Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1764
This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial.
Peer-review started: November 13, 2016
First decision: December 19, 2016
Revised: December 27, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: March 14, 2017
Processing time: 122 Days and 23.7 Hours
To address to what extent hypertrophy and hyperplasia contribute to liver mass restoration after major tissue loss.
The ability of the liver to regenerate is remarkable on both clinical and biological grounds. Basic mechanisms underlying this process have been intensively investigated. However, it is still debated to what extent hypertrophy and hyperplasia contribute to liver mass restoration after major tissue loss. We addressed this issue using a genetically tagged system. We were able to follow the fate of single transplanted hepatocytes during the regenerative response elicited by 2/3 partial surgical hepatectomy (PH) in rats. Clusters of transplanted cells were 3D reconstructed and their size distribution was evaluated over time after PH.
Liver size and liver DNA content were largely recovered 10 d post-PH, as expected (e.g., total DNA/liver/100 g b.w. was 6.37 ± 0.21 before PH and returned to 6.10 ± 0.36 10 d after PH). Data indicated that about 2/3 of the original residual hepatocytes entered S-phase in response to PH. Analysis of cluster size distribution at 24, 48, 96 h and 10 d after PH revealed that about half of the remnant hepatocytes completed at least 2 cell cycles. Average size of hepatocytes increased at 24 h (248.50 μm2 ± 7.82 μm2, P = 0.0015), but returned to control values throughout the regenerative process (up to 10 d post-PH, 197.9 μm2 ± 6.44 μm2, P = 0.11). A sizeable fraction of the remnant hepatocyte population does not participate actively in tissue mass restoration.
Hyperplasia stands as the major mechanism contributing to liver mass restoration after PH, with hypertrophy playing a transient role in the process.
Core tip: The ability of the liver to regenerate is remarkable on both clinical and biological grounds. It is still debated to what extent hypertrophy and hyperplasia contribute to liver mass restoration after major tissue loss. We addressed this issue using a genetically tagged system during the regenerative response elicited by 2/3 partial hepatectomy (PH) in rats. Analysis of cluster size distribution revealed that about half of the remnant hepatocytes completed at least 2 cell cycles. Average size of hepatocytes returned to control values throughout the regenerative process. Thus, hyperplasia stands as the major mechanism contributing to liver mass restoration after PH.
- Citation: Marongiu F, Marongiu M, Contini A, Serra M, Cadoni E, Murgia R, Laconi E. Hyperplasia vs hypertrophy in tissue regeneration after extensive liver resection. World J Gastroenterol 2017; 23(10): 1764-1770
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1764
The ability of the liver to regenerate is remarkable on both clinical and biological grounds. It allows this organ to maintain functional proficiency in spite of the multitude of food-born toxic insults it can be exposed to throughout life, given its anatomical position[1]. In addition, it represents one of the best systems for the mechanistic analysis of regulatory pathways controlling cell proliferation in vivo. Unsurprisingly, a vast scientific literature is dedicated to the detailed description of the process of liver regeneration, providing fundamental insights into its biological and molecular bases[2,3].
Partial (two/thirds) surgical hepatectomy (PH) is the most widely used experimental procedure to study liver regeneration. This model offers two important advantages: (1) it allows a relatively “clean” removal of hepatic parenchyma, due to the multilobular structure of the rodent liver, with no major interference of tissue necrosis and/or inflammation; and (2) The procedure is rapid (it can be performed in a few minutes) and the kinetics of the response is amenable to precise timing[4]. A large body of data is therefore available regarding the response of the liver following PH. The general consensus has been that, in order to restore the original mass, the majority of hepatocytes in the remaining lobes undergo one or two cell division cycles, resuming quiescence at the end of the process[2]. This conclusion is primarily based on reports describing the cumulative labelling of S phase cells[5-7], while direct data regarding the actual proportion of cells completing mitosis after S phase have been more difficult to obtain[6]. New insights into this issue were provided in an elegant study published a few years ago by Miyaoka et al[8], who followed the fate of single genetically tagged hepatocytes in the liver of mice during their response to PH. They reported that a significant fraction of hepatocytes (up to 40%) do not divide in the course of the regenerative response, while an increase in the size of single cells (hypertrophy) accounts for at least one third of the overall restoration of liver mass occurring after PH[8]. In spite of their challenging nature to current assumptions referred to above, to our knowledge these results have not been addressed so far. Taking advantage of an orthotopic system for rat hepatocyte transplantation that is utilized routinely by our research group[9], we probed into the hypothesis proposed by Miyaoka et al[8]. Our results support the conclusion that up to 1/3 of the remnant hepatocytes do not enter S-phase and/or divide in response to PH; however, hyperplasia is the main biological mechanism sustaining liver mass restoration in rats, while hypertrophy does not appear to contribute significantly to the process.
The syngeneic Fischer 344 rat strain was used for transplantation experiments. All animals were maintained on daily cycles of alternating 12 h light-darkness with food and water available ad libitum. They were fed Purina Rodent Lab Chow diet throughout the experiment and received humane care according to the criteria outlined in the National Institutes of Health Publication 86-23, revised 1985. Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Cagliari.
Hepatocytes were isolated using a two-step collagenase perfusion procedure as previously described[10,11]. To follow the fate of transplanted cells in the host liver, syngeneic donors expressing the green fluorescent protein (GFP+) were used. Heterozygous rats expressing GFP under ubiquitin C promoter (line 307 F455 Chr5) were obtained from Rat Resource and Research Center (University of Missouri, Columbia, MO) and they were bred to homozygosity before being utilized. Isolated cells were transplanted (Tx) into the liver of recipient animals (2 × 106 cells per animal in 0.2 mL) via a mesenteric vein[9].
Transplanted hepatocytes were then allowed to engraft and integrate in the recipient liver and one month later 2/3 partial hepatectomy (PH) was performed; groups of 5 animals each were killed at various time points thereafter, including 24, 48, 72, 96 h and 10 d post-operation. One group of intact animals was kept as control. Each animal received multiple doses of 5’-bromo-deoxyUridine (BrdU, 50 mg/kg, i.p.), every 6 h, starting at 24 h before killing; the last injection was given 1 h prior to euthanasia. Livers were excised and tissue samples were either immediately frozen or fixed for further analysis. Liver DNA content was measured according to published techniques[12].
For immunofluorescence analysis, liver tissues were fixed in 4% paraformaldehyde (PFA), cryoprotected in 30% sucrose solution for 24 h at 4 °C, and then frozen. Five μm-thick sections were blocked for 30’ with goat serum and incubated 1h at RT with Alexa Fluor 555®-conjugated Phalloidin (Thermo Fisher Scientific, Waltham, MA, United States). Nuclei were counterstained with DAPI (Abcam, Cambridge, MA, United States).
Immunohistochemical staining for GFP and BrdU, was performed on 5 μm-thick paraffin embedded sections, following de-wax and antigen retrieval with 0.01 mol/L pH 6 sodium citrate buffer. Slides were blocked for 30’, incubated with the primary antibody (GFP, Thermo Fisher Scientific; BrdU, Santa Cruz, CA, United States) overnight at 4 °C. Detection of specific signal was accomplished using an HRP/AEC detection IHC Kit (Abcam).
Three dimensional analysis of GFP+ clusters was performed on 10 consecutive serial sections by scanning slides with a Pathscan Enabler IV scanner (Meyer Instruments, Houston, TX, United States). Acquired images were overlayed and analyzed using Image-Pro Premier Software (Media Cybernetics, Rockville, MD, United States). Cell and nuclear size was measured on fluorescence images acquired with an Axio Imager Fluorescence Microscope (Zeiss, Oberkochen, Germany) using Image-Pro Premier Software.
Data were analyzed and plotted using GraphPad Prism (GraphPad Software, La Jolla, CA, United States). Results are presented as mean ± SE. Two-tailed Student t test was used to evaluate results, with a lowest level of significance of P < 0.05. Statistical review of the study was performed by Prof. Giacomo Diaz from the University of Cagliari.
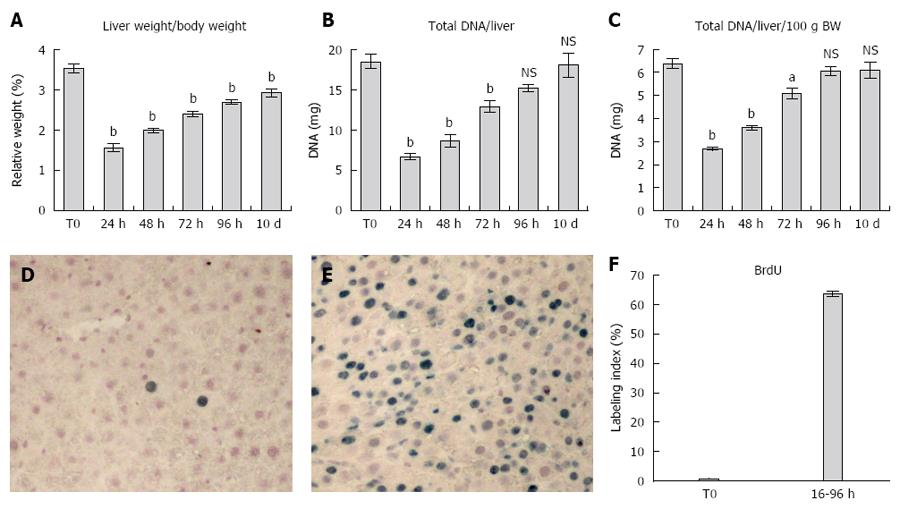
Relative liver weight increased gradually from day 1 to day 10 post-PH, returning to near-normal values at the latter time point (Figure 1A). A similar pattern was seen for the absolute and relative (i.e., expressed as percent body weight) liver DNA content: both parameters had largely recovered between 72 and 96 h after PH and attained levels comparable to normal by 10 d post-surgery (Figure 1B and C).
Panels D, E and F report data on the cumulative S-phase entry of hepatocytes during the first 96 h after PH. Both the figure in panel E and the plot in panel F clearly indicate that about one third of the hepatocytes have not entered S-phase as late as 96 h post-PH. Furthermore, this proportion is possibly still higher if referred to the remnant liver prior to the initiation of the proliferative response, in that at least a fraction of S-phase cells have divided and are therefore over-represented at 96 h post-PH.
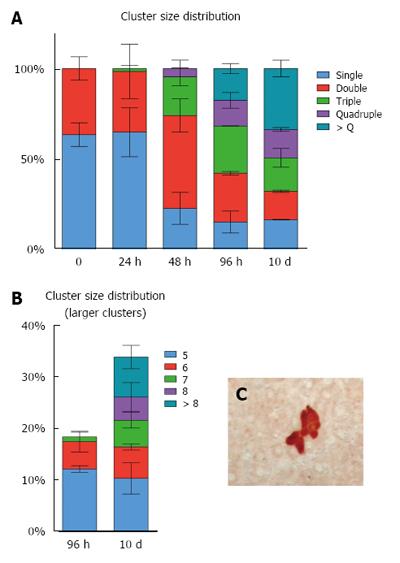
As detailed the Experimental Procedures, hepatocytes isolated from a syngeneic Fischer 344 rat donor expressing the GFP were transplanted into the liver of GFP-negative recipients, via a mesenteric vein. Four weeks later, PH was performed and the fate of GFP+ hepatocytes clusters was followed over time during the regenerative response of the liver. Each cluster was reconstructed in 3D through the analysis of 10 consecutive serial sections from each sample (see Experimental Procedures). Results are presented in Figure 2. At the time of PH, only single GFP+ cells and doublets (about 60% and 40%, respectively) were seen (Figure 3A). This proportion remained virtually unchanged at 24 h post-PH, while it had significantly shifted at 48 h, with a relative decrease of single GFP+ cells, an increase in doublets, a consistent appearance of triplets (about 20% of the total) and the first detection of four-cell sized clusters. Such progressive shift of GFP+ clusters towards higher size categories continued at 96 h and was still more prominent at 10 d post-PH (Figure 3A and B). Clusters of 5 GFP+ cells and larger were detected at 96 h (about 20% of the total, Figure 3C) and their proportion increased to approximately 35% at 10 d post-PH, when over 10% of GFP+ clusters comprised 8 or more hepatocytes, indicating that they resulted from multiple cell cycles.
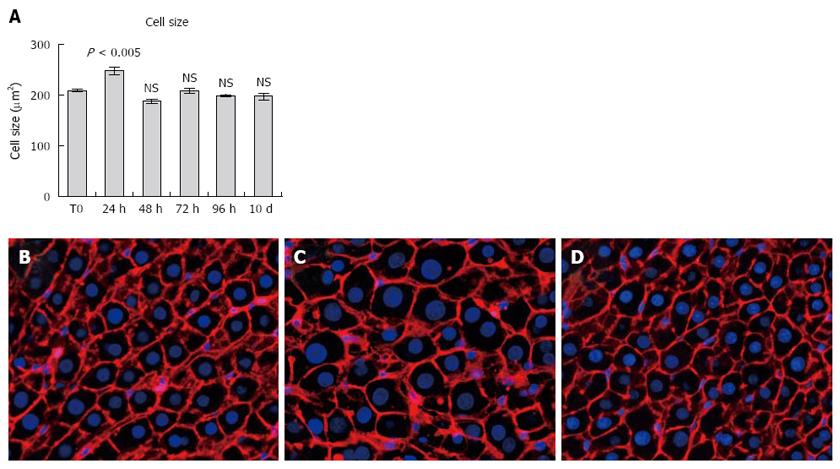
In Figure 4 (panels A through D) the average size of hepatocytes at various time points after PH, measured on 2D slides, is reported. The only evident change was observed at 24 h post-surgery, i.e., prior to the first wave of mitosis, when hepatocytes were significantly enlarged compared to any other time point considered. Importantly, no differences in size were recorded between hepatocytes in resting liver and those present at the end of the regenerative phase.
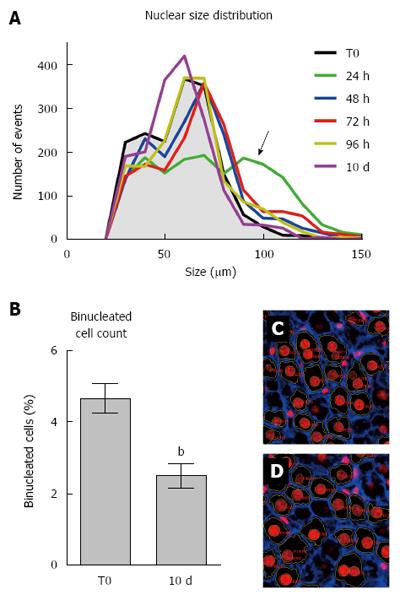
Size distribution of hepatocyte nuclei during the regenerative response was also similar at various time points post-PH, the only evident change being detected after 24 h (Figure 4A). In fact, at 24 h hepatocyte nuclei appeared to distribute in three different size categories, including a larger one, which is absent or minimally present in either control rat liver or at later time points after PH.
Finally, we estimated the percent of binucleated hepatocytes on 2D liver sections obtained prior to PH and at 10 d post-surgery (Figure 4, panels B through D). Although this is clearly an underestimate of the absolute numbers, results did indicate a significant drop (about 50%) in the proportion of binuclear hepatocytes following PH, in agreement with previous studies[8,13]. Such decrease has been generally attributed to cell division occurring in response to PH[13].
The remarkable ability of the liver to regenerate has intrigued humankind ever since the dawn of civilization, as exemplified by Greek mythology[14]. However, it was the work of Higgins and Anderson[15], describing the surgical procedure to perform PH, that set the stage for a detailed analysis of the process. Classical studies by Grisham[7], by Bucher’s research group[5,16] and by Fabrikant[6] established fundamental parameters of hepatic regeneration, including the kinetics of DNA synthesis in parenchymal and littoral cells and its critical dependence on the extent of tissue removal. The general agreement that emerged from these observations was that, in order for the liver to recover its original mass after PH, the large majority of hepatocytes had to undergo one round of DNA synthesis and cell division, followed by a smaller percentage of cells entering a second replication cycle[3]. In retrospect, it is worth noting that irrefutable evidence in support of this paradigm was not present in the available literature. In fact, the seminal papers referred to above report levels of about 60% resident hepatocytes entering S phase within 36-40 h post-PH, and an additional 22% doing so between 36 and 72 h post-surgery[5,7], with the possibility that the latter population could represent, at least in part, a fraction of the former. Furthermore, Rabes et al[17] reported that up to 80% of hepatocytes initiated S-phase during the first 40 h after PH; however, those studies were performed under continuous infusion of hydroxyurea, an S-phase blocker that might have recruited additional cells into cycle. Thus, the postulation that all residual hepatocytes enter the cell cycle at least once after PH has been rather inferential in nature.
A direct challenge to this widely accepted concept came from work by Miyaoka et al[8] reported a few years ago. The authors followed the fate of tagged single hepatocytes during their response to PH in mouse. They were able to observe that a significant fraction of hepatocytes (about 40%) do not divide in the course of the regenerative response, while an increase in the size of single cells (hypertrophy) accounts for at least one third of the overall restoration of liver mass occurring after PH[8].
Given the relevance of these findings, in the present investigation we probed into this issue using an experimental system of hepatocyte transplantation in the rat that is conceptually similar to the one of Miyaoka et al[8]. Single hepatocytes expressing GFP were injected into the liver of syngeneic Fischer 344 rats and their fate was traced over time following PH. Our findings indicate that hyperplasia stands as the main biological mechanism sustaining restoration of liver mass following PH in the rat, while hypertrophy does not appear to contribute to the process to any measurable extent.
These conclusions stem from the following observations. First, restoration of liver weight and liver DNA content is already prominent at day 4 and is virtually complete at day 10 post PH, as expected[18]. Secondly, the size distribution of GFP+ hepatocyte clusters at various time points post PH indicates that about 1 in 3 GFP+ clusters detected at day 10 comprise more than 4 cells. Given that at time zero all clusters were only 1 or 2 cells in size, the only possibility is that clones containing 5 cells and higher resulted from at least two cell division cycles of the original residual hepatocytes.
On the other hand, we confirmed that a sizeable proportion of the original hepatocytes do not enter S phase and/or do not appear to divide (Figure 1, panel F) for up to 10 d post-PH, when hepatic mass is largely recovered, in agreement with previous results[5-8]. In fact, about 1/3 of GFP+ clusters were still 1 to 2 cells in size at the end of the regenerative phase, indicating that they had not responded to the proliferative stimulus. Conversely, as already mentioned, a sub-population of the original hepatocytes divided at least twice, contributing substantially to the final liver mass. This is in line with data reported by Wu et al[19], documenting that at least 11% of residual hepatocytes divide thrice or more after PH[19,20]. Although S-phase and mitotic division are not necessarily coupled, neither in the liver or in other tissues[21], classical studies by Fabrikant indicated that at least the first wave of mitosis following PH in rat liver is preceded by DNA synthesis in virtually all dividing cells[6]. This implies that unconventional cell division, i.e., mitosis without prior S-phase[15], is not of prominent occurrence, if any, under these conditions.
Furthermore, mean hepatocyte size, measured in 2D, increased at 24 h after PH (Figure 3); however, no significant changes were observed at later time points and till the end of the regenerative process, in agreement with previously published results[22], implying that cell hypertrophy is not a major contributor to liver mass reconstitution after PH.
The reason(s) for the discrepancies between our present data and those of Miyaoka et al[8] are not apparent at this point. One likely possibility is that there might exist species-specificities in the overall strategies set in motion to respond to liver tissue loss in mice as compared to rats. It is well known that the kinetics of response to PH are substantially different between the two species[3], and such peculiarities appear to be cell autonomous, as if they are part of the overall genetic program of each species[23]. By analogy, a similar concept might also extend to the threshold level of tissue loss involved in activation of hypertrophy as opposed to hyperplasia as compensatory mechanisms for functional tissue mass restoration. More investigations are required to address this fundamental issue in rats and mice and, possibly, in humans.
In conclusion, we present evidence to indicate that restoration of rat liver mass following PH is attained largely via a hyperplastic response of the residual tissue. However, such response does not involve the totality of the residual hepatocyte population.
We thank Mrs. Anna Saba and Mr. Roberto Marras for their technical contribution.
The ability of the liver to regenerate is remarkable on both clinical and biological grounds. Basic mechanisms underlying this process have been intensively investigated. However, it is still debated to what extent hypertrophy and hyperplasia contribute to liver mass restoration after major tissue loss. We addressed this issue using a genetically tagged system.
Liver regenerative capacity declines with aging and its preservation is one of aims of current research.
A better understanding of such fundamental physiological response of the liver is important to devise better therapeutic strategies (e.g., through cell therapy of liver diseases).
The techniques described in this study can be applied to the field of regenerative medicine.
In this paper, the authors described the mechanism of hepatocyte regenerate the liver after acute liver injury model using partial hepatectomy as model. The authors clearly described hepatocytes undergo hypertrophy and hyperplasia after liver injury, with the occurrence of hypertrophy only observed within the first 24 h, and hepatocyte hyperplasia is mainly responsible for the remaining liver regeneration event. The authors use transplantation studies in rats as an alternative method to investigate the mechanism of hepatocyte regenerates the liver after acute liver injury.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): C
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lu WY S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Huang J, Rudnick DA. Elucidating the metabolic regulation of liver regeneration. Am J Pathol. 2014;184:309-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 3. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 4. | Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 570] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 5. | Bucher NL, Swaffield MN. The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res. 1964;24:1611-1625. [PubMed] |
| 6. | Fabrikant JI. The kinetics of cellular proliferation in regenerating liver. J Cell Biol. 1968;36:551-565. [PubMed] |
| 7. | Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842-849. [PubMed] |
| 8. | Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 9. | Serra MP, Marongiu F, Marongiu M, Contini A, Laconi E. Cell-autonomous decrease in proliferative competitiveness of the aged hepatocyte. J Hepatol. 2015;62:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506-520. [PubMed] |
| 11. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [PubMed] |
| 12. | Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DS, Pani P, Laconi E. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069-1074. [PubMed] |
| 13. | Gerlyng P, Abyholm A, Grotmol T, Erikstein B, Huitfeldt HS, Stokke T, Seglen PO. Binucleation and polyploidization patterns in developmental and regenerative rat liver growth. Cell Prolif. 1993;26:557-565. [PubMed] |
| 14. | Tiniakos DG, Kandilis A, Geller SA. Tityus: a forgotten myth of liver regeneration. J Hepatol. 2010;53:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Higgins G, Anderson GM. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186-202. |
| 16. | Bucher NL. Regeneration of mammalian liver. Int Rev Cytol. 1963;15:245-300. [PubMed] |
| 17. | Rabes HM, Iseler G, Czichos S, Tuczek HV. Synchronization of hepatocellular DNA synthesis in regenerating rat liver by continuous infusion of hydroxyurea. Cancer Res. 1977;37:1105-1111. [PubMed] |
| 18. | Bucher NL. Experimental aspects of hepatic regeneration. N Engl J Med. 1967;277:738-746 concl. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wu Y, Guo F, Liu J, Xiao X, Huang L, He D. Triple labeling with three thymidine analogs reveals a well-orchestrated regulation of hepatocyte proliferation during liver regeneration. Hepatol Res. 2011;41:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Tamori Y, Deng WM. Compensatory cellular hypertrophy: the other strategy for tissue homeostasis. Trends Cell Biol. 2014;24:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Papp V, Dezsö K, László V, Nagy P, Paku S. Architectural changes during regenerative and ontogenic liver growth in the rat. Liver Transpl. 2009;15:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Weglarz TC, Sandgren EP. Timing of hepatocyte entry into DNA synthesis after partial hepatectomy is cell autonomous. Proc Natl Acad Sci USA. 2000;97:12595-12600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |