Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2576
Peer-review started: August 11, 2015
First decision: September 29, 2015
Revised: October 25, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: February 28, 2016
Processing time: 198 Days and 21.1 Hours
AIM: To determine the significance of increased serum direct bilirubin level for lymph node metastasis (LNM) in Chinese rectal cancer patients, after those with known hepatobiliary and pancreatic diseases were excluded.
METHODS: A cohort of 469 patients, who were treated at the China-Japan Friendship Hospital, Ministry of Health (Beijing, China), in the period from January 2003 to June 2011, and with a pathological diagnosis of rectal adenocarcinoma, were recruited. They included 231 patients with LNM (49.3%) and 238 patients without LNM. Follow-up for these patients was taken through to December 31, 2012.
RESULTS: The baseline serum direct bilirubin concentration was (median/inter-quartile range) 2.30/1.60-3.42 μmol/L. Univariate analysis showed that compared with patients without LNM, the patients with LNM had an increased level of direct bilirubin (2.50/1.70-3.42 vs 2.10/1.40-3.42, P = 0.025). Multivariate analysis showed that direct bilirubin was independently associated with LNM (OR = 1.602; 95%CI: 1.098-2.338, P = 0.015). Moreover, we found that: (1) serum direct bilirubin differs between male and female patients; a higher concentration was associated with poor tumor classification; (2) as the baseline serum direct bilirubin concentration increased, the percentage of patients with LNM increased; and (3) serum direct bilirubin was associated with the prognosis of rectal cancer patients and higher values indicated poor prognosis.
CONCLUSION: Higher serum direct bilirubin concentration was associated with the increased risk of LNM and poor prognosis in our rectal cancers.
Core tip: Serum bilirubin has been associated with colorectal cancer; however, no information is available for the association between bilirubin and lymph node metastasis (LNM). This study was designed to determine the significance and prognostic value of increased serum direct bilirubin for Chinese rectal cancer patients with LNM, after exclusion of known hepatobiliary and pancreatic diseases. For the first time, this study found that higher serum direct bilirubin concentration was associated with an increased risk of LNM and poor prognosis in rectal cancer patients.
- Citation: Gao C, Fang L, Li JT, Zhao HC. Significance and prognostic value of increased serum direct bilirubin level for lymph node metastasis in Chinese rectal cancer patients. World J Gastroenterol 2016; 22(8): 2576-2584
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2576.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2576
Rectal cancer (RC) is a very common malignant tumor of the digestive tract, with a high incidence and high mortality. Its incidence has dramatically increased worldwide in recent years[1]. The presence of lymph node metastasis (LNM) has been identified as one most powerful risk factor for recurrence and overall survival of RCs[2,3]. The number of lymph nodes dissected is significantly linked with survival and mortality in RC patients[4,5]. For RC patients with LNM, some known risk factors have been identified, including younger age, cancer location, diameter of tumors, poor differentiation, surrounding infiltration and tumor invasion[6-12].
Historically, serum bilirubin has been considered to have no physiological function other than that of being the garbage of heme (bilirubin) metabolism. However, in recent years, some experimental and clinical research has demonstrated that serum bilirubin has several protective effects, including potent antioxidant, anti-inflammatory and anticancer activities[13-17]. In one study, the authors used their national, large health survey database and observed that patients with slightly increased serum bilirubin concentrations had a decreased risk of colorectal cancers (CRC)[14]. The results were supported by another exploratory case-control study that was performed in the Charles University in Prague of Czech Republic and was designed to determine the relationship between promoter variations of heme oxygenase-1 and bilirubin UDP-glucuronosyltransferase genes, serum bilirubin concentrations and the risk of CRC[13]. Moreover, experimental studies performed in cell lines of CRC showed that unconjugated bilirubin can induce their apoptosis[18].
However, no significant association was found in some epidemiological studies. One case-control study was performed in the United States that was designed to determine the differences of baseline serum bilirubin concentrations between patients with colon cancers and control patients without cancers[19]. This study involved 236 patients, including 118 colon cancer patients and 118 control patients without cancers. Their blood was collected and the serum bilirubin levels were measured 20 years before these patients developed colon cancers[19]. According to the results, no difference was found between their baseline serum bilirubin concentrations and the risk of development of colon cancers. This result was also supported by another cohort study that was designed to identify the association of total bilirubin levels with the risk of developing CRC in one representative United States population[15]. The study included 5487 cases and they were followed up for 88339 person-years. No relationship was found between their serum total bilirubin concentration and the risk of CRC, although some modified factors had been adjusted in the multivariable analysis.
For the association between serum bilirubin and LNM, no information is available. Our preliminary data showed that serum direct bilirubin, not total bilirubin, was related with LNM in RCs. The present was performed to determine the significance of increased serum direct bilirubin concentration for Chinese RC patients with LNM, after known hepatobiliary and pancreatic diseases were excluded.
A cohort of 469 patients, who were treated at our hospital (China-Japan Friendship Hospital, Ministry of Health, Beijing, China) in the period from January 2003 to June 2011, and with a pathological diagnosis of rectal adenocarcinoma, according to the diagnostic, exclusion and inclusion criteria, were recruited, including 231 patients with LNM (49.3%) and 238 patients without LNM. Follow-up for these patients was taken through to December 31, 2012.
According to our previous researches and current knowledge, the exclusion criteria were determined as followed: (1) patients with known hepatobiliary and pancreatic diseases; (2) patients that had been diagnosed with RC for more than two weeks or been subjected to any medical treatments associated with tumors before inclusion; (3) patients that had other tumors, including lymphoma and leukemia; (4) patients diagnosed with hereditary non-polyposis CRC and familial adenomatous polyposis; (5) patients that did not have complete clinical and pathological data, which would affect the statistical analysis; (6) patients that had used immunosuppressive agents and cytotoxic drugs during the past half of a year; (7) patients who died within three months after surgery; and (8) patients that had been diagnosed with serious conditions of other important systems or organs.
Our hospital Human Research Ethics Committee approved our study. This current research was performed in accordance with the Declaration of Helsinki.
The pathological/histological results were the only diagnostic criteria used for RC. Usually, patients were diagnosed preliminarily based on the biopsy of endoscopy examination, and confirmed by the results of surgical specimens. We only included those patients diagnosed with rectal adenocarcinoma. If two or more pathological/histological subtypes were shown in one sample and adenocarcinoma was considered to be occupying the leading pathological type, we also excluded them from our final analysis. Diagnosis of LNM was determined mainly by pathological/histological results after surgery (pathological LNM, pLNM). For the stage of LNM, cancer classification and distant metastasis of tumors, the results from pathological/histological findings were abbreviated as pN, pT and pM, respectively.
Based on our previous studies and current knowledge, we included the following parameters, mainly from clinical and pathological examinations: age, gender, body mass index (BMI), presence of hypertension, drinking, smoking, white blood cell count, hemoglobin, platelet count, total bilirubin, direct bilirubin (DBil), total bile acid, alanine aminotransferase (ALT), alkaline phosphatase (ALP), glutamic-oxalacetic transaminase (AST), gamma-glutamyl transpeptidase (GGT), serum cholinesterase, International normalized ratio/INR, prothrombin time, albumin level, potassium, serum sodium, creatinine, CA19-9, carcinoembryonic antigen (CEA), tumor differentiation, pN, pT, pM, lymph node-positive and total number of lymph nodes removed.
For these RC patients, the data were obtained after confirmed diagnosis, and data was excluded if it was obtained two weeks before or after the diagnosis. If multiple values were obtained for one parameter, we only recorded the first measured value. BMI was calculated based on the results of body weight and height. These clinical and pathological data were assessed by two authors carefully and independently.
We used the SPSS 17.0 software (Chicago, IL, United States) for statistical analyses. For statistical description, mean ± SD, median/inter-quartile range, and numbers and proportions were used for continuous variables with normal distributions, continuous variables with skewed distributions, and categorical variables, respectively. For statistical analysis, Independent-Samples t-test, Mann-Whitney non-parametric U-test, and three kinds of χ2 tests were used. According to the findings of univariate analysis, we used multivariate logistic regression to determine the independent relationship between serum direct bilirubin and pLNM.
For clinical practice, the continuous variables were changed to categorical variables, and the cutoff values were determined based on the combination of sensitivity, specificity, Youden index, their receiver operator characteristics (ROC) curves and areas under the curve (AUC). The values of CEA were lost for 77 RC patients; therefore, this parameter was excluded from the logistic regression analysis. In addition, our preliminary findings (data not shown) demonstrated no association between CEA and pLMN in these RC patients.
Moreover, using the χ2 test, we studied the association of serum direct bilirubin levels with certain clinical and pathological parameters/factors, including age, gender, tumor differentiation, pN, pT, pM, serum CEA and serum sodium. In addition, we studied the relationship between serum baseline direct bilirubin concentration and pLNM, when serum direct bilirubin was treated as one continuous variable. Follow-up was taken through to December 31, 2012. For prognostic significance, we used the Kaplan-Meier method and multivariate Cox regression model. The results were reported as the ORs with their 95%CI. Differences were considered statistically significant when P was < 0.05. All the quoted P values are two-sided.
Tables 1 and 2 show the basic and pathological characteristics of 469 rectal adenocarcinoma patients, including 231 patients with pLNM (49.3%) and 238 control cases without pLNM (50.7%). Their mean age was 62.1 ± 12.6 years old. Two hundred and eighty-six patients (61.0%) were male. One hundred and thirty-one patients (27.9%) had hypertension and 129 (27.5%) were smokers. Parameters for liver function were demonstrated, including total bilirubin, direct bilirubin, ALT, AST, ALP, GGT, serum cholinesterase, total bile acid, prothrombin time, INR, and albumin level. Among the 231 RC patients with LNM, 136 patients were determined as pathological lymph node stage 1 (pN1) and 95 patients were diagnosed with pN2. The mean number of lymph nodes removed was 13.3 ± 8.4. For pathological tumor classification (pT), 361 patients (77.0%) were diagnosed with pT3 or pT4.
| Characteristic | Total patients (n = 469)1 | RAC patients with LNM(n = 231)1 | RAC patients without LNM (n = 238)1 | P value |
| Male sex | 286 (61.0) | 146 (63.2) | 140 (58.8) | 0.331 |
| Mean age, yr | 62.1 ± 12.6 | 60.6 ± 12.8 | 63.6 ± 12.2 | 0.012 |
| BMI3, kg/m2 | 23.95 ± 3.54 | 23.90 ±3.67 | 24.0 ± 3.41 | 0.779 |
| Hypertension | 131 (27.9) | 58 (25.1) | 73 (30.7) | 0.179 |
| Alcohol intake | 29 (6.2) | 18 (7.8) | 11 (4.6) | 0.154 |
| Smoking | 129 (27.5) | 55 (23.8) | 74 (31.1) | 0.077 |
| White blood cell, × 109/L | 6.66 ± 2.10 | 6.70 ± 2.09 | 6.61 ± 2.11 | 0.666 |
| Hemoglobin, g/L | 132 ± 20 | 131 ± 20 | 132 ± 19 | 0.553 |
| Platelet count, × 109/L | 233 ± 79 | 235 ± 74 | 232 ± 84 | 0.659 |
| Total bilirubin2, mmol/L | 10.26 (7.70-14.40) | 10.26 (7.40-15.0) | 10.26 (8.13-13.68) | 0.451 |
| Direct bilirubin2, mmol/L | 2.30 (1.60-3.42) | 2.50 (1.70-3.42) | 2.10 (1.40-3.42) | 0.025 |
| ALT2, U/L | 16 (12-22) | 15 (12-22) | 16 (12-22) | 0.262 |
| AST2, U/L | 19 (16-23) | 18 (15-22) | 19 (16-24) | 0.076 |
| ALP2, U/L | 66 (55-81) | 66 (54-82) | 66 (55-80) | 0.905 |
| GGT2, U/L | 17 (13-25) | 18 (13-26) | 16 (12-23) | 0.136 |
| Serum cholinesterase4, U/L | 161.3 ± 50.0 | 158.1 ± 50.1 | 164.5 ± 50.0 | 0.170 |
| Total bile acid2, μmol/L | 4.4 (2.4-7.3) | 4.5 (2.4-8.5) | 4.2 (2.5-7.1) | 0.546 |
| Prothrombin time5, s | 13.2 ± 1.4 | 13.2 ± 1.7 | 13.2 ± 1.1 | 0.651 |
| International normalized ratio/INR5 | 1.02 ± 0.14 | 1.02 ± 0.17 | 1.01 ± 0.11 | 0.313 |
| Albumin level6, g/L | 42.3 ± 4.5 | 42.3 ± 4.4 | 42.2 ± 4.6 | 0.908 |
| Creatinine, mg/dL | 82.1 ± 18.0 | 80.8 ± 15.5 | 83.3 ± 20.1 | 0.128 |
| Serum sodium7, mmol/L | 141.0 ± 3.0 | 141.0 ± 3.1 | 142.0 ± 2.9 | 0.028 |
| Serum potassium7, mmol/L | 4.1 ± 0.5 | 4.1 ± 0.4 | 4.1 ± 0.5 | 0.282 |
| Carcinoembryonic antigen28, ng/mL | 4.33 (2.22-11.91) | 5.40 (2.40-13.95) | 3.50 (2.08-8.67) | 0.009 |
| Serum CA19-929, kU/L | 14.02 (7.20-28.63) | 15.10 (7.39-33.91) | 13.41 (7.10-23.10) | 0.109 |
| Characteristic | Total patient s(n = 469)1 | RAC patients with LNM (n = 231)1 | RAC patients without LNM (n = 238)1 | P value |
| Pathological lymph node stage | < 0.001 | |||
| pN0 | 238 (50.7) | 0 (0.0) | 238 (100.0) | - |
| pN1 | 136 (29.0) | 136 (58.9) | 0 (0.0) | - |
| pN2 | 95 (20.3) | 95 (41.1) | 0 (0.0) | - |
| Pathological tumor classification (pT) | < 0.001 | |||
| pT1 | 18 (3.8) | 1 (0.4) | 17 (7.1) | - |
| pT2 | 90 (19.2) | 21 (9.1) | 69 (29.0) | - |
| pT3 | 118 (25.2) | 54 (23.4) | 64 (26.9) | - |
| pT4 | 243 (51.8) | 155 (67.1) | 88 (37.0) | - |
| Pathological distant metastasis (pM) | ||||
| pM0 | 439 (93.6) | 213 (92.2) | 226 (95.0) | 0.224 |
| pM1 | 30 (6.4) | 18 (7.8) | 12 (5.0) | - |
| Tumor differentiation | ||||
| Moderately/well | 388 (82.7) | 166 (71.9) | 222 (93.3) | < 0.001 |
| Poorly | 57 (12.2) | 46 (19.9) | 11 (4.6) | - |
| Unknown | 24 (5.1) | 19 (8.2) | 5 (2.1) | - |
| Number of lymph node | ||||
| Lymph node-positive (metastasis) | 2.2 ± 4.5 | 4.5 ± 5.5 | - | - |
| Total number of lymph node removal | 13.3 ± 8.4 | 15.0 ± 9.1 | 11.6 ± 7.3 | < 0.001 |
| Results of follow-up | ||||
| Death | 72 (15.4) | 46 (19.9) | 26 (10.9) | 0.007 |
| Survival | 277 (59.1) | 118 (51.1) | 159 (66.8) | 0.001 |
| Lost | 120 (25.6) | 67 (29.0) | 53 (22.3) | 0.095 |
| Time of follow-up2 (mo) | 24.0 (9.0-44.3) | 20 (9.0-37.9) | 29.1 (10.5-48.5) | 0.013 |
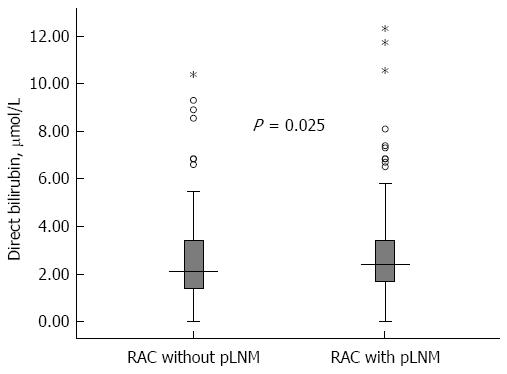
Among the 469 RC patients, their baseline serum direct bilirubin concentration (median and inter-quartile range) was 2.30 μmol/L (1.60-3.42 μmol/L). Univariate analysis showed that, compared with patients without LNM, the patients with LNM had an increased level of direct bilirubin (median/inter-quartile range 2.50/1.70-3.42 vs 2.10/1.40-3.42, P = 0.025) (Table 1 and Figure 1).
For the other basic characteristics shown in Table 1, compared with patients without LNM, the patients with LNM were younger (60.6 ± 12.8 year vs 63.6 ± 12.2 year, P = 0.012), had an increased level of CEA (5.40/2.40-13.95 vs 3.50/2.08-8.67, P = 0.009), and a decreased concentration of serum sodium (141 ± 3.1 mmol/L vs 142 ± 2.9 mmol/L, P = 0.028). The values of CEA were lost for 77 RC patients and those of serum sodium were lost for 12 patients. Pathological characteristics are shown in Table 2 and statistically significant differences were observed for tumor pathological classification (P < 0.001) and cancer differentiation (P < 0.001). In the 231 patients with LNM, 209 patients (90.5%) were diagnosed with pT3 or pT4, and 166 patients (71.9%) were diagnosed with moderate/good tumor differentiation; whereas in the 238 patients without LNM, 152 (63.9%) were diagnosed with pT3 or pT4, and 222 (93.3%) were diagnosed with moderate/good tumor differentiation.
The independent association between direct bilirubin and LNM was determined using a multivariate unconditional logistic regression model. Direct bilirubin, age and serum sodium were included, according to the findings of univariate analysis. Ultimately, we included 457 RC patients because the values of serum sodium were lost for 12 patients. For clinical practice, the continuous variables were changed to categorical variables, and the cutoff values were determined based on the combination of sensitivity, specificity, Youden index, ROC curves and AUC. Among the three variables that changed were direct bilirubin ≥ 2.60 μmol/L, age < 60 year and serum sodium ≤ 141 mmol/L. Multivariate analysis (Table 3) showed that the serum direct bilirubin concentration was independently related to pLNM in our RC patients (OR = 1.602, 95%CI: 1.098-2.338, P = 0.015).
| Variable | Adjusted OR | 95%CI | P value |
| Direct bilirubin ≥ 2.60 μmol/L | 1.602 | 1.098-2.338 | 0.015 |
| Age < 60 yr | 1.851 | 1.259-2.724 | 0.002 |
| Serum sodium ≤ 141 mmol/L | - | - | 0.090 |
We studied the relationship between direct bilirubin and some clinical and pathological parameters, using the χ2 test. Eight parameters were included based on the findings of univariate analysis, as shown in Tables 1 and 2, including age, gender, cancer differentiation, pN, pT, pM, serum CEA and serum sodium. Statistically significant differences were observed for gender (P < 0.001) and pathological cancer classification (P = 0.039) (Table 4). For patients with direct bilirubin ≥ 2.60 μmol/L, 148 (70.5%) were male, whereas 138 (53.3%) patients were male for those with direct bilirubin < 2.60 μmol/L; thus, serum direct bilirubin differs between male and female RC patients. For pathological tumor classification, compared with patients with direct bilirubin < 2.60 μmol/L, patients with direct bilirubin ≥ 2.60 μmol/L had an increased number of RC patients with pT3/pT4 (81.4% vs 73.4%, P = 0.039). However, no significant differences were found for other parameters, including the stage of pLNM.
| Variable | Direct bilirubin≥2.60μmol/L | Direct bilirubin < 2.60μmol/L | P value |
| Gender | |||
| Male | 148 (70.5) | 138 (53.3) | < 0.001 |
| Female | 62 (29.5) | 121 (46.7) | - |
| Age, yr | |||
| < 60 | 79 (37.6) | 102 (39.4) | 0.697 |
| ≥ 60 | 131 (62.4) | 157 (60.6) | - |
| Serum sodium, mmol/L | |||
| ≤ 141 | 101 (49.5) | 142 (56.1) | 0.159 |
| > 141 | 103 (50.5) | 111 (43.9) | - |
| Carcinoembryonic antigen, ng/mL | |||
| < 5.0 | 85 (50.0) | 129 (58.1) | 0.110 |
| ≥ 5.0 | 85 (50.0) | 93 (41.9) | - |
| Tumor differentiation | |||
| Moderately/well | 171 (86.8) | 217 (87.5) | 0.827 |
| Poorly | 26 (13.2) | 31 (12.5) | - |
| Pathological tumor classification (pT) | |||
| pT1/ pT2 | 39 (18.6) | 69 (26.6) | 0.039 |
| pT3/ pT4 | 171 (81.4) | 190 (73.4) | - |
| Pathological lymph node stage (pN) | |||
| pN1 | 65 (57.0) | 71 (60.7) | 0.571 |
| pN2 | 49 (43.0) | 46 (39.3) | - |
| Pathological distant metastasis (pM) | |||
| pM0 | 200 (95.2) | 239 (92.3) | 0.193 |
| pM1 | 10 (4.8) | 20 (7.7) | - |
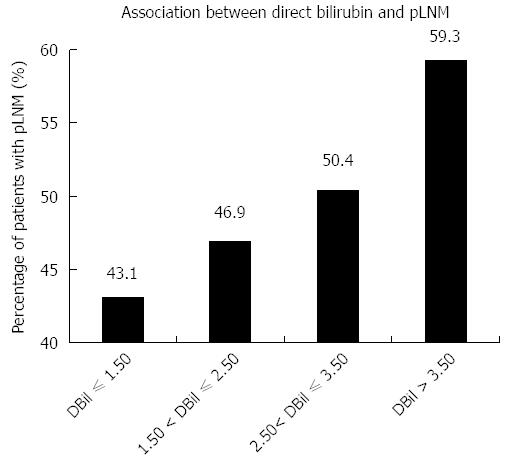
We studied the relationship between serum baseline direct bilirubin concentration and pLNM, when serum direct bilirubin level was treated as one continuous parameter. Based on the baseline concentration of serum direct bilirubin, combined with their median and inter-quartile range, 469 patients were classified into four sub-groups, including direct bilirubin ≤ 1.50 μmol/L, 1.50 μmol/L < direct bilirubin ≤ 2.50 μmol/L, 2.50 μmol/L < direct bilirubin ≤ 3.50 μmol/L, and direct bilirubin > 3.50 μmol/L (Figure 2 and Table 5). The percentages of patients with pLNM in each group were 43.1%, 46.9%, 50.4% and 59.3%, respectively. Thus, as the baseline serum direct bilirubin concentration increased, the percentage of patients with LNM increased (Figure 2). As demonstrated in Table 5, the same trends were found for their ORs and 95%CIs. Statistically significant differences were observed for comparison of patients with direct bilirubin ≤ 1.50 μmol/L and direct bilirubin > 3.50 μmol/L (OR = 1.926, 95%CI: 1.104-3.362, P = 0.020).
| Direct bilirubin/DBil (μmol/L) | Patients with LNM | Patients without LNM | OR (95%CI) | P value |
| DBil ≤ 1.50 | 50 (43.1) | 66 (56.9) | 1 (reference) | - |
| 1.50 < DBil ≤ 2.50 | 67 (46.9) | 76 (53.1) | 1.164 (0.711-1.905) | 0.547 |
| 2.50 < DBil ≤ 3.50 | 60 (50.4) | 59 (49.6) | 1.342 (0.803-2.244) | 0.261 |
| DBil > 3.50 | 54 (59.3) | 37 (40.7) | 1.926 (1.104-3.362) | 0.020 |
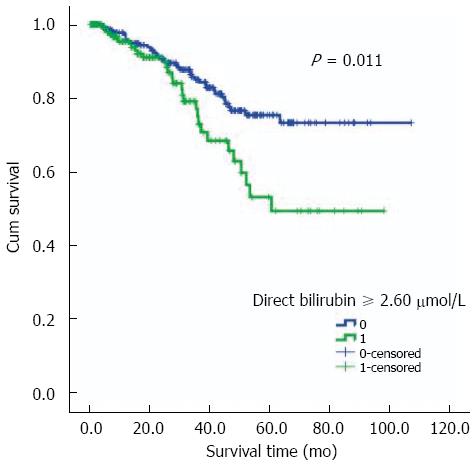
Follow-up for these patients was taken through to December 31, 2012. They were followed up for a median time of twenty-four months. The range of follow-up was 0.4-107.1 mo and their inter-quartile range time was 9.0-44.3 mo. Of the total 469 RC patients, 120 (25.6%) patients were lost from follow-up. No statistically significant difference was observed by comparison of the lost patients between the two groups with and without LNM (29.0% vs 22.3%, P = 0.095) (Table 2). In terms of death, the group of patients with LNM had a larger number and a higher percentage of deaths than the group without LNM (19.9% vs 10.9%, P = 0.007). As shown in Figure 3, the log rank test with the Kaplan-Meier curve demonstrated that serum direct bilirubin concentration was associated with the prognosis of RC patients (P = 0.011) and higher values indicated poor prognosis. These results were further confirmed by Cox regression model analysis. Furthermore, considering the significance of TNM staging for prognosis, we added the TNM staging to the modified Cox regression analysis and the result showed a similar independent association (HR = 1.142, 95%CI: 1.003-1.305, P = 0.048).
Our study showed that a higher concentration of serum baseline direct bilirubin was correlated with the increased risk of pLNM and poor prognosis in RC. Moreover, we found: (1) serum direct bilirubin differs between male and female patients. The percentage of male patients was higher in RC cases with direct bilirubin ≥ 2.60 μmol/L than those with direct bilirubin < 2.60 μmol/L; (2) a higher concentration of direct bilirubin was associated with poor tumor classification, patients with direct bilirubin ≥ 2.60 μmol/L had a higher percentage of patients with pT3/pT4; (3) as the baseline serum direct bilirubin concentration increased, the percentage of patients with LNM increased; and (4) direct bilirubin was associated with prognosis.
For the first time, baseline serum direct bilirubin concentration was associated with pLNM in RC. No unified opinion has been reached, although serum total bilirubin has been associated with CRC by some epidemiological researches. As described in the Background section, some studies demonstrated that elevated serum bilirubin concentration was correlated with a decreased risk of CRC[13,14], whereas other found no significant association[15,19]. However, different from these studies, our current research was designed to determine the relationship between serum direct bilirubin, not total bilirubin, and pLNM in RC, not CRC. Moreover, an increased serum bilirubin may be associated with a higher percentage of LNM, even though it has been correlated with a decreased risk of CRC. A similar association was described in hepatocellular carcinoma (HCC) and diabetes mellitus (DM). Recently, diabetes has been regarded as a new risk factor for hepatocellular carcinoma[20-22]; however, in the progression of cirrhosis to HCC, it may be inversely correlated with the incidence of HCC[23,24].
The major concern was the observational, cross-sectional, case-control design of our study. For the causal association in this design, no definite evidence can be provided. When serum direct bilirubin concentration was measured, these patients already had RC and LNM. Therefore, we only showed that these RC patients had LNM that was associated with the increased serum direct bilirubin level in the past. We have not resolved the clinical-associated issue of whether serum direct bilirubin level can be regarded as a new risk factor for the development of LNM in RC patients, much less whether serum direct bilirubin can promote the occurrence of LNM in RCs; thus, more well-designed prospective studies are required. However, for the first time, an association between direct bilirubin and LNM in RC was shown. In addition, we provide some additional evidences for this relationship, including: (1) higher serum direct bilirubin concentration was associated with poor tumor classification; (2) the percentage of LNM increased with the serum direct bilirubin concentration; and (3) serum direct bilirubin level was associated with prognosis and higher values indicated poor prognosis.
The second concern was that higher serum bilirubin concentration may be associated with different kinds of liver diseases. However, in our study the possible confounding effect associated with those liver diseases was eliminated by excluding patients with known hepatobiliary and pancreatic diseases and more than 95% of the included subjects had the normal hepatic enzyme levels. In addition, the values of serum bilirubin were found to vary slightly each day in healthy subjects[15]. All the patients were required to fast for the measurement of bilirubin.
Besides the association of serum total bilirubin with the risk of CRC, baseline serum bilirubin has also been correlated with both pharmacokinetics and toxicity[25]. Bilirubin undergoes glucuronidation by uridine diphosphate glucuronosyltransferase isoform 1A1 (UGT1A1)[26]. Gilbert’s syndrome represents an insufficiency of UGT1A1. Irinotecan, which is derived from camptothecin, can inhibit UGT1A1 topoisomerase I, and the latter has been confirmed to be useful for CRC[25]. One case report showed that two Gilbert’s syndrome patients experienced more serious side effects from irinotecan than others[27]. For toxicities and irinotecan in metastatic CRC patients, two studies demonstrated that serum bilirubin concentrations could be used to predict neutropenia, but not others[25,28]. However, in another cohort study that was designed for irinotecan and metastatic CRC, the authors performed a secondary analysis and found that the serum bilirubin level was not associated with overall drug-related toxicity[25].
Our study found that serum direct bilirubin differs between male and female patients, and the percentage of male patients is higher in RC cases with direct bilirubin ≥ 2.60 μmol/L than those with direct bilirubin < 2.60 μmol/L, leading to the deduction that serum bilirubin level is higher in men than in women, which was supported by another previously published study[14]. In that study, performed in the United States, the authors used the national, large health survey data to explore the demographic characteristics of serum bilirubin levels in the ordinary healthy population. They concluded that compared with women, the level of serum bilirubin of men is higher[14].
For the association between direct bilirubin level and pLNM in RC, the mechanism remains unclear. However, some data have suggested the mechanisms for the protective effect of bilirubin for the decreased risk of CRC. First, unconjugated bilirubin can induce cell apoptosis in colon cancer cell lines by triggering mitochondrial depolarization, suggesting that the occurrence and evolvement of CRC may be associated with the insufficiency of endogenous antioxidants, and the oxidative stress defense mechanisms may be deficient in CRC patients[18]. Secondly, the vascular cell adhesion molecule signaling pathway has been confirmed to be associated with the carcinogenesis of many kinds of cancers, and this pathway may be inhibited by bilirubin[29,30]. Whether these mechanisms are correlated with pLNM in RC requires further researches.
In conclusion, higher serum direct bilirubin concentration was associated with the increased risk of pLNM and poor prognosis in RC. The case-control design, single-center study and relatively limited number of study population require that our results should be validated.
Serum bilirubin has been associated with colorectal cancer (CRC); however, no information is available for the association between bilirubin and lymph node metastasis (LNM). This present study was performed to determine the significance of increased serum direct bilirubin concentration for Chinese rectal cancer (RC) patients with LNM.
Some experimental and clinical research has demonstrated that serum bilirubin has several protective effects including potent antioxidant, anti-inflammatory and anticancer activities. Several studies showed that patients with slightly increased serum bilirubin concentrations have a decreased risk of CRC. However, no significant association was also found in some epidemiological studies.
For the first time, this study found that higher serum direct bilirubin concentration was associated with the increased risk of LNM and poor prognosis in RC patients.
Serum direct bilirubin level may be used to predict LNM and prognosis.
Median values of direct bilirubin in this study were noted to be higher in lymph node positive patients than negative patients. It is well written paper but I would advice that it can still be improved with additional graphs by using other statistical packages such as STATA.
P- Reviewer: Uppara M S- Editor: Qi Y L- Editor: Stewart G E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 2. | Mekenkamp LJ, van Krieken JH, Marijnen CA, van de Velde CJ, Nagtegaal ID. Lymph node retrieval in rectal cancer is dependent on many factors--the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Peng J, Wu H, Li X, Sheng W, Huang D, Guan Z, Wang M, Cai S. Prognostic significance of apical lymph node metastasis in patients with node-positive rectal cancer. Colorectal Dis. 2013;15:e13-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kim YW, Kim NK, Min BS, Lee KY, Sohn SK, Cho CH. The influence of the number of retrieved lymph nodes on staging and survival in patients with stage II and III rectal cancer undergoing tumor-specific mesorectal excision. Ann Surg. 2009;249:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB, Cummings B, Gunderson L, Macdonald JS, Mayer RJ. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157-163. [PubMed] |
| 6. | Saraste D, Gunnarsson U, Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer. 2013;49:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Chang HC, Huang SC, Chen JS, Tang R, Changchien CR, Chiang JM, Yeh CY, Hsieh PS, Tsai WS, Hung HY. Risk factors for lymph node metastasis in pT1 and pT2 rectal cancer: a single-institute experience in 943 patients and literature review. Ann Surg Oncol. 2012;19:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Ding PR, An X, Cao Y, Wu XJ, Li LR, Chen G, Lu ZH, Fang YJ, Wan DS, Pan ZZ. Depth of tumor invasion independently predicts lymph node metastasis in T2 rectal cancer. J Gastrointest Surg. 2011;15:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Fujita S, Yamamoto S, Akasu T, Moriya Y. Risk factors of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Colorectal Dis. 2009;24:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Wu ZY, Wan J, Li JH, Zhao G, Yao Y, Du JL, Liu QF, Peng L, Wang ZD, Huang ZM. Prognostic value of lateral lymph node metastasis for advanced low rectal cancer. World J Gastroenterol. 2007;13:6048-6052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Gao C, Li JT, Fang L, Wen SW, Zhang L, Zhao HC. Pre-operative predictive factors for intra-operative pathological lymph node metastasis in rectal cancers. Asian Pac J Cancer Prev. 2013;14:6293-6299. [PubMed] |
| 12. | Gao C, Li JT, Fang L, Xu YY, Zhao HC. Drug allergy and the risk of lymph node metastasis in rectal cancer. PLoS One. 2014;9:e106123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Jirásková A, Novotný J, Novotný L, Vodicka P, Pardini B, Naccarati A, Schwertner HA, Hubácek JA, Puncochárová L, Šmerhovský Z. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int J Cancer. 2012;131:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Ioannou GN, Liou IW, Weiss NS. Serum bilirubin and colorectal cancer risk: a population-based cohort study. Aliment Pharmacol Ther. 2006;23:1637-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [PubMed] |
| 17. | Temme EH, Zhang J, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12:887-894. [PubMed] |
| 18. | Keshavan P, Schwemberger SJ, Smith DL, Babcock GF, Zucker SD. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int J Cancer. 2004;112:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Ko WF, Helzlsouer KJ, Comstock GW. Serum albumin, bilirubin, and uric acid and the anatomic site-specific incidence of colon cancer. J Natl Cancer Inst. 1994;86:1874-1875. [PubMed] |
| 20. | Zhang H, Gao C, Fang L, Yao SK. Increased international normalized ratio level in hepatocellular carcinoma patients with diabetes mellitus. World J Gastroenterol. 2013;19:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Gao C, Zhao HC, Li JT, Yao SK. Diabetes mellitus and hepatocellular carcinoma: comparison of Chinese patients with and without HBV-related cirrhosis. World J Gastroenterol. 2010;16:4467-4475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8:465-473. [PubMed] |
| 23. | Gao C, Fang L, Zhao HC, Li JT, Yao SK. Potential role of diabetes mellitus in the progression of cirrhosis to hepatocellular carcinoma: a cross-sectional case-control study from Chinese patients with HBV infection. Hepatobiliary Pancreat Dis Int. 2013;12:385-393. [PubMed] |
| 24. | Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Meyerhardt JA, Kwok A, Ratain MJ, McGovren JP, Fuchs CS. Relationship of baseline serum bilirubin to efficacy and toxicity of single-agent irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Iyer L, Das S, Janisch L, Wen M, Ramírez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43-47. [PubMed] |
| 27. | Wasserman E, Myara A, Lokiec F, Goldwasser F, Trivin F, Mahjoubi M, Misset JL, Cvitkovic E. Severe CPT-11 toxicity in patients with Gilbert’s syndrome: two case reports. Ann Oncol. 1997;8:1049-1051. [PubMed] |
| 28. | Freyer G, Rougier P, Bugat R, Droz JP, Marty M, Bleiberg H, Mignard D, Awad L, Herait P, Culine S. Prognostic factors for tumour response, progression-free survival and toxicity in metastatic colorectal cancer patients given irinotecan (CPT-11) as second-line chemotherapy after 5FU failure. CPT-11 F205, F220, F221 and V222 study groups. Br J Cancer. 2000;83:431-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol. 2005;174:3709-3718. [PubMed] |
| 30. | Wu TC. The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer Res. 2007;67:6003-6006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |