Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2725
Peer-review started: September 23, 2015
First decision: October 14, 2015
Revised: November 1, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: March 7, 2016
Processing time: 162 Days and 13.1 Hours
The prevalence of hepatic cirrhosis in Europe and the United States, currently 250 patients per 100000 inhabitants, is steadily increasing. Thus, we observe a significant increase in patients with cirrhosis and portal hypertension needing liver resections for primary or metastatic lesions. However, extended liver resections in patients with underlying hepatic cirrhosis and portal hypertension still represent a medical challenge in regard to perioperative morbidity, surgical management and postoperative outcome. The Barcelona Clinic Liver Cancer classification recommends to restrict curative liver resections for hepatocellular carcinoma in cirrhotic patients to early tumor stages in patients with Child A cirrhosis not showing portal hypertension. However, during the last two decades, relevant improvements in preoperative diagnostic, perioperative hepatologic and intensive care management as well as in surgical techniques during hepatic resections have rendered even extended liver resections in higher-degree cirrhotic patients with portal hypertension possible. However, there are few standard indications for hepatic resections in cirrhotic patients and risk stratifications have to be performed in an interdisciplinary setting for each individual patient. We here review the indications, the preoperative risk-stratifications, the morbidity and the mortality of extended resections for primary and metastatic lesions in cirrhotic livers. Furthermore, we provide a review of literature on perioperative management in cirrhotic patients needing extrahepatic abdominal surgery and an overview of surgical options in the treatment of hepatic cirrhosis.
Core tip: Liver resections in patients with underlying hepatic cirrhosis and portal hypertension still represent a medical challenge with regard to perioperative morbidity, surgical management and postoperative outcome. However, the increasing incidence of liver cirrhosis on the one hand, and the ongoing improvements in surgical technique and perioperative management on the other hand have up until today rendered even extended hepatic resections in these patients possible. Especially in primary and metastatic liver malignancies, surgery often presents the only curative approach and thus potential short- and long-term benefits and risks have to be evaluated carefully and interdisciplinary from a surgical, oncological and hepatologist point of view.
- Citation: Hackl C, Schlitt HJ, Renner P, Lang SA. Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol 2016; 22(9): 2725-2735
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2725
Liver cirrhosis, the end-stage of chronic liver fibrosis, shows a prevalence of 250 patients per 100000 inhabitants[1,2]. Pathological alterations of liver parenchyma and hepatic perfusion lead to a decrease in hepatocyte function and an increase in transhepatic perfusion resistance, resulting in portal hypertension. Over time, up to 20% of cirrhotic patients develop a hepatocellular carcinoma[1].
Causes of liver cirrhosis are manifold (Table 1). In Europe and the United States, alcoholic liver disease is the predominant cause of hepatic cirrhosis, followed by chronic viral hepatitis (B/C) and nonalcoholic fatty liver disease. In many African and Asian countries, chronic viral hepatitis remains the main cause of liver cirrhosis[1].
| Alcoholic liver disease |
| Chronic viral hepatitis (hepatitis B and C) |
| Non-alcoholic fatty liver disease |
| Primary and secondary biliary cirrhosis |
| Primary sclerosing cholangitis |
| Hemochromatosis |
| Autoimmune hepatitis |
| Wilson’s disease |
| α1-Antitrypsin deficiency |
| Celiac disease |
| Right-sided heart failure |
| Granulomatous liver disease |
| Congenital malformation syndromes |
Phenotypically, liver cirrhosis can show a variety of symptoms from almost asymptomatic stage to liver failure. In advanced cirrhosis, live-threatening complications are bleeding from esophageal varices, hydroptic decompensation (ascites), spontaneous bacterial peritonitis and hepatic encephalopathy. A first evaluation of the cirrhotic stage can be estimated by the Child-Pugh-Turcotte Score (CPT) using the INR (International Normalized Ratio), the serum bilirubine, the serum albumine, the grade of hepatic encephalopathy and the grade of ascites[3]. Although the CPT score is a valuable method to categorize patients into individuals with compensated (CPT A), mildly (CPT B) or severely decompensated (CPT C) cirrhosis, it can be influenced by the subjective assessment of the parameters “ascites” and “encephalopathy”. Furthermore, it does not include the prognostic relevant assessment of renal function.
Another system evaluating the mortality of a patient with liver cirrhosis is the Model of End Stage Liver Disease (MELD) score, currently used for organ allocation in liver transplantation[4,5]. It is reproducibly calculated from creatinine, bilirubin and INR, and does not include less precise parameter such as ascites or varices. Since a continuous deterioration of each individual parameter results in a continuous increase in MELD score, risk discrimination of individual patients is more accurate than using the CPT score[6,7]. However, neither the CPT- nor the MELD-score were originally created for surgical settings. While the CPT classification was developed to estimate the liver-specific overall survival of cirrhotic patients, the MELD-score was developed for estimating the mortality after TIPS (transjugular portosystemic stent shunt) -placement[4,5]. A main drawback in surgical risk stratification is that both scoring systems do not include features of portal hypertension except for ascites in the CPT classification.
Portal hypertension is defined as an increased pressure gradient between portal vein and hepatic veins [= portal-pressure gradient (PPG) also called the porto-caval gradient/porto-systemic gradient]. The hepatic venous pressure gradient (HVPG) is an indirect measurement of the real PPG, which allows obviating the risks linked with the direct puncture of the portal vein[8]. Under physiological conditions, the HVPG is 1 to 5 mmHg. Subclinical portal hypertension is defined as a HVPG of 5 to 9 mmHg. Clinically relevant portal hypertension shows a HVPG of ≥ 10 mmHg. Reasons for portal hypertension are shown in Table 2. In Western countries, hepatic cirrhosis accounts for > 90% of cases of portal hypertension.
| Prehepatic etiology of portal hypertension |
| Portal vein thrombosis |
| External portal vein compression |
| Intrahepatic etiology of portal hypertension |
| Hepatic cirrhosis (of any origin) |
| Congenital hepatic fibrosis |
| Schistosomiasis |
| Idiopathic non-cirrhotic portal hypertension |
| Posthepatic etiology of portal hypertension |
| Budd-Chiari’s syndrome |
| Sinusoidal obstruction syndrome |
| Cirrhose cardiaque |
Gold standard for assessing the HVPG is the angio-invasive measurement of the free hepatic venous pressure (FHVP) in the right hepatic vein as well as the wedge hepatic venous pressure (WHVP) at the same location[8]. HVPG is then defined as the difference of WHVP and FHVP. Since this invasive method may not be available everywhere and might also cause complications, translational studies are currently performed to establish ultrasound-based elastography for assessing the degree of portal hypertension. So far some promising progress has been made using Fibroscan® measurements. Here, a cut-off value of 13.6 kPa has been described as being 90% sensitive to detect clinically relevant portal hypertension, with excellent specificity at a higher cut-off value of 21.1 kPa. Thus, invasive HVPG measurements to diagnose clinically relevant portal hypertension may be limited to patients without esophageal varices and with a Fibroscan® measurement result between 13.0 and 21.1 kPa[9]. Ongoing clinical assessments will further refine this technique[10,11]. Meanwhile, surrogate markers such as thrombocytopenia < 100/nL, splenomegaly > 12 cm, the endoscopic confirmation of esophageal varices (that do not occur in HVPG < 10 mmHg[12]), clinical signs of collateralization such as the presence of caput medusae and presence as well as extent of ascites are used to define portal hypertension[13].
While taking the medical history of a patient with liver cirrhosis, a main focus should lie on prior episodes of decompensation or variceal hemorrhage, ongoing alcohol abuse and further comorbidities such as congestive gastropathy, cardiovascular complaints (coronary artery sclerosis, cirrhotic cardiomyopathy), pulmonary disorders (chronic obstructive pulmonary disease (COPD), hepato-pulmonary syndrome, pleural effusion), restricted renal function (hepato-renal syndrome), diabetes and malnutrition[14,15]. During physical examination, signs of portal hypertension such as ascites and caput medusae may be seen. Preoperative laboratory screening should include a full blood count (anemia, thrombocytopenia), coagulation tests (INR), renal function parameters (creatinine, GFR, electrolytes) as well as liver function parameters. Radiologic examinations should consist of (if possible: contrast-enhanced) ultrasound (parenchymal liver morphology, focal lesions, liver perfusion, umbilical vein recanalization, elastography if available) and contrast-enhanced CT/MRI scans (morphology of lesions indicating liver resection, staging, morphology of collaterals, volumetric assessment of remnant liver).
Several methods have been established to specifically evaluate the liver function prior to hepatic resections[16]. Among them, the LiMaX (maximum liver function capacity) test, which analyses the liver-specific, cytochrome P450-based metabolism of 13C-methacetin[17,18], and the Indocyanine-green-Clearance (ICG-Clearance) test[19-21] are most commonly used. Ishizawa et al[22] have reported the combination of ascites, bilirubine and indocyanine-green-clearance to be excellent predictors of resectability for hepatocellular carcinoma (HCC) independent of portal hypertension. However, LiMaX-Test and Indocyanine-green-clearance both analyse the global liver function and cannot distinguish the expected post-surgical liver remnant function. A method that may distinguish this remnant liver function is functional scintigraphy and functional MRI using Gd-EOP-DTPA contrast enhancement[23-25]. Nonetheless, up until today, little experience of these assays in patients with liver cirrhosis and portal hypertension exists and future prospective studies are warranted.
A critical issue is the remaining liver remnant after resection. In general, CT-based volumetric assessment is used to estimate the volume of liver that remains after resection. Upon liver cirrhosis, a minimum of 40% well-perfused liver tissue must remain in situ to maintain an adequate function[26]. However, this is not an absolute value and should be taken with caution.
Since liver cirrhosis and portal hypertension significantly increase perioperative morbidity and mortality[27,28], a thorough pre-operative risk evaluation is recommended. The American Society of Anaesthesiologists Score (ASA-Score), although not originally meant specifically for liver surgery, has been shown to reliably predict 7-d mortality of patients with liver cirrhosis after abdominal surgery[28]. In addition, CPT and MELD score are used to predict the mortality after major abdominal surgery. Thus, mortality rates of 10%, 30% and 80% were observed for patients classified as CPT A, CPT B and CPT C, respectively[29,30]. However, recent improvements in surgical technique and perioperative management have significantly reduced mortality especially in CPT A and CPT B patients[12,22,29-33]. The impact of portal hypertension on perioperative morbidity and long-term survival has lately been discussed controversially. Berzigotti et al[12] have shown a relative risk of 2.0 for 3- and 5-year mortality and a relative risk of 3.0 for perioperative clinical cirrhotic decompensation in patients undergoing liver resection for HCC with and without portal hypertension. Similar results were shown for subgroups of patients undergoing resection of a single small HCC in CPT A cirrhosis. In contrast, other reports state that portal hypertension is no prognostic parameter for perioperative mortality and long-term survival in multivariate analyses[34]. A matched-pair analysis of 241 cirrhotic patients undergoing liver resections for HCC showed no significant difference in perioperative morbidity and long-term postoperative outcome in patients with or without portal hypertension and identified the MELD-score and the extent of liver surgery as only specific prognostic parameters[34]. Using the MELD score as predictor, mortality after abdominal surgery has been described to be 5%-10% for MELD ≤ 11, 25%-54% for MELD 12%-25% and 55%-80% for MELD ≥ 26[35-37]. Furthermore, a study analysing liver resections in cirrhotic patients and showing an overall-mortality of 16%, defined a MELD score of 9 as “cut-off” point: none of the patients with less than 9 points died in the perioperative period whereas a 29% mortality rate was observed in patients with 9 or more points[32]. Based on these analyses, the Mayo Clinic offers a web-based risk evaluation of 7-, 30-, 90-, 360- and 1800-d mortality for cirrhotic patients after abdominal, cardiac and orthopedic surgery (http://www.mayoclinic.org/meld/mayomodel9.html)[28]. A synopsis of preoperative risk scores is shown in Table 3. In conclusion, elective abdominal surgery in CPT C patients or patients with a MELD > 15 is only feasible with significantly increased morbidity and mortality. In these patients, potential therapeutic alternatives as well as morbidity and mortality in case of conservative therapy have to be carefully assessed and risks vs potential benefits evaluated for each individual patient. In CPT A and MELD < 9 patients, elective surgery including liver resections can be performed with adequate risk-to benefit ratio. Patients showing a CPT B classification and a MELD score of 9-15, the risk-to benefit ratio has to be carefully discussed for and with each patient. Of note, portal hypertension is not adequately included in all of these staging systems and has to be taken into consideration in the context of liver resection. In particular, thrombocytopenia as one of the features of portal hypertension has been associated with a significant increase in postoperative major complications, postoperative liver failure and finally with a more than 3-times higher 60-d mortality[22,38].
| Score | Groups | Initial function | Mortality assessment | Mortality after liver resection |
| ASA | 1-6 | Perioperative risk stratification for any patient | Predictor of 7-d mortality | Not specifically defined |
| CPT | A-C | Overall survival in patients with liver cirrhosis | A: 10%, B: 30%, C: 80%; predictor for 30- and 90-d mortality | A: < 9%, no data for B and C |
| MELD | 0-40 | Mortality of TIPS-placement | 0-11: 5%-10%, 12%-25: 25%-54%, > 26: 55%-80%; predictor for 30- and 90-d mortality | ≤ 8: 0%; > 8: 29% |
| Mayo | Mortality after abdominal, orthopedic and cardiac surgery | 7-, 30-, 90-, 360-, 1800-d mortality | Not specifically defined |
The most frequent indication for hepatic resections in cirrhotic patients with and without portal hypertension is HCC. In addition, cholangiocellular adenocarcinoma (CCA) and liver metastases may be reasons for liver resection upon cirrhosis although several studies indicate that cirrhosis reduces the risk of metastasis formation[39,40]. Benign hepatic lesions are very rare indications for hepatic surgery in these high-risk patients.
According to the European Association for the Study of the Liver (EASL) and American Association for the Study of the Liver (AASLD) guidelines for HCC therapy, surgical resections are restricted to patients without portal hypertension, patients showing a normal bilirubin level and a singular tumor nodule[12]. In addition, the Barcelona Clinic Liver Cancer (BCLC) classification recommends curative liver resections for HCC in cirrhotic patients only in early tumor stages and in CPT A cirrhosis without portal hypertension[41]. In contrast, several studies world-wide have shown that HCC resections can be performed in cirrhotic patients with portal hypertension showing acceptable mortality rates with clear oncologic benefits[22,33,34]. Furthermore, Torzilli et al[42] could show that in centres specialized in complex liver surgery, 50% of patients undergoing liver resection in cirrhosis are resected outside the BCLC criteria with 5-year overall survival rates of 57% and 38% for BCLC B and BCLC C patients, respectively. Several authors could show that hepatic resections in patients diagnosed with HCC in advanced cirrhosis result in significantly better long-term survival compared to transarterial chemoembolization[43-46] and that tumor size alone should not be an exclusion criteria for curative surgery[47]. In a randomized controlled clinical trial, Yin et al[46] performed liver resections or transarterial chemoembolizations in 173 HCC patients exceeding the Milan criteria. The authors described a significantly better long-term survival in patients undergoing liver resection (3-year overall survical rate 51.5% vs 18.1%). Thus, advanced liver cirrhosis and portal hypertension should not be considered as absolute contraindication in liver resections but must be evaluated individually for each patient in the context of the hepatic resection planned and further comorbidities of the patient.
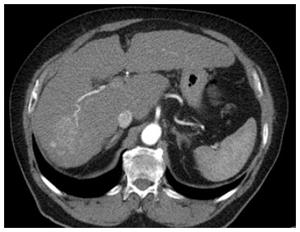
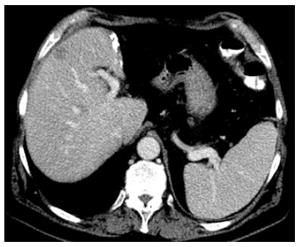
In a cirrhotic liver, a minimum of 40% well-perfused liver-volume must remain in situ after resection[26]. However, in our institution these radical resections of up to 60% liver volume are restricted to CPT A, MELD < 9 patients with no or only mild portal hypertension (Figure 1). Some reports indicate that anatomic hepatic resections for HCC show a trend towards increased disease-free and overall survival compared to non-anatomic resections[48-50] whereas others do not see a benefit from anatomic resections[51-53]. However, to avoid postoperative liver failure due to insufficient liver remnant, we would prefer hepatic resections to be as parenchymal-sparing as possible. In particular, the latter strategy may enable liver resections in patients with advanced cirrhosis (CPT B) and with (mild) portal hypertension[54]. Lately, local ablation using radiofrequency has been shown to be an adequate alternative therapy for small malignant hepatic lesions < 2 cm with similar long-term oncologic outcome as surgical resection but significantly reduced peri-interventional morbidity[9]. Thus, for patients with small malignant liver lesions in cirrhosis and portal hypertension local ablation has to be considered as an alternative (and potentially better) option (see Figure 2).
Standard access for cirrhotic as well as non-cirrhotic patients in liver surgery is the upper abdomen midline incision extended by right-upper quadrant traverse laparotomy (“inversed L-shape”). In cases where right-posterior segmentectomy has to be performed and where clamping of the suprahepatic inferior caval vein is not possible through inversed L-shape incision, a thoraco-abdominal incision is recommended[55-57]. In minor resections, a midline incision may suffice. The recanalized umbilical vein must be carefully ligated or preserved if possible. First studies have also established laparoscopic resections in cirrhotic patients. In specialized centres, even extended liver resections can be performed in a minimally invasive technique with removal of the resected liver using a Pfannenstiel incision[58].
For parenchymal dissections, all techniques used in non-cirrhotic patients can be used in cirrhotic patients with or without portal hypertension. “Selective” dissection techniques are based on discriminating the higher tissue resistance of blood vessels and bile ducts compared to liver parenchyma. Selective dissection techniques are blunt dissection using a pair of scissors and selective application of clips, water jet dissection and clips, stapler dissection and ultrasound-aspiration (CUSAR) and clips[59-64]. In our institution, blunt selective dissection by scissors and clips is the preferred dissection technique. In any case, care must be taken of thorough bleeding control during resection. Post-resection application of fibrin glue or collage fleece has not shown any benefit on blood loss and need for blood transfusions[65].
Blood loss and need for blood transfusions have been defined as independent predictors of postoperative morbidity and mortality[66-68]. In cases of problematic blood loss during parenchymal dissection, hilar vascular occlusion can be applied (Pringle manoeuvre). Of note, the Pringle manoeuvre is not recommended in cirrhotic patients but sometimes is necessary to avoid extensive blood loss. If needed, collateral vessels in the liver hilum should be preserved during vascular clamping. If vascular clamping cannot be avoided, selective clamping of a hepatic lobe, section or segment is recommended to limit ischemic and reperfusion damage[55,69]. Moreover, hilar vascular occlusion should be performed as short as possible. In cases where longer occlusion is needed, intermittent occlusion, e.g., intervals of 15 min of ischemia, followed by 5 min of reperfusion - has shown to be tolerated better than continuous clamping[70-74]. Total hepatic vascular exclusion, i.e., including occlusion of the infra- and suprahepatic caval vein, should be avoided since a significant increase in perioperative morbidity and mortality has been observed even in non-cirrhotic patients[75]. A setting were total hepatic vascular exclusion may be necessary is the resection of a hepatocellular carcinoma invading the retrohepatic caval vein and needing (partial) resection and replacement of the retrohepatic caval vein.
During hepatic resection, a close cooperation and communication with the anaesthetic team is essential. Since intrahepatic sinusoidal pressure correlates with the pressure in the inferior caval vein, which again correlates with the central venous pressure (CVP), blood loss during parenchymal dissection can be significantly decreased by reducing the CVP to approximately 5 mmHg[55,69,76]. Reduction of the CVP to lower (even negative) values is not recommended due the increased risk of air embolism[55]. Restrictive volume management, reduction of tidal volume and positive end-expiratory pressure, diuretics and reverse Trendelenburg positioning are options to reduce the CVP[77]. A surgical method to reduce CVP is partial clamping of the infrahepatic caval vein[78]. Finally, in case of increased blood loss during hepatic resection, a decent management including not only blood transfusions but also substitution of non-cellular blood components (e.g., fresh frozen plasma, coagulation factors) is mandatory. It has been observed that a restrictive transfusion policy for cirrhotic patients with anemia therapeutically targeted to a Hb between 7-9 g/dL is associated with a better clinical outcome[79]. This should be considered in order to avoid an overtransfusion policy that can further increase portal pressure as consequence of high hepatic inflow.
Routine placement of drainage systems in hepatic resections is controversially discussed. In minor resections of non-cirrhotic livers in patients without portal hypertension, drainage placement is not recommended[80]. In major resections, prophylactic drainage placement has been associated with increased septic complications and prolonged time to discharge[81]. However, other publications show a significantly reduced intraabdominal pressure for temporary drainage of increased postoperative ascites and improved wound healing after drainage placement[82,83].
Compared to liver resections in otherwise healthy patients, resections in cirrhotic livers in patients with portal hypertension show a markedly increased postoperative morbidity of 22%-50%[22,33,34,66].
Postoperative liver failure is the most serious and life-threatening complication after liver resection. Several definitions for “liver failure” have been proposed over the last 20 years. So far, the commonly used definitions, though initially not developed to score liver resections in cirrhotic livers, are the “50-50 criteria”, the International Study Group of Liver Surgery (ISGLS)-criteria and the “peakBili > 7” criteria (see Table 4)[84-86].
| Definition | Time of scoring | |
| “50-50” | bilirubin > 50 μmol/L, prothrombin time < 50% (INR > 1.7) → mortality of 50% | POD 5 |
| ISGLS | Increased INR and hyperbilirubinemia | On or after POD 5 |
| ISGLS A | No intervention necessary | |
| ISGLS B | Non-invasive intervention necessary | |
| ISGLS C | Invasive intervention necessary | |
| peakBili > 7 | Maximum hyperbilirubinemia > 7 mg/dL any day after surgery - predictor for 90-d mortality | Any POD |
Liver transplantation as only causal therapy of liver failure is often no option in these patients. Treatment must therefore focus on optimal perfusion of remnant liver, adequate infection prophylaxis and intensive care management of fluid balance, electrolytes, coagulation and kidney function.
Ascites is a major problem after hepatic resections in cirrhotic patients with portal hypertension, even if no postoperative liver failure occurs. Ascites production often suspends after 5-7 d. Supportive therapies consist of diuretics (e.g., spironolactone), restrictive volume management and substitution of albumin. In cases of persisting ascites, a microbiological examination is recommended to detect and treat infections early. In individual patients, TIPS placement (alternatively: splenic artery embolization) must be considered.
The rate of infection-associated complications is significantly increased after liver resections in cirrhotic patients with portal hypertension compared to liver resections in otherwise healthy livers[87]. Main foci here are pneumonia and superinfected ascites. In suspected infection, no delay in antibiotic treatment must be made. In cases of superinfected ascites, recommended empirical treatment is the use of third generation cephalosporins such as ceftriaxone or cefotaxime[88,89], alternatively the use of carbapenems[90]. Prior sampling and microbiological culture of the ascitic fluid is recommended for adequate adaption of the antibiotic regime depending on the antimicrobial resistance patterns. Control sampling and microbiologic culture of the ascitic fluid 48 h after start of antibiotic treatment is recommended.
Due to the frequently underlying malnutrition and postoperative ascites, the rate of wound healing disorders in cirrhotic patients is significantly higher than in non-cirrhotic patients[91]. Some reports indicate that abdominal closure with a running suture prevents wound dehiscence. Moreover, placement of an intraabdominal drainage to prevent fluid collection and to lower the intraabdominal pressure has been proposed although this is discussed controversially (see above).
Postoperative hemorrhage in cirrhotic patients can be caused by superficial wound bleeding, bleeding from the resection site as well as by GI bleedings. Rapid diagnostic assessment and interdisciplinary surgical-medical therapy including supplementation of coagulation products are essential in these potentially life-threatening complications.
A recent analysis of 2046 patients treated in 10 high-volume centres for liver surgery has shown that only 50% of patients were operated within the BCLC recommendations, i.e., BCLC stage 0-A and no portal hypertension. 36% and 14% of patients underwent liver resection in BCLC stage B and C, respectively[42]. In this publication, 5-year overall survival rates after HCC resections in BCLC A/B/C cirrhosis were 61%, 57% and 38% and 5-year disease free survival of 21%, 27% and 18%. Thus, the authors conclude that liver resections for HCC can be performed even in higher degrees of cirrhosis with acceptable long-term outcome and that the BCLC/EASL/AASLD guidelines should thus be updated. Furthermore, several publications could show that portal hypertension is no prognostic factor for long-term survival or HCC recurrence in multivariate analyses[25,34]. In contrast, Berzigotti et al[12] determined portal hypertension as an independent factor for decreased long-term survival and increased perioperative decompensation after HCC resection. However, since many cirrhotic patients diagnosed with HCC do not qualify for liver transplantation, taking an increased operative risk must carefully be discussed for and with each individual patient in the light of the potential long-term oncologic outcome.
Only liver transplantation (LT) is the causal therapy for patients diagnosed with portal hypertension and advanced liver cirrhosis[92]. However, not all cirrhotic patients are candidates for LT. In particular, patients suffering from severe complications from portal hypertension such as variceal hemorrhage and subsequent failure of conservative, endoscopic and angiographic interventions are referred to TIPS placement[93,94]. Only if TIPS placement is technically not feasible, shunt surgery may be considered. Interestingly, several studies comparing TIPS and shunt surgery show a significantly reduced recurrence of hemorrhage and less occlusive complications for shunt surgery[95]. However, due to the high perioperative morbidity and mortality, shunt surgery should only be considered for CPT A or well-compensated CPT B patients[96]. Depending on the region of the portal-venous system requiring relief of vascular pressure and influenced by possible anastomotic sites, several shunting techniques have been developed (see Table 5). If possible, only a partial bypass of the portalvenous flow is recommended to minimize the risk of encephalopathy.
| Shunt | Bypass of portal venous flow | Recurrent haemorrhage/complications |
| Porto-caval; end-to-side | Complete | Low rate of recurrent haemorrhage (< 5%) |
| Low degree of shunt occlusion | ||
| 40% encephalopathy | ||
| Increase in ascites | ||
| Porto-caval, side-to-side +/- interposition graft | Partial | Recurrent haemorrhage 5% |
| Low degree of shunt occlusion (5%) | ||
| 5% encephalopathy | ||
| Distal splenorenal shunt (Warren) | Partial | Recurrent haemorrhage 5%-8% |
| Shunt occlusion 10% | ||
| Selective decompression of gastroesophageal varices |
More rarely used shunting techniques are the meso-caval side-to-side shunt (+/- interposition graft), the proximal end-to-side splenorenal shunt (Linton), the spleno-renal side-to-side shunt (Cooley), the coronario-cava- end-to-side shunt (Inokuchi) and the mesenterico-portal Rex-Shunt. Because shunt surgery is no longer a common practice, it should only be performed where there is sufficient expertise at specialized centers.
In conclusion, hepatic resections in cirrhotic patients with portal hypertension show a significantly increased mortality and morbidity. However, due to ongoing improvements in surgical technique and intensive care management, surgery is feasible in selected patients with adequate long-term outcome. Patients have to be carefully screened for the degree of liver cirrhosis (CPT A-C, MELD-score), grade of portal hypertension (thrombocyte count, splenomegaly, presence of collaterals and varices, elastography, HPVG-measurement) and comorbidities. To us, portal hypertension is not an absolute contraindication for liver resections in cirrhotic patients. A close interaction with anaesthesiology and the critical care team is mandatory for optimal perioperative management. Main indications for liver resections in cirrhotic patients are HCC, but also CCA and, rarely, hepatic metastases. Elective surgery should not only be restricted to patients with CPT A cirrhosis, but should also be offered to very well selected patients with more advanced cirrhosis. During surgery, abdominal access should be made carefully to preserve potential collaterals. Careful dissection to prevent intraoperative blood loss is necessary and the extent of liver resection should be restricted to a minimum. Moreover, the therapeutic alternative of local ablation (radiofrequency ablation, irreversible electroporation among others) by an experienced interventional radiologist should always be considered. In case of surgery, the Pringle-manoeuvre should be avoided but may help to bridge critical situations. During the postoperative period, close interdisciplinary management by the hepatobiliary surgical team, critical care specialists and hepatologists is warranted for optimized stabilisation of these critical patients and for early and appropriate management of complications.
P- Reviewer: Cerwenka HR, Kaiser GM, Makisalo H S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 2. | Sørensen HT, Thulstrup AM, Mellemkjar L, Jepsen P, Christensen E, Olsen JH, Vilstrup H. Long-term survival and cause-specific mortality in patients with cirrhosis of the liver: a nationwide cohort study in Denmark. J Clin Epidemiol. 2003;56:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5737] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 4. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 5. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 6. | La Mura V, Nicolini A, Tosetti G, Primignani M. Cirrhosis and portal hypertension: The importance of risk stratification, the role of hepatic venous pressure gradient measurement. World J Hepatol. 2015;7:688-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Pagliaro L. MELD: the end of Child-Pugh classification? J Hepatol. 2002;36:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Hernandez-Gea V, Turon F, Berzigotti A, Villanueva A. Management of small hepatocellular carcinoma in cirrhosis: focus on portal hypertension. World J Gastroenterol. 2013;19:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 395] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 11. | Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, Festi D, Pinna AD. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256:706-712; discussion 712-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2015;61:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 14. | Rai R, Nagral S, Nagral A. Surgery in a patient with liver disease. J Clin Exp Hepatol. 2012;2:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Sabbagh C, Fuks D, Regimbeau JM. Non-hepatic gastrointestinal surgery in patients with cirrhosis. J Visc Surg. 2014;151:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr. 2014;3:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 17. | Jara M, Bednarsch J, Lock JF, Malinowski M, Schulz A, Seehofer D, Stockmann M. [Enhancing safety in liver surgery using a new diagnostic tool for evaluation of actual liver function capacity - The LiMAx test]. Dtsch Med Wochenschr. 2014;139:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, Lehmann S, Niehues SM, Schwabe M, Lemke AJ. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Gupta S, Chawla Y, Kaur J, Saxena R, Duseja A, Dhiman RK, Choudhary NS. Indocyanine green clearance test (using spectrophotometry) and its correlation with model for end stage liver disease (MELD) score in Indian patients with cirrhosis of liver. Trop Gastroenterol. 2012;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Leevy CM, Smith F, Longueville J, Paumgartner G, Howard MM. Indocyanine green clearance as a test for hepatic function. Evaluation by dichromatic ear densitometry. JAMA. 1967;200:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Sheng QS, Lang R, He Q, Yang YJ, Zhao DF, Chen DZ. Indocyanine green clearance test and model for end-stage liver disease score of patients with liver cirrhosis. Hepatobiliary Pancreat Dis Int. 2009;8:46-49. [PubMed] |
| 22. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 23. | Farkas SA, Schlitt HJ. [Operative therapy of hepatocellular carcinoma]. Radiologe. 2014;54:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Verloh N, Haimerl M, Rennert J, Müller-Wille R, Nießen C, Kirchner G, Scherer MN, Schreyer AG, Stroszczynski C, Fellner C. Impact of liver cirrhosis on liver enhancement at Gd-EOB-DTPA enhanced MRI at 3 Tesla. Eur J Radiol. 2013;82:1710-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Verloh N, Haimerl M, Zeman F, Schlabeck M, Barreiros A, Loss M, Schreyer AG, Stroszczynski C, Fellner C, Wiggermann P. Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol. 2014;24:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 26. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 27. | Bhangui P, Laurent A, Amathieu R, Azoulay D. Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol. 2012;57:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Teh SH, Nagorney DM, Stevens SR, Offord KP, Therneau TM, Plevak DJ, Talwalkar JA, Kim WR, Kamath PS. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 29. | Garrison RN, Cryer HM, Howard DA, Polk HC. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-655. [PubMed] |
| 30. | Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122:730-735; discussion 735-736. [PubMed] |
| 31. | Hyder O, Pulitano C, Firoozmand A, Dodson R, Wolfgang CL, Choti MA, Aldrighetti L, Pawlik TM. A risk model to predict 90-day mortality among patients undergoing hepatic resection. J Am Coll Surg. 2013;216:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207-1215; discussion 1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 34. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192-204; discussion 205. [PubMed] |
| 36. | Hoteit MA, Ghazale AH, Bain AJ, Rosenberg ES, Easley KA, Anania FA, Rutherford RE. Model for end-stage liver disease score versus Child score in predicting the outcome of surgical procedures in patients with cirrhosis. World J Gastroenterol. 2008;14:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for End-Stage Liver Disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg. 2005;242:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC, McMasters KM, Cho CS, Winslow ER, Wood WC. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212:638-648; discussion 648-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Dahl E, Rumessen J, Gluud LL. Systematic review with meta-analyses of studies on the association between cirrhosis and liver metastases. Hepatol Res. 2011;41:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Cai B, Liao K, Song XQ, Wei WY, Zhuang Y, Zhang S. Patients with chronically diseased livers have lower incidence of colorectal liver metastases: a meta-analysis. PLoS One. 2014;9:e108618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Eguchi S, Kanematsu T, Arii S, Omata M, Kudo M, Sakamoto M, Takayasu K, Makuuchi M, Matsuyama Y, Monden M. Recurrence-free survival more than 10 years after liver resection for hepatocellular carcinoma. Br J Surg. 2011;98:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 43. | Chang WT, Kao WY, Chau GY, Su CW, Lei HJ, Wu JC, Hsia CY, Lui WY, King KL, Lee SD. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Liu PH, Hsia CY, Lee YH, Hsu CY, Huang YH, Su CW, Lee RC, Lin HC, Huo TI. Surgical resection versus transarterial chemoembolization for BCLC stage C hepatocellular carcinoma. J Surg Oncol. 2015;111:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, Volk M, Giannini EG, Ciccarese F, Piscaglia F. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 46. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 47. | Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, Luciani A, Van Nhieu JT, Azoulay D, Cherqui D. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62:1131-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012;19:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, Shen F, Pinna AD. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 50. | Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2011;396:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Tang YH, Wen TF, Chen X. Anatomic versus non-anatomic liver resection for hepatocellular carcinoma: a systematic review. Hepatogastroenterology. 2013;60:2019-2025. [PubMed] |
| 53. | Tomimaru Y, Eguchi H, Marubashi S, Wada H, Kobayashi S, Tanemura M, Umeshita K, Doki Y, Mori M, Nagano H. Equivalent outcomes after anatomical and non-anatomical resection of small hepatocellular carcinoma in patients with preserved liver function. Dig Dis Sci. 2012;57:1942-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Duan YF, Li XD, Sun DL, Chen XM, An Y, Zhu F. A preliminary study on surgery for hepatocellular carcinoma patients with portal hypertension. Am J Surg. 2015;210:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Chouillard EK, Gumbs AA, Cherqui D. Vascular clamping in liver surgery: physiology, indications and techniques. Ann Surg Innov Res. 2010;4:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Donadon M, Costa G, Gatti A, Torzilli G. Thoracoabdominal approach in liver surgery: how, when, and why. Updates Surg. 2014;66:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Sato H, Sugawara Y, Yamasaki S, Shimada K, Takayama T, Makuuchi M, Kosuge T. Thoracoabdominal approaches versus inverted T incision for posterior segmentectomy in hepatocellular carcinoma. Hepatogastroenterology. 2000;47:504-506. [PubMed] |
| 58. | Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D, Scatton O. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles: The Caudal Approach. Ann Surg. 2015;261:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 59. | Fasulo F, Giori A, Fissi S, Bozzetti F, Doci R, Gennari L. Cavitron Ultrasonic Surgical Aspirator (CUSA) in liver resection. Int Surg. 1992;77:64-66. [PubMed] |
| 60. | Rau HG, Duessel AP, Wurzbacher S. The use of water-jet dissection in open and laparoscopic liver resection. HPB (Oxford). 2008;10:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Rau HG, Wichmann MW, Schinkel S, Buttler E, Pickelmann S, Schauer R, Schildberg FW. [Surgical techniques in hepatic resections: Ultrasonic aspirator versus Jet-Cutter. A prospective randomized clinical trial]. Zentralbl Chir. 2001;126:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Buell JF, Gayet B, Han HS, Wakabayashi G, Kim KH, Belli G, Cannon R, Saggi B, Keneko H, Koffron A. Evaluation of stapler hepatectomy during a laparoscopic liver resection. HPB (Oxford). 2013;15:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Cannon RM, Saggi B, Buell JF. Evaluation of a laparoscopic liver resection in the setting of cirrhosis. HPB (Oxford). 2014;16:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Hoffmann K, Müller-Bütow V, Franz C, Hinz U, Longerich T, Büchler MW, Schemmer P. Factors predictive of survival after stapler hepatectomy of hepatocellular carcinoma: a multivariate, single-center analysis. Anticancer Res. 2014;34:767-776. [PubMed] |
| 65. | Hoots WK, Buchanan GR, Parmley RT, Alperin JB, Kletzel M, Sexauer CL. Comprehensive care for patients with hemophilia: an expanded role in reducing risk for human immunodeficiency virus. Tex Med. 1991;87:73-75. [PubMed] |
| 66. | Capussotti L, Muratore A, Amisano M, Polastri R, Bouzari H, Massucco P. Liver resection for hepatocellular carcinoma on cirrhosis: analysis of mortality, morbidity and survival--a European single center experience. Eur J Surg Oncol. 2005;31:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 68. | Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860-869; discussion 869-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 369] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 69. | Yang Y, Zhao LH, Fu SY, Lau WY, Lai EC, Gu FM, Wang ZG, Zhou WP. Selective hepatic vascular exclusion versus pringle maneuver in partial hepatectomy for liver hemangioma compressing or involving the major hepatic veins. Am Surg. 2014;80:236-240. [PubMed] |
| 70. | Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 245] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 71. | Brooks AJ, Hammond JS, Girling K, Beckingham IJ. The effect of hepatic vascular inflow occlusion on liver tissue pH, carbon dioxide, and oxygen partial pressures: defining the optimal clamp/release regime for intermittent portal clamping. J Surg Res. 2007;141:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 369] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 73. | Capussotti L, Nuzzo G, Polastri R, Giuliante F, Muratore A, Giovannini I. Continuous versus intermittent portal triad clamping during hepatectomy in cirrhosis. Results of a prospective, randomized clinical trial. Hepatogastroenterology. 2003;50:1073-1077. [PubMed] |
| 74. | Lesurtel M, Lehmann K, de Rougemont O, Clavien PA. Clamping techniques and protecting strategies in liver surgery. HPB (Oxford). 2009;11:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Cherqui D, Malassagne B, Colau PI, Brunetti F, Rotman N, Fagniez PL. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg. 1999;230:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 77. | Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Rahbari NN, Koch M, Zimmermann JB, Elbers H, Bruckner T, Contin P, Reissfelder C, Schmidt T, Weigand MA, Martin E. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Ann Surg. 2011;253:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1069] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 80. | Gurusamy KS, Samraj K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2007;CD006004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, Poon RT, Wong J. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Fuster J, Llovet JM, Garcia-Valdecasas JC, Grande L, Fondevila C, Vilana R, Palacin J, Tabet J, Ferrer J, Bruix J. Abdominal drainage after liver resection for hepatocellular carcinoma in cirrhotic patients: a randomized controlled study. Hepatogastroenterology. 2004;51:536-540. [PubMed] |
| 83. | Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Tanigawa N. Re-evaluation of the necessity of prophylactic drainage after liver resection. Am Surg. 2011;77:539-544. [PubMed] |
| 84. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 85. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862; discussion 862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 86. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1729] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 87. | Pessaux P, Msika S, Atalla D, Hay JM, Flamant Y. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg. 2003;138:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 88. | Gómez-Jiménez J, Ribera E, Gasser I, Artaza MA, Del Valle O, Pahissa A, Martínez-Vázquez JM. Randomized trial comparing ceftriaxone with cefonicid for treatment of spontaneous bacterial peritonitis in cirrhotic patients. Antimicrob Agents Chemother. 1993;37:1587-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Rimola A, Navasa M, Arroyo V. Experience with cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis. Diagn Microbiol Infect Dis. 1995;22:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 91. | de Goede B, Klitsie PJ, Hagen SM, van Kempen BJ, Spronk S, Metselaar HJ, Lange JF, Kazemier G. Meta-analysis of laparoscopic versus open cholecystectomy for patients with liver cirrhosis and symptomatic cholecystolithiasis. Br J Surg. 2013;100:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 92. | Rikkers LF, Jin G, Langnas AN, Shaw BW. Shunt surgery during the era of liver transplantation. Ann Surg. 1997;226:51-57. [PubMed] |
| 93. | Glowka TR, Kalff JC, Schäfer N. Clinical Management of Chronic Portal/Mesenteric Vein Thrombosis: The Surgeon’s Point of View. Viszeralmedizin. 2014;30:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Tripathi D, Helmy A, Macbeth K, Balata S, Lui HF, Stanley AJ, Redhead DN, Hayes PC. Ten years’ follow-up of 472 patients following transjugular intrahepatic portosystemic stent-shunt insertion at a single centre. Eur J Gastroenterol Hepatol. 2004;16:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Clark W, Hernandez J, McKeon B, Villadolid D, Al-Saadi S, Mullinax J, Ross SB, Rosemurgy AS. Surgical shunting versus transjugular intrahepatic portasystemic shunting for bleeding varices resulting from portal hypertension and cirrhosis: a meta-analysis. Am Surg. 2010;76:857-864. [PubMed] |
| 96. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1031] [Article Influence: 68.7] [Reference Citation Analysis (0)] |