Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1804
Peer-review started: October 25, 2016
First decision: December 2, 2016
Revised: December 25, 2016
Accepted: January 17, 2017
Article in press: January 17, 2017
Published online: March 14, 2017
Processing time: 141 Days and 21.2 Hours
To verify whether curcumin (Cur) can treat inflammatory bowel disease by regulating CD8+CD11c+ cells.
We evaluated the suppressive effect of Cur on CD8+CD11c+ cells in spleen and Peyer’s patches (PPs) in colitis induced by trinitrobenzene sulfonic acid. Mice with colitis were treated by 200 mg/kg Cur for 7 d. On day 8, the therapeutic effect of Cur was evaluated by visual assessment and histological examination, while co-stimulatory molecules of CD8+CD11c+ cells in the spleen and PPs were measured by flow cytometry. The levels of interleukin (IL)-10, interferon (IFN)-γ and transforming growth factor (TGF)-β1 in spleen and colonic mucosa were determined by ELISA.
The disease activity index, colon weight, weight index of colon and histological score of experimental colitis were obviously decreased after Cur treatment, while the body weight and colon length recovered. After treatment with Cur, CD8+CD11c+ cells were decreased in the spleen and PPs, and the expression of major histocompatibility complex II, CD205, CD40, CD40L and intercellular adhesion molecule-1 was inhibited. IL-10, IFN-γ and TGF-β1 levels were increased compared with those in mice with untreated colitis.
Cur can effectively treat experimental colitis, which is realized by inhibiting CD8+CD11c+ cells.
Core tip: CD11c is highly expressed in CD8+ and CD8- dendritic cells (DCs). Overaccumulation of CD8+ DCs is seen in colonic mucosa in experimental colitis and patients with inflammatory bowel disease (IBD). CD8+CD11c+ cells may be a potential strategy to explore the mechanism of action of drugs in IBD. The immunosuppressant curcumin (Cur) plays a therapeutic role in various immune diseases, including IBD and rheumatoid arthritis. However, it is unclear whether Cur regulates level of CD8+CD11c+ cells to treat IBD. We found that the therapeutic effect of Cur in experimental colitis was closely related to decreased levels of CD8+CD11c+ cells.
- Citation: Zhao HM, Han F, Xu R, Huang XY, Cheng SM, Huang MF, Yue HY, Wang X, Zou Y, Xu HL, Liu DY. Therapeutic effect of curcumin on experimental colitis mediated by inhibiting CD8+CD11c+ cells. World J Gastroenterol 2017; 23(10): 1804-1815
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1804.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1804
As one of integrin family, CD11c is a type I transmembrane protein that mediates adherence between leukocytes and endothelial cells, and participates in exudation and phagocytosis of leukocytes. It is suggested that CD11c induces tissue injury and the inflammatory response[1]. Importantly, CD11c is a specific marker in dendritic cells (DCs) and is highly expressed in CD8+ and CD8- DCs[2].
As professional antigen-presenting cells, DC precursors capture antigens and promote T-cell migration to regions of the draining lymph nodes where they can mature into functional DCs and present antigens to initiate T-cell-mediated immune responses[3]. Increasingly, it has been reported that DCs are critical to maintaining intestinal immunity and mucosal immune tolerance to resist the pathogenicity of commensal microorganisms, which is one of the pivotal inflammatory etiologies of induced inflammatory bowel diseases (IBD)[4].
High expression of co-stimulatory molecules and major histocompatibility complex (MHC) II of DCs, which is a known marker of DC maturation, and a “danger signal” of induced inflammatory mucosal damage in the gut, occurs in the colonic mucosa of animal models of colitis[5,6]. Moreover, DCs can develop from both myeloid and lymphoid progenitors. In mice, CD8+ DCs have been designated as lymphoid DCs, and CD8- DCs as myeloid DCs[7]. More importantly, CD8+ DCs predominantly stimulate T helper (Th)1-inducing cytokines such as interleukin (IL)-12p70 and IL-12p40, which can lead to Th1 differentiation[8], and have been reported to play a key role in controlling viral infection[3,9,10]. Overaccumulation of CD8+ DCs induces inflammatory injury in the colonic mucosa when they migrate into Peyer’s patches (PPs) in experimental colitis and IBD patients[11,12]. Thus, CD8+ DCs may be a potential therapeutic target to explore the mechanisms of clinical treatment of IBD.
Many studies have indicated that CD11c expressed in DCs can promote maturation and activation of DCs; present antigen for CD4+ or CD8+ T cells; accelerate T-cell activation and proliferation; and produce various cytokines[13-15]. CD11c+ DCs are depleted by diphtheria toxin during treatment of experimental colitis, induced indirectly by CD4+ CD62L+ T cells, with oligodeoxynucleotides containing unmethylated cytosine-guanosine[16]. These results suggest that CD11c+ DCs play an important role in the pathogenetic process of IBD.
Curcumin (Cur) is the major constituent of turmeric powder that is extracted from the rhizomes of Curcuma longa L. Cur has a long history of effectively treating chronic colitis by blocking nuclear factor-κB signaling in human IBD and experimental colitis, including trinitrobenzene sulfonic acid (TNBS)-induced and dextran sulfate sodium (DSS)-induced experimental colitis[17-19]. Multifunctional Cur has exhibited antioxidant, anti-inflammatory, antimutagenic, and anticarcinogenic activities, as well as antiplatelet, hypoglycemic, cholesterol-lowering, antibacterial, wound-healing and antifungal effects[17,20-22]. In addition, Shirley et al[23], have shown that Cur prevents DCs from responding to immunostimulants and DC-mediated induction of CD4+ T-cell proliferation by blocking maturation marker expression, cytokine and chemokine expression, and reducing migration and endocytosis. Shirley et al[23] also concluded that Cur might play a therapeutic role as an immunosuppressant in the treatment of various immune diseases including IBD and rheumatoid arthritis. In our previous study, we found that Cur repaired colonic structure, decreased colonic weight and histological injury score, and recovered colonic length, indicating that Cur restored damaged colonic mucosa in mice with TNBS-induced colitis[24]. However, it is unclear whether Cur can regulate the expression levels of CD8+CD11c+ cells to treat IBD.
In the present study, we investigated the effects of Cur on CD8+CD11c+ cells in the spleen and PPs in a murine model of TNBS-induced colitis to explore the possible therapeutic mechanisms of Cur in experimentally induced IBD.
Nine to twelve-week-old male C57BL/6 mice (20-24 g) were purchased from the Animal Center of Peking University Health Science Center (Animal Certificate No.: SCXK 2012-0001). Mice were housed in a special room with a humidity of 50% ± 5% and an equal 12-h light/dark cycle at 20 ± 2 °C throughout the experimental period. Animals were allowed free access to a commercial diet and clean water ad libitum. All animals were allowed to acclimatize for 4 d before the start of the experiment. The experimental protocols (JZ2015-016) were approved by the Biomedical Ethics Committee Experimental Animal Ethics Branch of Jiangxi University of Traditional Chinese Medicine.
Colitis was induced according to the procedure described previously by Huang et al[25], Sałaga et al[26], Fina et al[27] and Bai et al[28]. Mice were fasted for 12 h. Each mouse was anesthetized with pentobarbital sodium (40 mg/kg), following which, 100 mg/kg TNBS (Sigma-Aldrich, St. Louis, MO, United States; 100 g/L dissolved in 0.3 mL 50% ethanol) was instilled via a rubber catheter that was inserted approximately 4 cm into the colon via the anus. The rubber catheter was modified with numerous holes positioned over the final 4 cm of its length. The instillation procedure required only a few seconds, following which the mice were maintained in a head-down position for 5 min to prevent solution leakage. Mice in the Normal group received 50% ethanol of the same volume that was delivered using the same technique as described above.
To explore the effect of Cur (purity ≥ 95% by HPLC; Gangrun Biotechnology, Nanjing, China) on CD8+CD11c+ cells in colitis mice, C57BL/6 mice (20-24 g) were randomized into four groups of eight with comparable average body weight: Normal group (receiving ethanol only, and not treated); TNBS group (received TNBS and were not treated); TNBS + Cur group [received TNBS and 100 mg/kg/d Cur intragastrically (i.g.)]; and TNBS + mesalazine (Mes) group (received TNBS and mesalazine at 300 mg/kg/d i.g.). Before administration, Cur was dissolved in 5% dimethylsulfoxide (DMSO) in physiological saline, which was used as a vehicle. Twenty-four hours after colitis was induced, mice in the TNBS + Cur group were administered Cur, and in the TNBS + Mes group, they were administered Mes for 7 d until the mice were killed. Mice in the Normal and TNBS groups received the same volume of 5% DMSO in physiological saline daily (which was the vehicle for Cur) until the end of the experiment.
Disease activity index (DAI) was analyzed according to the previous study[29,30], which was the combined score of weight loss, stool consistency, and bleeding. The criteria for DAI scores are described in Table 1. The changes in growth rate, stool consistency, and gross bleeding or occult blood in the feces were scored daily from 0 to 4 for each animal after TNBS treatment.
| Score | Decrease in growth or weight loss | Stool consistency | Occult/gross rectal bleeding |
| 0 | 0% | Normal | Normal |
| 1 | 1%-5% | Normal | Occult blood+ |
| 2 | 5%-10% | Loose stools | Occult blood++ |
| 3 | 10%-15% | Loose stools | Occult blood+++ |
| 4 | > 15% | Diarrhea | Gross bleeding |
On day 8, all mice were killed after being anesthetized with pentobarbital sodium (40 mg/kg) by intraperitoneal injection. The colon was removed rapidly and its length was measured, opened longitudinally, rinsed with phosphate-buffered saline (PBS), assessed immediately for weight, and the weight index of the colon was calculated (colonic weight/body weight × 100%). Segments of the colon were fixed in 4% paraformaldehyde for at least 7 d. Subsequently, colon tissues were dehydrated, embedded in paraffin, sectioned at 5 μm and mounted onto slides. These sections were stained with hematoxylin and eosin.
A histological damage score was determined according to the criteria of Nicole and Schmidt et al[31]. The histological score included inflammatory cell infiltration and tissue damage. Scores for infiltration were as follows: 0: no infiltration; 1: increased number of inflammatory cells in the lamina propria; 2: inflammatory cells extending into the submucosa; and 3: transmural inflammatory cell infiltration. The scores of tissue damage were as follows: 0: no mucosal damage; 1: discrete epithelial lesions; 2: erosions or focal ulcerations; and 3: severe mucosal damage with extensive ulceration extending into the bowel wall.
PPs were separated and collected from the small intestine to the terminal rectum. To prepare single-cell suspensions, spleens or PPs were minced and digested in 2 mg/mL collagenase D (Roche Diagnostics, Basel Switzerland) in 1% fetal calf serum (FCS)/RPMI 1640 for 15 min at 37 °C. Next, 10 mM EDTA was added for the last 5 min, and the cell suspensions were then pipetted up and down several times and filtered through a fine-mesh sieve. The cell suspensions were centrifuged at 380 ×g at 4 °C for 5 min and suspended at a density of 106-107/mL in 3% FCS/PBS. Remnant supernatants of spleen and PPs were used separately to analyze the levels of cytokines by ELISA.
After removal of RBC, splenic and PPs cells were labeled with V450-anti-mouse CD8a+antibody (0.125 μg/100 μL; BD Biosciences, San Jose, CA, United States) and APC/Cy7 anti-mouse CD11c (eBioscience, San Diego, CA, United States), respectively, at 37 °C in the dark. Cells were centrifuged at 380 ×g at 4 °C for 5 min, and fixed in 1% paraformaldehyde/PBS. In addition, fluorescence-activated cell sorting analysis was performed on a FACSCalibur flow cytometer (BD Biosciences).
Cell suspensions were stained according to the appropriate isotypic control antibody match of different fluorochromes and incubated for 30 min with V450-anti-mouse CD8a+ antibody (0.125 μg/100 μL; BD Biosciences), APC/Cy7 anti-mouse CD11c (eBioscience), PerCP/Cy5.5 anti-mouse I-A/I-E (MHC II), PE anti-mouse CD40, APC anti-mouse CD154 (i.e., CD40 ligand), FITC anti-mouse CD54, and PerCP/Cy5.5 anti-mouse CD205. Limits for the quadrant markers were based on negative populations and isotype controls.
The levels of IL-10, IFN-γ and TGF-β1 in spleen and colonic mucosa supernatants were measured using ELISA (eBioscience).
Data were expressed as mean ± SEM. The statistical significance was evaluated by analysis of variance followed by Tukey’s test for multiple comparisons using GraphPad Prism version 5.0 (La Jolla, CA, United States). Nonparametric data were analyzed with the Mann-Whitney U test. P < 0.05 was considered statistically significant.
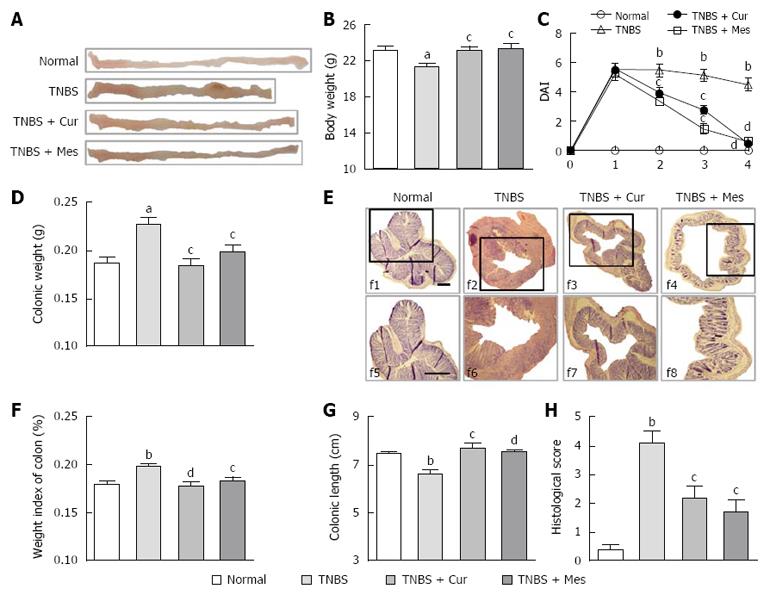
The body weight of mice and the disease activity index of experimental colitis in the TNBS group were significantly decreased compared with the Normal, TNBS + Cur and TNBS + Mes groups (Figure 1B and C). Colonic weight and the weight index of the colon from the TNBS groups were higher than those in the Normal group, but lower than in the TNBS + Cur and TNBS + Mes groups (Figure 1D and E). However, the colonic length in the colitis mice was shorter in the TNBS group compared with the Normal, TNBS + Cur, and TNBS + Mes groups (Figure 1A and G). Histological evaluation of colonic sections from untreated mice with colitis showed that TNBS-induced colitis was characterized by a loss of mucosal architecture, thickening of the colon wall, cryptic abscesses, ulcer formation, and extensive inflammatory cell infiltration in the colonic mucosa (Figure 1F). Treatment with Cur and Mes inhibited these pathological symptoms and and kept histo-progressive restoration, reduced inflammatory cell infiltration in the mucosa and submucosa, and maintained the integrity of colonic mucosa (Figure 1F). We observed visually ulceration, hyperemia and edema in the colonic mucosa in colitis mice without treatment, which were ameliorated in mice treated with Cur and Mes (Figure 1A). Moreover, the histological scores in the colon of mice from the Normal, TNBS + Cur, and TNBS + Mes groups were significantly lower than those in untreated mice with colitis (Figure 1F and H). All results demonstrated that Cur effectively treated experimental colitis.
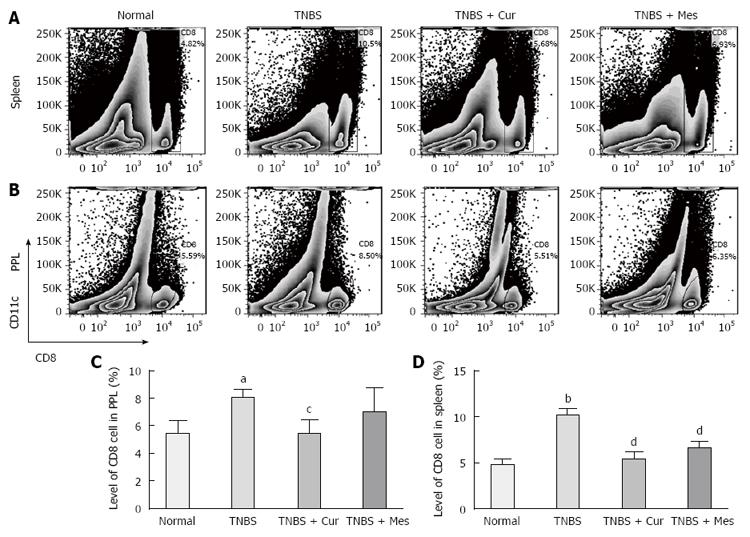
We analyzed the numbers of CD8+CD11c+ cells in the spleen and PPs of mice with colitis (Figure 2). Data clearly indicated a significantly increased tendency in this parameter in the spleen (Figure 2A and D) and PPs (Figure 2A and C) in the TNBS group as compared with the Normal group. Significantly, after 7 d treatment with Cur, the numbers of CD8+CD11c+ cells in the spleen and PPs in the TNBS + Cur and TNBS + Mes groups were decreased dramatically as compared with the TNBS group.
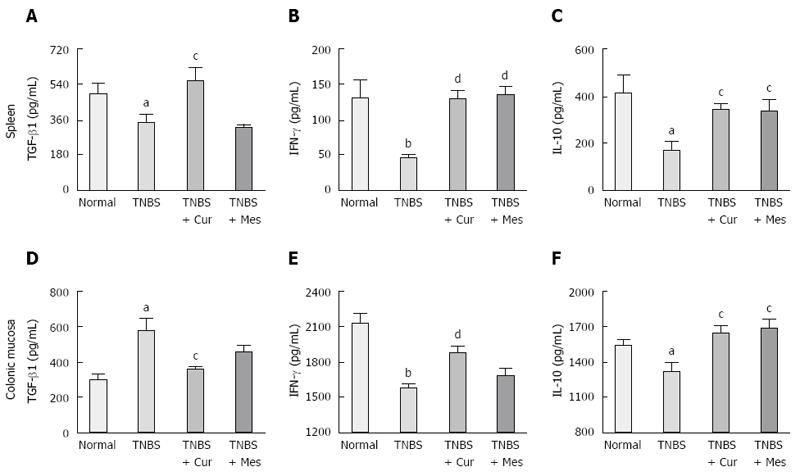
To understand the effects of activated CD8+CD11c+ cells in the development of murine colitis, the secretion of IL-10, IFN-γ and TGF-β1 was determined (Figure 3). There was significantly increased expression of TGF-β1 in the colonic mucosa of untreated colitis mice (Figure 3B). In addition, the secretion of TGF-β1 in the colonic mucosa in the TNBS + Cur and TNBS + Mes groups was lower than that in the TNBS group. However, expression of TGF-β1 in the spleen of mice treated with Cur and Mes was higher than that in the TNBS group compared with the Normal group, levels of IFN-γ (Figure 3B and E) and IL-10 (Figure 3C and F) in the spleen and colonic mucosa in untreated colitis mice were decreased 7 d after TNBS-induced colitis. In the colonic mucosa and spleen, the expression of both IL-10 and IFN-γ was increased in colitis mice treated with Cur and Mes as compared with untreated colitis mice.
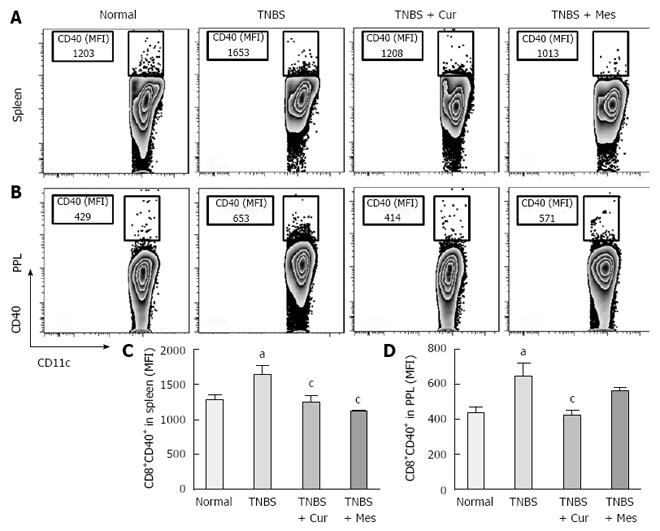
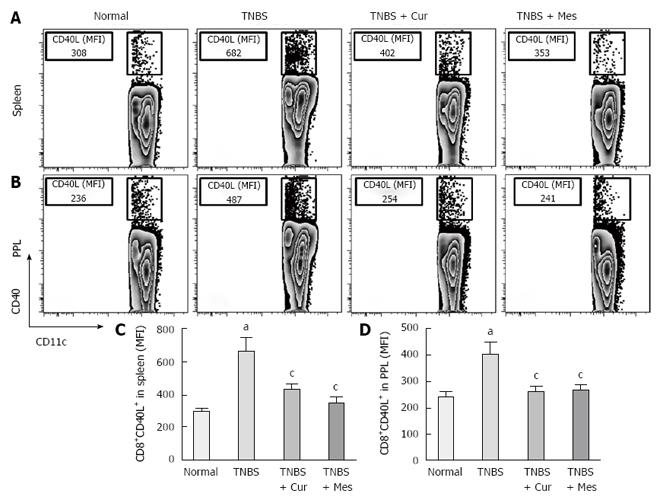
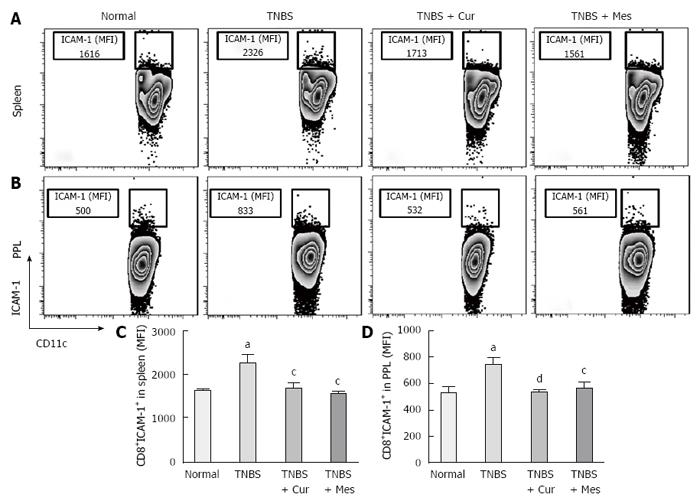
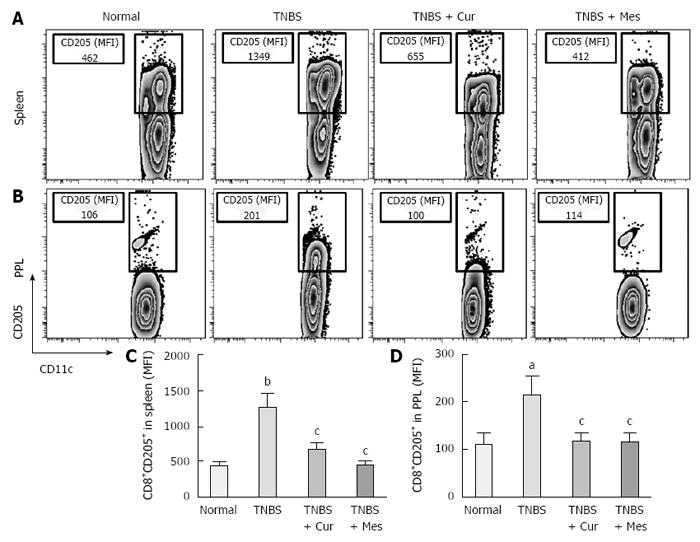
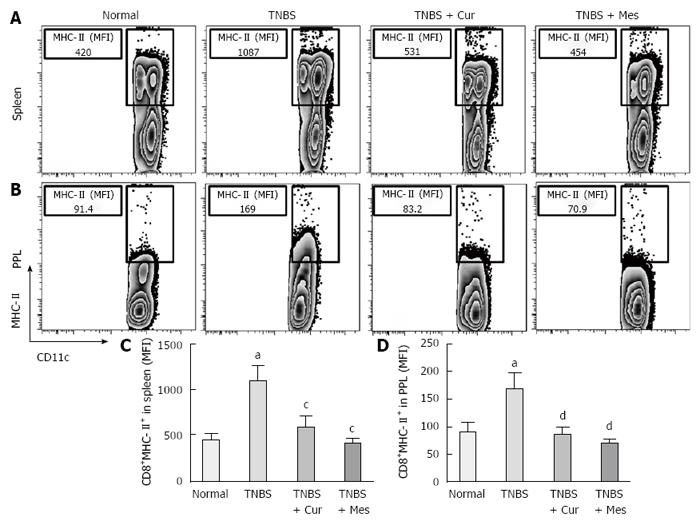
Expression of co-stimulatory molecules of CD8+CD11c+ cells, including CD40 (Figure 4), CD40L (Figure 5), CD54 (Figure 6), CD205 (Figure 7) and MHC II (Figure 8), was detected in normal spleen and PPs. Expression increased after TNBS-induced colitis. Treatment with Cur decreased expression of CD40 (Figure 4), CD40L (Figure 5), CD54 (Figure 6), CD205 (Figure 7) and MHC II (Figure 8) in the spleen and PPs.
In the present study, the DAI, colonic weight, weight index of the colon, and histological score of colonic of experimental colitis were significantly decreased after Cur treatment, while the body weight and colonic length were recovered. The results indicate that Cur can effectively treat experimental colitis. The numbers of CD8+CD11C+ cells in the spleen and PPs were decreased, which showed that the therapeutic effect of Cur on colitis was related to the number of CD8+CD11C+ cells.
As a positive regulatory factor, CD11c, which is an adhesion molecule in the CD11/CD18 family, participates in conglutination, migration, antigenic recognition and presence of DCs, and activates CD4+, CD8+ T cells to regulate the immune response. CD11c can promote DC activation and maturity by elevating expression of co-stimulatory molecules[32,33]. Activated CD11c+ DCs secrete a large number of inflammatory factors (including IL-1β, IL-6, IL-12 and IL-20) and promote CD4+ T cells transformation into Th 1 cells, inducing inflammatory injury[34]. In the present study, the total number of CD8+CD11c+ cells in the spleen and PPs increased in colitis mice, which decreased with Cur treatment. Our previous study indicated that Cur alleviated inflammatory injury in the colonic mucosa of colitis mice, using the same model as in the present study[24]. These results show that Cur exerts its therapeutic effect on TNBS-induced colitis by decreasing the number of CD8+CD11c+ cells.
Previous research has shown that CD8 + DCs play an important role in the development of experimental colitis and human IBD[11,12]. Our study showed that co-stimulatory molecules of DCs were increased in the spleen and PPs in untreated colitis mice, and Cur attenuated expression of MHC II, CD205, CD40, CD40L and CD54 (ICAM-1) in CD8+ DCs in the spleen and PPs. Our previous and present studies show that Cur can treat experimental colitis induced by TNBS or DSS[35,36]. The present study proves that Cur regulates the levels of CD8+ DCs to treat TNBS-induced colitis.
DCs are essential in the activation of the adaptive immune system[37], and can be distinguished into myeloid and lymphoid DCs based on the cell-surface expression of CD8[8,9], which is one of the most important DC subset markers. Research has previously demonstrated that lymphoid DCs express CD8 in mice, whereas myeloid DCs do not[38-40]. Thus, DCs in the spleen and PPs of mice are considered to be CD8+ DCs, which were identified in the present study[39,41].
Overwhelming evidence suggests that activation of CD8 + DCs is a significant pathway to generate specific CD8+ T-cell immune responses[42,43]. The complex pathway includes activation of Toll-like receptor 3[44], MHC and co-stimulatory molecule expression. MHC can promote DCs to migrate into lymphoid tissues such as the spleen and PPs, and accelerate antigen presentation, activation and maturation of DCs.
Researchers have previously incorporated MHC II as a phenotypic segregation marker for ex vivo analysis of DCs under inflammatory settings such as influenza[44]. Waithman et al[45] have shown that many of the CD8+ DC subpopulations undergo a phenotypic change from CD11chigh MHC IIint in naïve mice to CD11cint MHC IIhigh mice infected with influenza A virus. According to MHC II and CD11c expression levels, CD8+ DCs, which are a classic migratory DC phenotype, could be segregated into both lymphoid-resident DC subsets and migratory subsets found at inflammatory zones[46-48]. Based on the high expression of MHC II, CD8+ DCs capture antigens and promote T-cell migration at regions of the draining lymph nodes where they mature into functional DCs and present antigens to initiate primary immune responses[49,50]. In the process of maturation and activation of CD8+ DCs, co-stimulatory molecules are highly expressed and include expression of CD205, CD24, CD40 and CD40L[2].
As a symbol of maturation and activation, DCs express co-stimulatory molecules including members of the tumor necrosis factor (TNF)/TNF receptor protein family, CD40/CD40L and OX40/OX40L, and members of the immunoglobulin superfamily including ICAM-1/lymphocyte function-associated antigen (LFA-)1, and CD28/cytotoxic T lymphocyte associated antigen 4/B7. Collectively, these cell-surface expressed protein receptors and their cognate ligands regulate the balance between Th1 and Th2 responses, and were found to be highly expressed in human and animal colitis[51]. For example, CD40/CD40L signaling can stimulate DCs to secrete IL-12, and direct the differentiation of CD4+ T cells into Th1 cells. Similar functions are present in the context of ICAM-1/LFA-1 signaling and the B7-1 molecular signaling pathway (i.e., the B7/CD28 signal)[52,53].
More importantly, CD8+ DCs predominantly produce Th1-promoting cytokines like IL-12 p70 and IL-12 p40, while CD8+ DCs lead to Th1 differentiation with reduced secretion of IFN-γ and IL-10[8,54,55], and enhanced secretion of the proinflammatory cytokine IL-6, which is associated with autoimmunity and chronic inflammatory diseases[56]. These cytokines were previously shown to be closely related to the pathogenesis of IBD[57,58]. Therefore, we have experimental evidence to believe that CD8+ DCs played a critical role in the development of TNBS-induced colitis in our study. This was confirmed by the increased numbers of CD8+ DCs in the spleen and PPs in untreated colitis mice. The results showed high expression of MHC II, CD205, CD40, CD40L and ICAM-1. These co-stimulatory molecules and MHC II promoted CD8+ DCs to migrate into the colonic mucosa. Here, CD8+ DCs secreted proinflammatory cytokines and suppressed anti-inflammatory cytokine production, and ultimately induced inflammatory injury in the colonic mucosa.
Seven days after administration of Cur, the total number of CD8+CD11c+ cells was decreased, and the expression of these co-stimulatory molecules of DCs was inhibited. Although it is uncertain that Cur regulated the function of CD8+CD11c+ cells, Shirley et al[23] indicated that Cur prevented DCs from inducing CD4+ T-cell proliferation by blocking maturation marker expression, cytokine and chemokine secretion, and reducing migration and endocytosis of DCs.
The present study suggested that Cur restricted the quantity and activation of CD8+CD11c+ cells by downregulating expression of the co-stimulatory molecules of DCs in an attempt to improve the level of anti-inflammatory cytokines (i.e., IL-10, IFN-γ and TGF-β1). These data suggest a therapeutic role for Cur as an immunosuppressant in the treatment of IBD. However, the level of TGF-β1 in the colonic mucosa was decreased by Cur, which is contrary to that seen in the spleen. We speculated that overproduction of TGF-β1 in the colonic mucosa was related to the chronicity and fibrosis of experimental colitis. Thus, Cur might inhibit fibroplasia at the base of the colonic ulcer. However, the signaling pathway remains unknown under conditions in which Cur controls maturation and migration of CD8+ DCs. Future work is important in this area in an attempt to explore the pathway that regulates the function of CD8+ DCs by TGF-β1 signaling.
In conclusion, we demonstrated that Cur effectively treated experimental colitis, which was realized by inhibiting CD8+CD11c+ cells.
CD11c is a specific marker of dendritic cells (DCs) and is highly expressed in CD8+ and CD8- DCs. Overaccumulation of CD8+ DCs is seen in colonic mucosa in experimental colitis and patients with inflammatory bowel disease (IBD).
CD8+ DCs predominantly stimulate T helper (Th)1-inducing cytokines like interleukin (IL)-12p70 and IL-12p40, which can lead to Th1 differentiation, and have been reported to play a key role in controlling viral infection. Overaccumulation of CD8+ DCs induces inflammatory injury in the colonic mucosa when they migrate into Peyer’s patches in experimental colitis and in patients with IBD. Thus, CD8+ DCs may be a potential therapeutic target to explore the mechanisms of clinical treatment of IBD.
The present study is believed to be the first to show that curcumin (Cur) can effectively treat experimental colitis, which was realized by inhibiting CD8+CD11c+ cells.
It is known that Cur has a long history of effectively treating human IBD and experimental colitis. Cur prevents DC-mediated induction of CD4+ T-cell proliferation by blocking expression of maturation markers, cytokines and chemokines. However, it is unclear whether Cur can regulate expression of CD8+CD11c+ cells to treat IBD. The present study suggests that Cur can treat experimental colitis, via inhibition of CD8+CD11c+ cells.
CD11c is a type I transmembrane protein that mediates adherence between leukocytes and endothelial cells, and participates in exudation and phagocytosis of leukocytes.
The manuscript is presented in an easy understandable manner. The topic in the manuscript is very well explained. But it requires substantial corrections for the acceptance. According to detailed experimental data and reliable results, the present study had proved that Cur effectively treated experimental colitis, which was realized by inhibiting CD8+CD11c+ cells.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bolboaca SD, Gopu B, Sharaf IA S- Editor: Yu J L- Editor: Ma JY E- Editor: Wang CH
| 1. | Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785-21788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 987] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 2. | Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 486] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52:1522-1529. [PubMed] |
| 5. | Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795-823. [PubMed] |
| 7. | Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978-2986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 619] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | Maldonado-López R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(-) dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 2001;167:4345-4350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Vogt A, Mahé B, Costagliola D, Bonduelle O, Hadam S, Schaefer G, Schaefer H, Katlama C, Sterry W, Autran B. Transcutaneous anti-influenza vaccination promotes both CD4 and CD8 T cell immune responses in humans. J Immunol. 2008;180:1482-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Smith CM, Belz GT, Wilson NS, Villadangos JA, Shortman K, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437-4440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Sabado RL, Bhardwaj N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy. 2010;2:37-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Guo YM, Hirokawa M, Takahashi N, Fujishima M, Fujishima N, Komatsuda A, Tagawa H, Ohyagi H, Michishita Y, Ubukawa K. Delayed addition of tumor necrosis factor (TNF) antagonists inhibits the generation of CD11c+ dendritic cells derived from CD34+ cells exposed to TNF-alpha. Int J Hematol. 2010;91:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol. 2008;38:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 16. | Hofmann C, Dunger N, Grunwald N, Hämmerling GJ, Hoffmann P, Schölmerich J, Falk W, Obermeier F. T cell-dependent protective effects of CpG motifs of bacterial DNA in experimental colitis are mediated by CD11c+ dendritic cells. Gut. 2010;59:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787-809. [PubMed] |
| 18. | Salh B, Assi K, Templeman V, Parhar K, Owen D, Gómez-Muñoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235-G243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 749] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 21. | Martelli L, Ragazzi E, di Mario F, Martelli M, Castagliuolo I, Dal Maschio M, Palù G, Maschietto M, Scorzeto M, Vassanelli S. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil. 2007;19:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 22. | Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 429] [Article Influence: 22.6] [Reference Citation Analysis (3)] |
| 23. | Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS. Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun. 2008;374:431-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Zhao HM, Xu R, Huang XY, Cheng SM, Huang MF, Yue HY, Wang X, Zou Y, Lu AP, Liu DY. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World J Gastroenterol. 2016;22:5374-5383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Huang LY, He Q, Liang SJ, Su YX, Xiong LX, Wu QQ, Wu QY, Tao J, Wang JP, Tang YB. ClC-3 chloride channel/antiporter defect contributes to inflammatory bowel disease in humans and mice. Gut. 2014;63:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Sałaga M, Mokrowiecka A, Zakrzewski PK, Cygankiewicz A, Leishman E, Sobczak M, Zatorski H, Małecka-Panas E, Kordek R, Storr M. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH). J Crohns Colitis. 2014;8:998-1009. [PubMed] |
| 27. | Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038-1048. [PubMed] |
| 28. | Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, Bernstein CN, Peng Z. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Ghia JE, Blennerhassett P, El-Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G711-G718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Si JM, Liu WL, Cai JT, Du Q, Wang LJ, Gao M. Induction of experimental acute ulcerative colitis in rats by administration of dextran sulfate sodium at low concentration followed by intracolonic administration of 30% ethanol. J Zhejiang Univ Sci B. 2007;8:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Schmidt N, Gonzalez E, Visekruna A, Kühl AA, Loddenkemper C, Mollenkopf H, Kaufmann SH, Steinhoff U, Joeris T. Targeting the proteasome: partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut. 2010;59:896-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Lin Y, Roberts TJ, Sriram V, Cho S, Brutkiewicz RR. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur J Immunol. 2003;33:2736-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Singh-Jasuja H, Thiolat A, Ribon M, Boissier MC, Bessis N, Rammensee HG, Decker P. The mouse dendritic cell marker CD11c is down-regulated upon cell activation through Toll-like receptor triggering. Immunobiology. 2013;218:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Fujiwara D, Chen L, Wei B, Braun J. Small intestine CD11c+ CD8+ T cells suppress CD4+ T cell-induced immune colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G939-G947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Zhang M, Deng C, Zheng J, Xia J, Sheng D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol. 2006;6:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Liu L, Liu YL, Liu GX, Chen X, Yang K, Yang YX, Xie Q, Gan HK, Huang XL, Gan HT. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int Immunopharmacol. 2013;17:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Mackern-Oberti JP, Llanos C, Vega F, Salazar-Onfray F, Riedel CA, Bueno SM, Kalergis AM. Role of dendritic cells in the initiation, progress and modulation of systemic autoimmune diseases. Autoimmun Rev. 2015;14:127-139. [PubMed] |
| 38. | Beijer MR, Molenaar R, Goverse G, Mebius RE, Kraal G, den Haan JM. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8- CD4- and CD4+ dendritic cells. Eur J Immunol. 2013;43:1608-1616. [PubMed] |
| 39. | Rutella S, Locatelli F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2011;17:3761-3775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 481] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 880] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 42. | Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153-162. [PubMed] |
| 43. | Langlois RA, Varble A, Chua MA, García-Sastre A, tenOever BR. Hematopoietic-specific targeting of influenza A virus reveals replication requirements for induction of antiviral immune responses. Proc Natl Acad Sci USA. 2012;109:12117-12122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Davey GM, Wojtasiak M, Proietto AI, Carbone FR, Heath WR, Bedoui S. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J Immunol. 2010;184:2243-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Waithman J, Zanker D, Xiao K, Oveissi S, Wylie B, Ng R, Tögel L, Chen W. Resident CD8(+) and migratory CD103(+) dendritic cells control CD8 T cell immunity during acute influenza infection. PLoS One. 2013;8:e66136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 47. | Ballesteros-Tato A, León B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216-224. [PubMed] |
| 48. | Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 561] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 49. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10720] [Article Influence: 397.0] [Reference Citation Analysis (0)] |
| 50. | Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729-10734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 51. | Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Sheng KC, Pietersz GA, Wright MD, Apostolopoulos V. Dendritic cells: activation and maturation--applications for cancer immunotherapy. Curr Med Chem. 2005;12:1783-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Vainer B. Intercellular adhesion molecule-1 (ICAM-1) in ulcerative colitis: presence, visualization, and significance. APMIS Suppl. 2010;1-43. [PubMed] |
| 54. | Kamijo R, Shapiro D, Gerecitano J, Le J, Bosland M, Vilcek J. Biological functions of IFN-gamma and IFN-alpha/beta: lessons from studies in gene knockout mice. Hokkaido Igaku Zasshi. 1994;69:1332-1338. [PubMed] |
| 55. | Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 56. | Johnston JA, O’Shea JJ. Matching SOCS with function. Nat Immunol. 2003;4:507-509. [PubMed] |
| 57. | Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, Calame K. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 58. | Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466-474. [PubMed] |