 describes different downstaging treatments for each patient.
The UNOS status was 2A in 5 patients (17.8%), 2B in 15 patients (53.6%), and 3 in 8 patients (28.6%).
Donor characteristics were reported on Table 3 and the overall serological state of the recipient/donor is shown in the Table 4.
| Case | Age | Gender | AB0 Gr. | BMI (kg/m2) | Cause of death | Time ICU (d) | Sodium (mEq/mL) | Vasopressors | Histologic activity index | Graft steatosis macro | |
| Grading | Staging | ||||||||||
| 1 | 59 | M | 0 | 26 | CH | 2 | 165 | No | 1 | 0 | 20% |
| 2 | 13 | F | B | 19 | T | 21 | 161 | Yes | 2 | 0 | 0% |
| 3 | 69 | M | A | 24 | CH | 3 | 152 | No | 0 | 0 | 0% |
| 4 | 72 | F | A | 27 | CH | 7 | 150 | No | 1 | 0 | 35% |
| 5 | 66 | M | 0 | 22 | CH | 5 | 137 | Yes | 3 | 1 | 0% |
| 6 | 60 | M | 0 | 26 | CH | 4 | 158 | No | 2 | 0 | 0% |
| 7 | 73 | M | A | 26 | CH | 2 | 151 | Yes | 2 | 1 | 0% |
| 8 | 51 | M | B | 23 | CH | 6 | 149 | Yes | 3 | 1 | 10% |
| 9 | 54 | M | A | 24 | T | 13 | 160 | Yes | 2 | 1 | 10% |
| 10 | 72 | F | A | 23 | T | 2 | 148 | No | 2 | 0 | 0% |
| 11 | 60 | F | B | 29 | CH | 8 | 162 | Yes | 3 | 1 | 5% |
| 12 | 65 | M | A | 29 | CH | 3 | 140 | Yes | 4 | 1 | 30% |
| 13 | 50 | M | 0 | 24 | CH | 12 | 141 | Yes | 2 | 1 | 3% |
| 14 | 48 | M | A | 23 | T | 2 | 143 | No | 4 | 1 | 5% |
| 15 | 26 | M | A | 23 | T | 1 | 145 | Yes | 1 | 0 | 0% |
| 16 | 52 | F | A | 28 | CH | 1 | 155 | Yes | 4 | 1 | 0% |
| 17 | 79 | F | A | 24 | CH | 19 | 136 | No | 0 | 0 | 0% |
| 18 | 46 | F | B | 29 | CH | 2 | 158 | No | 0 | 0 | 0% |
| 19 | 61 | M | A | 29 | CH | 3 | 156 | No | 1 | 1 | 10% |
| 20 | 53 | F | A | 25 | CH | 6 | 154 | Yes | 2 | 1 | 4% |
| 21 | 44 | F | A | 24 | CH | 6 | 144 | Yes | 4 | 1 | 5% |
| 22 | 23 | F | A | 21 | other | 7 | 149 | Yes | 1 | 1 | 0% |
| 23 | 57 | F | 0 | 24 | CH | 4 | 147 | Yes | 0 | 0 | 0% |
| 24 | 59 | M | A | 24 | CH | 1 | 160 | Yes | 0 | 0 | 5% |
| 25 | 35 | F | 0 | 23 | CH | 1 | 140 | Yes | 1 | 0 | 0% |
| 26 | 36 | M | A | 25 | T | 2 | 146 | Yes | 3 | 1 | 0% |
| 27 | 23 | M | A | 29 | T | 2 | 147 | Yes | 0 | 0 | 0% |
| 28 | 66 | F | 0 | 27 | CH | 3 | 151 | Yes | 1 | 0 | 10% |
| Case | HBsAg | HBsAb | HBcAb | HBeAg | HBeAb | HBV DNA | HDV | HDV RNA | HCV Ab | Therapy pre-LT | Mutation |
| R1 | + | - | + | - | + | - | - | - | - | Lam | No |
| D1 | + | - | + | - | + | + | - | - | - | No | - |
| R2 | + | - | + | - | + | - | - | - | - | Lam | No |
| D2 | + | - | + | - | + | + | - | - | - | No | - |
| R3 | + | - | + | - | + | + | - | - | - | Lam + Adef | Yes |
| D3 | + | - | + | - | + | + | - | - | - | No | - |
| R4 | + | - | + | + | - | - | - | - | - | Lam | No |
| D4 | + | - | + | - | + | - | - | - | - | No | - |
| R5 | + | - | + | - | + | + | - | - | - | Lam + Adef | Yes |
| D5 | + | - | + | - | + | - | - | - | - | No | - |
| R6 | + | - | + | - | + | - | + | - | - | Lam | No |
| D6 | + | - | + | - | + | + | - | - | - | No | - |
| R7 | - | + | + | - | - | - | - | - | + | No | No |
| D7 | + | - | + | + | - | + | - | - | + | No | - |
| R8 | - | + | + | - | - | - | - | - | + | No | No |
| D8 | + | - | + | - | + | + | - | - | - | No | - |
| R9 | + | - | + | + | - | + | - | - | - | Lam | No |
| D9 | + | - | + | + | - | + | - | - | - | No | - |
| R10 | + | - | + | - | + | - | - | - | - | Lam | No |
| D10 | + | - | + | + | - | + | - | - | - | No | - |
| R11 | - | + | + | - | - | - | - | - | + | No | No |
| D11 | + | - | + | + | - | + | - | - | + | No | - |
| R12 | + | + | + | - | + | + | - | - | - | Lam | No |
| D12 | + | - | + | - | + | + | - | - | - | No | - |
| R13 | + | - | + | - | + | - | - | - | - | Lam | No |
| D13 | + | - | + | + | - | + | - | - | - | No | - |
| R14 | + | - | + | - | + | + | - | - | - | Lam | No |
| D14 | + | - | + | - | + | - | - | - | - | No | - |
| R15 | - | - | + | - | + | - | - | - | - | No | No |
| D15 | + | - | + | - | + | + | - | - | - | No | - |
| R16 | + | - | + | - | + | + | - | - | - | Lam | No |
| D16 | + | - | + | - | + | + | - | - | - | No | - |
| R17 | + | - | + | - | + | + | - | - | - | Lam + Adef | Yes |
| D17 | + | - | + | + | - | + | - | - | - | No | - |
| R18 | + | - | + | - | + | + | - | - | - | Lam | No |
| D18 | + | - | + | - | + | - | - | - | - | No | - |
| R19 | + | - | + | + | - | + | - | - | - | Adefovir | No |
| D19 | + | - | + | + | - | + | - | - | - | No | - |
| R20 | + | - | + | - | + | + | - | - | - | Lam + Adef | No |
| D20 | + | - | + | + | - | + | - | - | - | No | - |
| R21 | + | - | + | - | + | - | - | - | - | NA | No |
| D21 | + | - | + | + | - | + | - | - | - | No | - |
| R22 | + | - | + | - | + | - | - | - | - | Lam | No |
| D22 | + | - | + | + | - | + | - | - | - | No | - |
| R23 | + | - | + | - | + | - | - | - | - | Lam | No |
| D23 | + | - | + | - | + | - | - | - | - | No | - |
| R24 | + | - | + | - | + | + | - | - | - | Lam | Yes |
| D24 | + | - | + | - | + | - | - | - | - | No | - |
| R25 | - | + | + | - | + | - | - | - | + | No | No |
| D25 | + | - | + | - | + | - | - | - | - | No | - |
| R26 | + | - | + | - | + | - | - | - | - | Lam | No |
| D26 | + | - | + | + | - | + | - | - | - | No | - |
| R27 | + | - | + | - | + | - | - | - | - | Adef | Yes |
| D27 | + | - | + | - | + | + | - | - | - | No | - |
| R28 | + | - | + | - | + | - | + | - | - | Lam | No |
| D28 | + | - | + | + | - | + | - | - | - | No | - |
The median age was 52.6 years (range: 13-79, SD ± 16.9). 13 donors were female (46.4%) while 15 donors were male (53.6%). The death causes are reported on the Table 3. The average body mass index (BMI) of donors was 25 (range: 19.5-29.4; SD ± 2.6) All the patients were HBsAg-positive. 21 donors (75%) were HBV-DNA positive while 7 (25%) were HBV-DNA-negative. 2 (7.1%) donors were anti-HCV positive but both were HCV-RNA negative.
None was HDV co-infected. Five patients (17.9%) were HBsAg negative, and 4 (14.3%) were HCV co-infected (Table 4).
Data on pre-perfusion histologic features of the biopsies are shown in Table 3. Most of the HBsAg positive grafts had a HAI inflammatory grade between 0-2 (71.4%), followed by an HAI inflammatory grade between 3-4 (28.6%). None of the grafts used had an HAI inflammatory grade score ≥ 5.
In particular, 6 donors (21.4%) had a grading score 0; 7 donors (25%) had a grading score 1; 7 donors (25%) had a grading score 2; 4 donors (14.3%) had a grading score 3; 4 donors (14.3%) had a Grading score 4. All the grafts had a fibrosis stage ≤ 1.
For 14 (50%) grafts the staging was 0. Macrosteatosis of the grafts are reported on the Table 3.
Cold ischemia time was in an average of 429 min (range: 255-632) and the warm ischemia time (WIT) was around 39.7 min (range: 30-55). The average hematic loss was 2307 mL (range: 300-13000). The mean length of stay in the Intensive Care Unit (ICU) was 5.5 d (range: 0-22), while the average Hospital stay was 21.4 d with a range from 6 to 143.
None primary non-function (PNF), re-LT, early or late hepatic artery thrombosis occurred after liver transplantation.
Two (7.1%) patients who received an HBsAg-positive donor liver had acute cellular rejection with a total of 1 event respectively for each patient.
Biliary complication occurred in seven patients (25%); in particular five biliary stenosis and two biliary leakages.
Five patients (17.9%) developed a major infection, 2 patients (7.1%) had an Hepatitis C recurrence.
Recurrence of HBV infection, confirmed histologically, occurred in 4 (14.3%) patients who received HBsAg positive grafts. The mean time of onset of HBV recurrence was 2.1 (± 1.4) mo.
The average follow-up was 63.6 mo (range: 0.1-119.4). The 6 deceased patients died not for the Hepatitis B recurrence but for different reasons. In particular, the cause and time of death were respectively: 1 patient for severe sepsis (0.4 mo), 1 patient for cardiac arrest (0.1 mo), 1 patient for HCV recurrence (11.8 mo), 2 patients for HCC recurrence (3.5 and 13 mo, respectively) and one patient for Merkel cell carcinoma (45 mo).
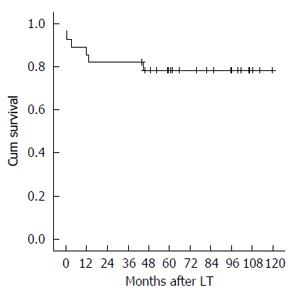
The 1-, 3- and 5-year graft and patient survival resulted of 85.7%, 82.1% and 78.4% (Figure 2).
Liver transplantation is an established therapeutic modality for patients with end-stage liver disease or/and hepatocellular carcinoma. However, in recent years the number of patients needing a transplant increase overcoming the supply: as a result, the mean waiting time is now longer than before, with higher mortality rates of patients waiting for an organ. It is estimated that 15% to 20% of patients on the waiting list die each year without receiving a suitable organ.
Several strategies have been developed by transplant physicians to face this increased demand: innovative ways of expanding the donor pool are the use of split and live donor LT. Another approach is the use of organs from “less-than-perfect donors”, also called “suboptimal donors”. Non-heart-beating donors and donors older than 65 years belong to such donors, as well as steatosic liver allografts and patients with previous exposure to HBV or HCV. Also the selection HBsAg positive donors represents a way to expand the pool of transplantable grafts.
On the other hand, living donor (LD) LT was adopted in Eastern countries to counterbalance the lack of deceased donors due to cultural reasons. Living donors and split liver transplantation have been used to contrast the donor shortage, but they have failed to significantly decrease the number of patients on the wait list. Those two approaches have ethical issues and technical complexities that make them less than ideal ways to expand the donor pool[13-15]. In addition, living donor programs have been activated in a small minority of transplant centers, and more institutions have been forced to resort to the use of other marginal organ donors.
As a matter of fact, wider acceptance criteria can assure more donors available for transplantation and several guidelines are available to classify donors as standard or ECD[16-20]. Two main categories of ECD can be identified: the first one includes grafts with risk of dysfunction due to direct or indirect liver injury, the second accounts for the risk of disease transmission between donor and recipient.
In the first case should be taken into account that those grafts must be carefully evaluated and transplanted in recipients capable to overcome the increased physiologic stress.
The ECD liver disease transmission risk is broken into 2 separate categories: (1) viral transmission of HCV, HBV, HTLV-1, and HTLV-2; and (2) malignancy transmission. Our previously reported results are consistent with other studies showing that it is safe to allocate grafts from HCV positive donors into HCV positive recipients[21-25]. The HCV positive donor liver must have no evidence of cirrhosis or stage > 1 fibrosis. It is clear that HCV-positive livers should be declassified as ECDs.
HBV scenario: About 2 billion people have serological evidence of present or past HBV infection worldwide, and a prevalence of more than 350 million cases of chronic infection is estimated[26].
The selection criteria of the recipient of HBcAb-positive donors are currently debated, while it has been demonstrated that a lifelong antiviral therapy is needed after transplantation of those grafts[23,27,28]. The majority of chronic HBV infections is nowadays present in the Western Pacific region[29], while a recent survey from Korea showed an overall HBsAg prevalence of 3.7%. This group of ECD is currently underestimated due to the high risk of HBV reactivation and to the paucity of clinical data, and up-to-now they are not used in most of the transplant centers.
Because of the existing shortage of organs, the increased demand for LT, and given the possible implications in terms of extension of the donor pool, the use of HBsAg-positive grafts should be studied to assess safety policies. To date, only a few studies exist regarding the effect of donor HBsAg positivity on survival (Table 5). These available reports yield conflicting results and are limited by small sample sizes and short follow-up[30-38].
Gonzalez-Peralta et al[31] were the first to report a successful LT of an HBsAg-positive graft into HBV negative recipient, who shortly afterwards turns HBsAg positive. Several reports in literature attested the use of HBIg and antiviral drugs against HBV such as lamivudine, adefovir dipivoxil, and tenofovir in recipients with HBsAg-positive grafts[30,32-36,38]. Loggi et al[37] reported a series of 10 HBsAg-positive grafts with HBIg and nucleos(t)ide analogue prophylaxis. In their experience only one patient died due to HCV recurrence over a mean follow-up period of 36.8 mo. In a cohort with 8 patients out of 10 positive for HBsAg after LT, no patient ever had any signs of active HBV hepatitis.
However, there was no comparison of outcomes between HBsAg-positive graft recipients with and without HBIg prophylaxis.
Using comprehensive clinical data from the SRTR database, Li et al[39] failed to identify any significant association between the use of HBsAg-positive donors and post-transplant graft or patient survival, after adjusting for other predictors of post-transplant survival. Their results demonstrate that HBsAg-positive donors for liver transplantation are safe and comparable in terms of outcomes and long-term survival to the use of HBsAg-negative grafts. Furthermore, other studies clearly showed that using HBIg may improve post-transplant survival in recipients with HBsAg-positive grafts.
Several innovations have been introduced during the last two decades to improve the outcomes of patients receiving LT for HBV-related liver disease, such as the administration of HBIg since the early 1990s and lamivudine in late 1990s[40-45]. Although there is now a consensus in favor of the use of HBIg in HBV-positive recipients, its application in HBV positive donors is still unclear.
Our study shows that the use of HBsAg positive grafts is a safe procedure when carried out in combination with appropriate antiviral therapy and when the graft fibrosis is ≤ 1 and the grading score is ≤ 4.
The 4 Hepatitis B recurrences that we have followed during the post-LT didn’t influence the graft and patient survival. From our own experience, there were no cases of PNF and the infectious and biliary complications were similar to the cases of HBsAg negative graft recipients.
However, our research shows some relevant limitations: first, even if it represents the major European study, the number of patients is still too low and therefore it doesn’t allow to establish ultimate conclusions. Then, we have chosen to focus only on a descriptive kind of analysis while for the future it will be necessary to perform comparative studies and matched analysis. Second, the lack of a common serial protocol hepatic biopsies has not allowed to examine the histological evolution of these grafts as well as a serial protocol for the dosage of the HBsAg quantification and the HBV-DNA level.
Despite the small number of cases, our results suggest that the utilization of grafts from deceased HBsAg positive donors, according to our allocation criteria, is feasible and HBV can be controlled with graft stability if selection of grafts and postoperative antiviral treatment are appropriately managed.
This way it could be possible to expand the donor pool, especially in the high-endemic areas where a large proportion of patients are highly viremic and HBeAg positive.
Long-term follow-up data and large-scale multicenter studies are required to confirm our findings.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boletis IN, Elsiesy H, Jin B S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M, Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF, Carrillo-Pérez DL. Diagnosis and treatment of hepatocellular carcinoma: An update. World J Hepatol. 2015;7:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Dodson SF, de Vera ME, Bonham CA, Geller DA, Rakela J, Fung JJ. Lamivudine after hepatitis B immune globulin is effective in preventing hepatitis B recurrence after liver transplantation. Liver Transpl. 2000;6:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann H, Samuel D, Zeuzem S, Villeneuve JP. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007;13:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Ueda Y, Marusawa H, Kaido T, Ogura Y, Ogawa K, Yoshizawa A, Hata K, Fujimoto Y, Nishijima N, Chiba T. Efficacy and safety of prophylaxis with entecavir and hepatitis B immunoglobulin in preventing hepatitis B recurrence after living-donor liver transplantation. Hepatol Res. 2013;43:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kim WR, Terrault NA, Pedersen RA, Therneau TM, Edwards E, Hindman AA, Brosgart CL. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137:1680-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Dao DY, Seremba E, Ajmera V, Sanders C, Hynan LS, Lee WM. Use of nucleoside (tide) analogues in patients with hepatitis B-related acute liver failure. Dig Dis Sci. 2012;57:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, Garrity ER, Roberts JP, Wynn JJ, Metzger RA. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2:701-711. [PubMed] |
| 9. | Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D’Alessandro A, Dec GW, Edwards NM, Higgins RS, Jeevanandum V, Kauffman M. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28-29, 2001, Crystal City, Va. Circulation. 2002;106:836-841. [PubMed] |
| 10. | Freeman RB, Edwards EB. Liver transplant waiting time does not correlate with waiting list mortality: implications for liver allocation policy. Liver Transpl. 2000;6:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Brandsaeter B, Höckerstedt K, Friman S, Ericzon BG, Kirkegaard P, Isoniemi H, Olausson M, Broome U, Schmidt L, Foss A. Fulminant hepatic failure: outcome after listing for highly urgent liver transplantation-12 years experience in the nordic countries. Liver Transpl. 2002;8:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 12. | Venettoni S, Grigioni W, Grossi P, Gianelli Castiglione A, Nanni Costa A. Criteria and terms for certified suitability of organ donors: assumptions and operational strategies in Italy. Ann Ist Super Sanita. 2007;43:279-286. [PubMed] |
| 14. | Miller C, Florman S, Kim-Schluger L, Lento P, De La Garza J, Wu J, Xie B, Zhang W, Bottone E, Zhang D. Fulminant and fatal gas gangrene of the stomach in a healthy live liver donor. Liver Transpl. 2004;10:1315-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Miller CM. Regulation and oversight of adult living donor liver transplantation. Liver Transpl. 2003;9:S69-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Bernat JL, D’Alessandro AM, Port FK, Bleck TP, Heard SO, Medina J, Rosenbaum SH, Devita MA, Gaston RS, Merion RM. Report of a National Conference on Donation after cardiac death. Am J Transplant. 2006;6:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 365] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 497] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 18. | Renz JF, Kin C, Kinkhabwala M, Jan D, Varadarajan R, Goldstein M, Brown R, Emond JC. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg. 2005;242:556-563; discussion 563-565. [PubMed] |
| 19. | Mor E, Klintmalm GB, Gonwa TA, Solomon H, Holman MJ, Gibbs JF, Watemberg I, Goldstein RM, Husberg BS. The use of marginal donors for liver transplantation. A retrospective study of 365 liver donors. Transplantation. 1992;53:383-386. [PubMed] |
| 20. | Wall WJ, Mimeault R, Grant DR, Bloch M. The use of older donor livers for hepatic transplantation. Transplantation. 1990;49:377-381. [PubMed] |
| 21. | Ballarin R, Cucchetti A, Spaggiari M, Montalti R, Di Benedetto F, Nadalin S, Troisi RI, Valmasoni M, Longo C, De Ruvo N. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011;91:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Marroquin CE, Marino G, Kuo PC, Plotkin JS, Rustgi VK, Lu AD, Edwards E, Taranto S, Johnson LB. Transplantation of hepatitis C-positive livers in hepatitis C-positive patients is equivalent to transplanting hepatitis C-negative livers. Liver Transpl. 2001;7:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Saab S, Chang AJ, Comulada S, Geevarghese SK, Anselmo RD, Durazo F, Han S, Farmer DG, Yersiz H, Goldstein LI. Outcomes of hepatitis C- and hepatitis B core antibody-positive grafts in orthotopic liver transplantation. Liver Transpl. 2003;9:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Saab S, Ghobrial RM, Ibrahim AB, Kunder G, Durazo F, Han S, Farmer DG, Yersiz H, Goldstein LI, Busuttil RW. Hepatitis C positive grafts may be used in orthotopic liver transplantation: a matched analysis. Am J Transplant. 2003;3:1167-1172. [PubMed] |
| 25. | Vargas HE, Laskus T, Wang LF, Lee R, Radkowski M, Dodson F, Fung JJ, Rakela J. Outcome of liver transplantation in hepatitis C virus-infected patients who received hepatitis C virus-infected grafts. Gastroenterology. 1999;117:149-153. [PubMed] |
| 26. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] |
| 27. | Donataccio D, Roggen F, De Reyck C, Verbaandert C, Bodeus M, Lerut J. Use of anti-HBc positive allografts in adult liver transplantation: toward a safer way to expand the donor pool. Transpl Int. 2006;19:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Jain A, Orloff M, Abt P, Kashyap R, Mohanka R, Lansing K, Kelley M, Bozorgzadeh A. Use of hepatitis B core antibody-positive liver allograft in hepatitis C virus-positive and -negative recipients with use of short course of hepatitis B immunoglobulin and Lamivudine. Transplant Proc. 2005;37:3187-3189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB, Treleaven S, van Konkelenberg R, Wiersma S. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine. 2006;24:1975-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Bahde R, Hölzen JP, Wolters HH, Schmidt HH, Bock CT, Lügering A, Spieker T, Senninger N, Brockmann JG. Course of a HBsAg positive liver transplantation in a hepatitis B and D virus coinfected recipient. Ann Hepatol. 2011;10:355-360. [PubMed] |
| 31. | González-Peralta RP, Andres JM, Tung FY, Fang JW, Brunson ME, Davis GL, Lau JY. Transplantation of a hepatitis B surface antigen-positive donor liver into a hepatitis B virus-negative recipient. Transplantation. 1994;58:114-116. [PubMed] |
| 32. | Ho JK, Harrigan PR, Sherlock CH, Steinbrecher UP, Erb SR, Mo T, Chung SW, Buczkowski AK, Intaraprasong P, Scudamore CH. Utilization of a liver allograft from a hepatitis B surface antigen positive donor. Transplantation. 2006;81:129-131. [PubMed] |
| 33. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Oh HB, Moon DB, Ha TY, Lim YS, Jung DH. Five-year follow-up of a hepatitis B virus-positive recipient of hepatitis B surface antigen-positive living donor liver graft. Liver Transpl. 2006;12:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Jiang L, Yan L, Li B, Wen T, Zhao J, Jiang L, Yang J, Xu M, Wang W. Successful use of hepatitis B surface antigen-positive liver grafts in recipients with hepatitis B virus-related liver diseases. Liver Transpl. 2011;17:1236-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Jiao Z, Zhang Y, Han L, Zeng Y, Yan L. Four-year follow-up of two chronic hepatitis B recipients of hepatitis B surface antigen-positive cadaveric liver grafts from asymptomatic carriers. Hepatol Res. 2011;41:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Loggi E, Bihl F, Chisholm JV, Biselli M, Bontadini A, Vitale G, Ercolani G, Grazi GL, Pinna AD, Bernardi M. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J Hepatol. 2009;50:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Loggi E, Micco L, Ercolani G, Cucchetti A, Bihl FK, Grazi GL, Gitto S, Bontadini A, Bernardi M, Grossi P. Liver transplantation from hepatitis B surface antigen positive donors: a safe way to expand the donor pool. J Hepatol. 2012;56:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Soejima Y, Shimada M, Taketomi A, Yoshizumi T, Uchiyama H, Ikegami T, Nakamuta M, Maehara Y. Successful living donor liver transplantation using a graft from a hepatitis B surface antigen-positive donor. Liver Int. 2007;27:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Li Z, Hu Z, Xiang J, Zhou J, Yan S, Wu J, Zhou L, Zheng S. Use of hepatitis B surface antigen-positive grafts in liver transplantation: a matched analysis of the US National database. Liver Transpl. 2014;20:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, Bismuth H. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 746] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 41. | Terrault NA, Zhou S, Combs C, Hahn JA, Lake JR, Roberts JP, Ascher NL, Wright TL. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996;24:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 43. | Han SH, Ofman J, Holt C, King K, Kunder G, Chen P, Dawson S, Goldstein L, Yersiz H, Farmer DG. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl. 2000;6:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Marzano A, Salizzoni M, Debernardi-Venon W, Smedile A, Franchello A, Ciancio A, Gentilcore E, Piantino P, Barbui AM, David E. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001;34:903-910. [PubMed] |
| 45. | Zheng S, Chen Y, Liang T, Lu A, Wang W, Shen Y, Zhang M. Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transpl. 2006;12:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Franchello A, Ghisetti V, Marzano A, Romagnoli R, Salizzoni M. Transplantation of hepatitis B surface antigen-positive livers into hepatitis B virus-positive recipients and the role of hepatitis delta coinfection. Liver Transpl. 2005;11:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Krishnamoorthi R, Manickam P, Cappell MS. Liver transplantation of hepatitis B surface antigen positive donors to hepatitis B core antibody recipients: analysis of 27 patients. Minerva Gastroenterol Dietol. 2014;60:113-118. [PubMed] |
| 48. | Saidi RF, Jabbour N, Shah SA, Li YF, Bozorgzadeh A. Liver transplantation from hepatitis B surface antigen-positive donors. Transplant Proc. 2013;45:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Choi Y, Choi JY, Yi NJ, Lee K, Mori S, Hong G, Kim H, Park MS, Yoo T, Suh SW. Liver transplantation for HBsAg-positive recipients using grafts from HBsAg-positive deceased donors. Transpl Int. 2013;26:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Ju W, Chen M, Guo Z, Wang D, Zhu X, Huang J, He X. Allografts positive for hepatitis B surface antigen in liver transplant for disease related to hepatitis B virus. Exp Clin Transplant. 2013;11:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Yu S, Yu J, Zhang W, Cheng L, Ye Y, Geng L, Yu Z, Yan S, Wu L, Wang W. Safe use of liver grafts from hepatitis B surface antigen positive donors in liver transplantation. J Hepatol. 2014;61:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Jeng LB, Thorat A, Yang HR, Yeh CC, Chen TH, Hsu CH, Hsu SC, Poon KS, Li PC, Lai HC. Successful use of hepatitis B surface antigen-positive liver grafts - an effective source for donor organs in endemic areas: a single-center experience. Ann Transplant. 2015;20:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |