Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2566
Peer-review started: September 17, 2015
First decision: October 14, 2015
Revised: October 30, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: February 28, 2016
Processing time: 164 Days and 20.7 Hours
AIM: To observe the efficacy and mechanism of grain-sized moxibustion at different acupoints in a rat model of ulcerative colitis (UC).
METHODS: Sprague-Dawley rats were randomly divided into control, UC model, grain-sized moxibustion at a single acupoint (CV 12), grain-sized moxibustion at two acupoints (CV 12 and CV 4), grain-sized moxibustion at three acupoints (CV 12, CV 4, and ST 36), and medication groups (n = 8/group). The UC model was established by enema of trinitrobenzene sulfonic acid. Direct moxibustion was used once a day for 7 d. Disease activity index (DAI) was evaluated before and after the treatment. Morphologic changes of intestinal tissue were observed under an optical microscope. The expression of tumor necrosis factor (TNF)-α and p38 mitogen-activated protein kinase (p38MAPK) in colonic tissue was detected using Western blot, and the levels of occludin and zonula occludens-1 (ZO-1) mRNAs were detected using reverse transcription PCR.
RESULTS: Compared with the control group, the intestinal mucosae were incomplete in the model group, glandular structures were irregular, and submucosae were edematous, hyperemic, and infiltrated with inflammatory cells. The DAI scores and expression of TNF-α and p38MAPK were increased significantly in the model group compared to controls (Ps < 0.01), while the mRNA levels of occludin and ZO-1 were reduced significantly (Ps < 0.01). Compared with the model group, colonic mucosa and the arrangement of glands were complete and regular in the treatment groups. DAI scores and the expression of TNF-α and p38MAPK were reduced significantly in moxibustion groups compared to controls (Ps < 0.01), while the mRNA levels of occludin and ZO-1 were increased significantly (Ps < 0.01). The improvements in the above indices in the three acupoints group and the medication group were superior to those in the single and two acupoints groups (all P < 0.05).
CONCLUSION: Reduction of TNF-α and p38MAPK and increased expression of occludin and ZO-1 in colonic tissue represent a potential mechanism for improved intestinal mucosal tissue repair with grain-sized moxibustion.
Core tip: The efficacy of different acupoint prescriptions for grain-sized moxibustion on inflammatory responses and the mucosal barrier of colonic tissue was assessed in ulcerative colitis rats. Treatment with moxibustion reduced expression of tumor necrosis factor-α and p38 mitogen-activated protein kinase, with increased levels of occludin and zonula occludens-1 mRNA in colonic tissue of rats. These data suggest that the improved mucosal barrier function of colonic tissue and decreased invasion of inflammatory factors are mechanisms for grain-sized moxibustion in repairing the intestinal mucosal tissue. The therapeutic effect of grain-sized moxibustion was greater with three acupoints compared to one or two acupoints.
- Citation: Ma TM, Xu N, Ma XD, Bai ZH, Tao X, Yan HC. Moxibustion regulates inflammatory mediators and colonic mucosal barrier in ulcerative colitis rats. World J Gastroenterol 2016; 22(8): 2566-2575
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2566
Ulcerative colitis (UC) is a kind of chronic digestive system disease with clinical manifestations of continuous or recurrent abdominal pain, diarrhea, mucus, bloody purulent stool, and others. In Western medicine, aminosalicylic acid drugs, hormone drugs, or immunosuppressive agents are often used in the treatment of UC, which can control and relieve symptoms in the short term[1], but have more adverse reactions in the long term[2].
Moxibustion is a therapy that treats and prevents diseases using moxa floss. The combustion of the moxa floss permits transmission of heat to the acupoints or other parts of the body that have various pathologic changes. It is an external therapy to treat or prevent diseases and promote health of the body. Moxibustion is safe and effective in treatment of UC[3-6]. Studies have shown that the mechanisms of moxibustion mainly include the regulation of the immune function[7,8], apoptosis[9,10], and expression of protein in acupoint areas[11,12].
In order to improve the clinical efficacy of moxibustion in the treatment of UC, specific moxibustion therapy and a combination of acupoints should be performed first[13]. Grain-sized moxibustion has the advantage of short treatment duration, with only a mild burning sensation and slight scarring of the skin; however, there is still a lack of relevant experimental studies on grain-sized moxibustion in the treatment of UC. Therefore, in this study, the effects of grain-sized moxibustion at different acupoints on inflammatory mediators and mucosal barrier of colonic tissue in UC rats were investigated to explore the similarities and differences of the efficacy and mechanisms, and to provide a reasonable acupoint prescription for grain-sized moxibustion in treatment of UC in the clinic.
Fifty-two, healthy, clean-grade Sprague-Dawley rats (26 males, 26 females) provided by Liaoning Changsheng Biotechnology Co., Ltd. were selected, weighing 200 ± 20 g [license number: SYXK (Liao)2013-0009]. Principles of humane animal use for experiments[14] were strictly followed during the experimental design and implementation. According to a random number table, the 52 rats were divided into two groups, 8 in a control group and 44 in model replication groups. After modeling, two rats were sacrificed to verify whether models were successfully established. Next, model rats were randomly divided into model, grain-sized moxibustion at single acupoint, grain-sized moxibustion at two acupoints, grain-sized moxibustion at three acupoints, and medication groups (final n = 8/group).
Main reagents: 5% trinitrobenzene sulfonic acid (TNBS) (batch No. 22972; Sigma-Aldrich, St. Louis, MO, United States); salazosulfapyridine colon-soluble capsules (batch No. G.Y.Z.Z. H20051173; Guangdong Qianji Pharmaceutical Co., Ltd., China); reverse transcription kit (DBI), rabbit anti-rat tumor necrosis factor (TNF)-α and p38 mitogen-activated protein kinase (p38MAPK) antibodies and reverse transcription (RT)-PCR kit (Beijing TransGen Biotech Co., Ltd.).
Main instruments: Paraffin slicing machine (LEICA RM2235; Leica Microsystems, Wetzlar, Germany); digital microscope (BX41; Olympus Corp., Shinjuku, Tokyo, Japan); electrophoresis apparatus (EPS300; Shanghai Tanon Science & Technology Co., Ltd.); gel imaging analysis system (Tanon5200, Shanghai Tanon Science & Technology Co., Ltd.); PCR amplifier (Bio-Rad Laboratories, Hercules, CA, United States).
The modified modeling method was used according to the previous reports[15]. Briefly, 5% TNBS and absolute ethyl alcohol were mixed in the proportion of 1:1. Rats in the model replication group were fasted for 24 h but allowed free access to water. The rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (3 mL/kg). Anesthesia was achieved when the breath and heartbeat of rats were smooth and steady. Then, an enemator with diameter of 0.2 cm was gently inserted 6 cm into the anus, and the TNBS/ethanol mixture was injected slowly using a 2-mL syringe at a dose of 60 mg/kg, which was followed by an injection of 0.3 mL of air. The tails of rats were lifted and the rats were placed on a 45° slope for 30 s. Then, the rats were put into the cage in a supine position, and allowed to wake up naturally and feed freely. On the 7th day, two rats were randomly selected and sacrificed for sampling and observation. The model was successfully established when an intestinal adhesion had formed and an ulcer scar was obvious with macroscopic observation.
After establishment of the model, Zhōngwăn (CV 12) was selected for the single acupoint group, CV 12 and Guānyuán (CV 4) were selected for the two acupoints group, and CV 12, CV 4, and Zúsānlĭ (ST 36) were selected for rats in the three acupoints group. Rats were fixed. Hair on the treatment area was shaved and vaseline was applied. A grain-sized moxa cone (1 mg of pure moxa punk with a base of 2.5-3.0 mm and a height of 4-5 mm) was put on the acupoints using tweezers and ignited with a line-incense stick (the burning time of each cone was 10-12 s, with temperature of 48-52 °C at acupoints). A new moxa cone was applied when the prior one was burned up. Seven grain-sized moxa cones were applied at each acupoint for a total of 7 d.
Sulfasalazine (SASP) solution was administrated by gavage in the medication group. The dose of SASP colon-soluble capsules (0.25 g/capsule) was calculated according to the equivalent dose-ratio table of human and animal body surface area. The conversion factor was 0.018 and the dosage for a rat with weight of 200 g was 0.054 g/d (2.16 mL of 0.025 g/mL SASP solution), once daily for 7 consecutive days.
Rats in the model and control groups were taken and fixed at the same time as that in moxibustion groups but without any treatment.
A disease activity index (DAI) score was applied for the assessment of the severity of colonic inflammation in rats before and after treatment; DAI = (rate of weight loss + viscosity of stool + status of hematochezia)/3[16]. The details are shown in Table 1.
| Rate of weight loss (%) | Viscosity of stool | Hematochezia | Score (points) |
| None | Normal | Normal | 0 |
| ≤ 5 | 1 | ||
| 5-10 | Loose stool | Positive occult blood | 2 |
| 10-15 | 3 | ||
| > 15 | Liquid stool | Bloody stool with macrography | 4 |
After the last treatment, rats in each group were fasted for 24 h but allowed free access to water. Rats were euthanized by an overdose injection (0.5 mL/100 g) of 10% chloral hydrate. Then, 2 cm of colonic tissue was rapidly removed and divided into two parts: one segment was frozen at -80 °C for index testing, while the other segment was immersed and fixed in a 4% paraformaldehyde solution and stored at 4 °C for hematoxylin-eosin staining.
Six rats in each group were randomly selected. The corresponding intestinal tissues stored at 4 °C were taken, dehydrated in an alcohol series, embedded in paraffin, and sectioned. Then, sections were deparaffinated, stained with hematoxylin-eosin, dehydrated again, transparentized, and mounted. Morphologic changes of intestinal tissue were observed under an optical microscope.
A piece of frozen intestinal tissue with the same code number in pathologic examination was taken, and the expression levels of TNF-α and p38MAPK were detected by Western blot. Protein extraction reagent was added in a ratio of 1:10 between net weight of tissue and lysate. The sample was centrifuged at a speed of 10000 r/min for 15 min, and the obtained supernatant was total protein. Coomassie brilliant blue method was applied for the quantitative determination of total protein. Electrophoresis was performed with each well containing 20 μg of total protein. Then, protein was transferred to a PVDF membrane using a semi-dry transfer printing method. Primary antibody (1:200) was incubated overnight at 4 °C followed by secondary antibody (1:2000) for 1 h at room temperature. Electrochemiluminescence was used for detection, and film was exposed and scanned. The gray values of target protein and internal reference protein bands were read through a gel imaging analysis system, and the ratio of the gray value of the target protein band to that of internal reference protein band was taken as the relative expression level.
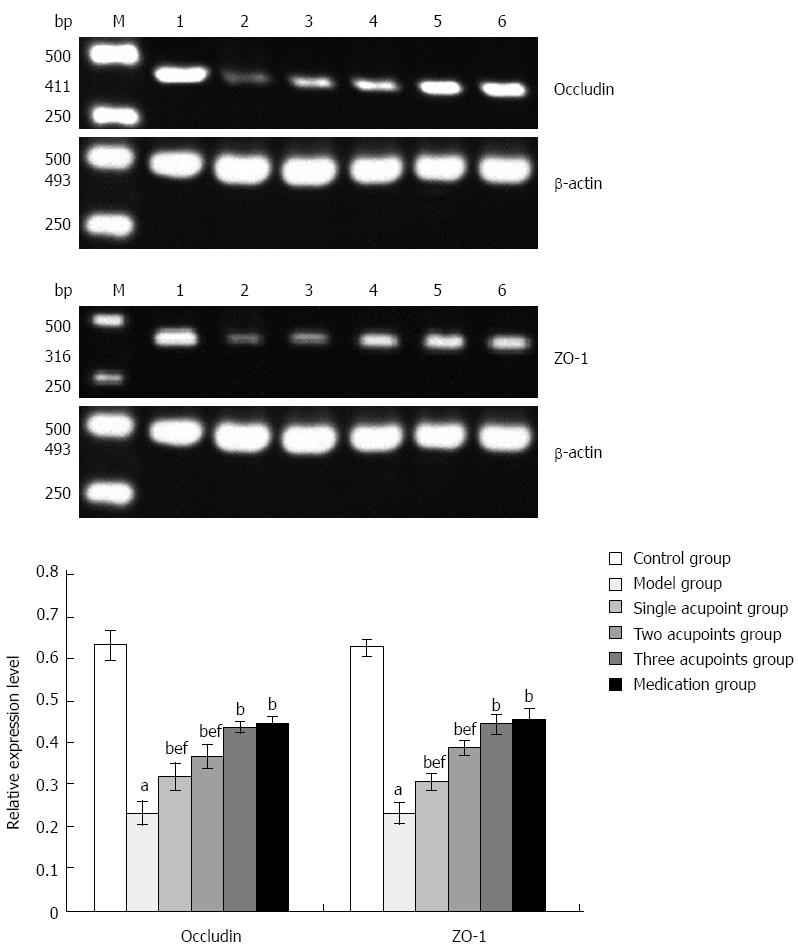
A piece of frozen intestinal tissue with the same code number in pathologic examination was taken, and the levels of occludin and zonula occludens-1 (ZO-1) mRNAs were detected by RT-PCR using target and internal reference gene primers. Images were collected using a gel imaging analysis system to determine the integral optical density value of each gene. The ratio of integral optical density value of the target gene band to that of the internal reference gene band was taken as the relative expression level.
Data were analyzed using SPSS 16.0 software (SPSS Inc., Chicago, IL, United States). Measurement data are expressed as mean ± SD. One-way analysis of variance was carried out for the comparison of means of multiple groups and least significant differences were calculated for intergroup comparisons. P < 0.05 indicated statistical significance.
On the 7th day after modeling, two rats in the model replication group exhibited accidie, hogback, listlessness, abdominal distension, and significant weight loss, and were excluded due to suspected intestinal obstruction.
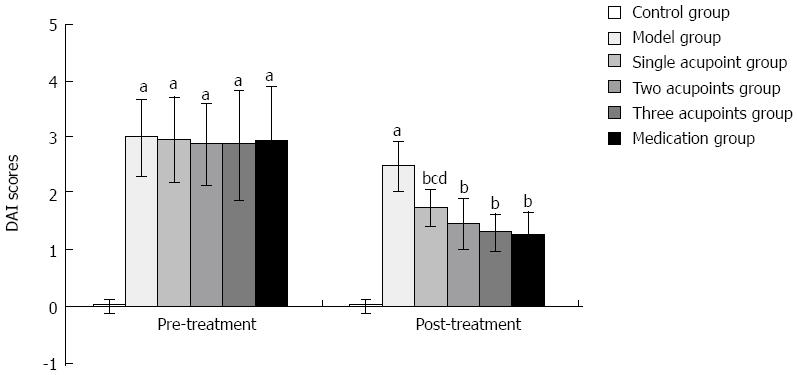
Before treatment, the DAI index was increased significantly in the model and treatment groups when compared with the control group (all P < 0.01). After treatment, the DAI decreased significantly in each treatment group when compared with the model group (all P < 0.01). The difference between the single acupoint group and the three acupoints group, and the difference between the single acupoint group and the medication group were statistically significant (all P < 0.05), while the difference was not statistically significant when compared among the rest treatment groups (Figure 1).
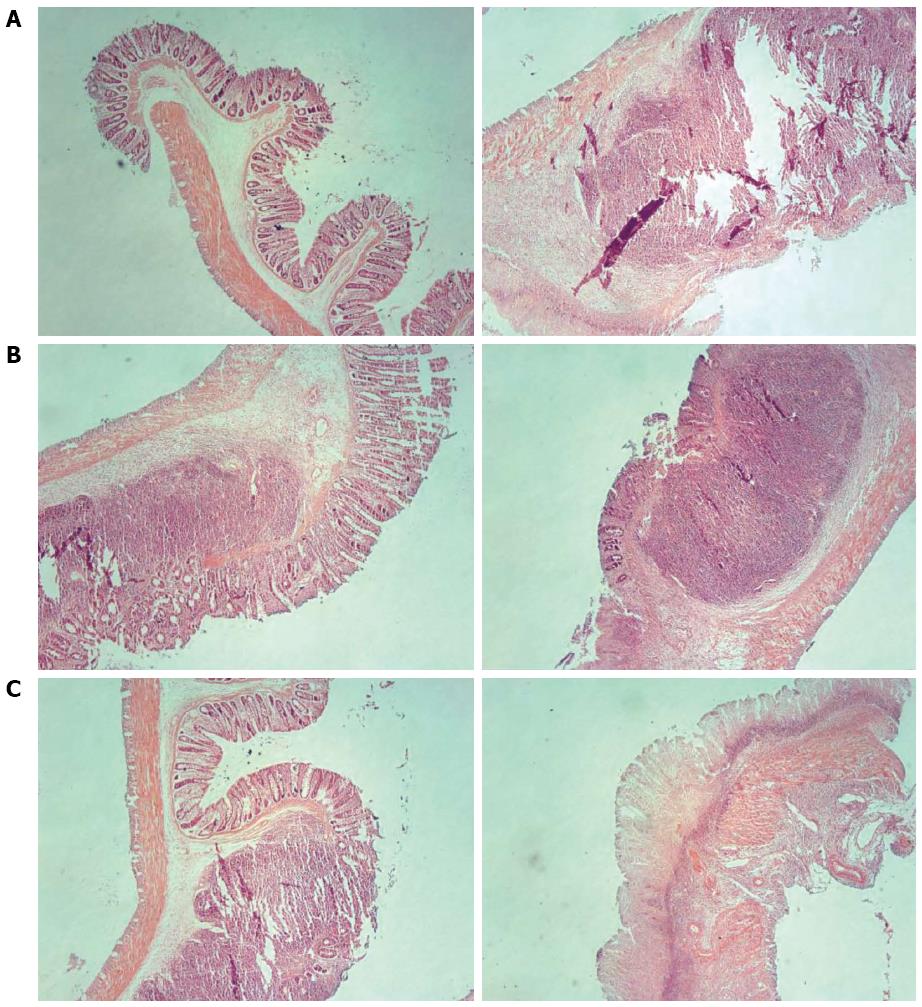
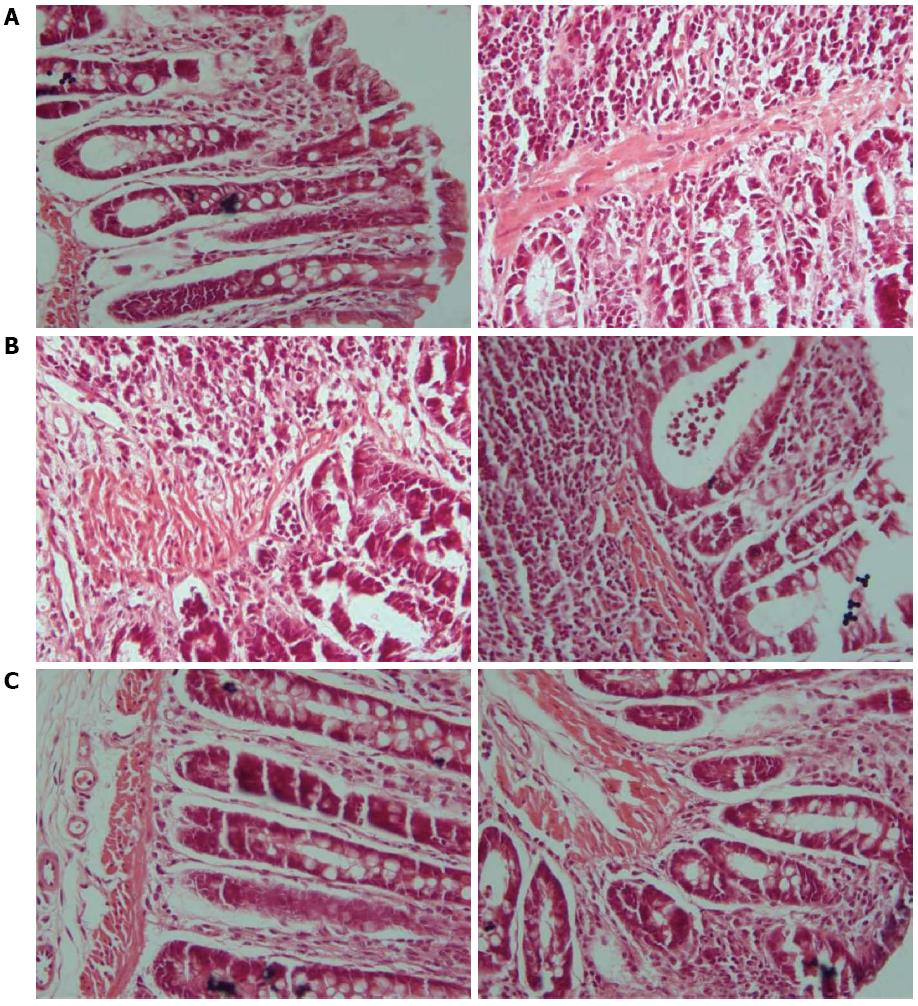
In rats in the control group, intestinal mucosae were complete, the arrangement of glands was regular, structure was clear, and there was no edema, hyperemia, or tissue necrosis. In rats in the model group, intestinal mucosae were deficient, the arrangement of glands was irregular, and submucosae were edematous, hyperemic, and infiltrated with inflammatory cells. In each treatment group, the arrangement of glands was relatively regular, the arrangement of mucosae was somewhat intact, and there were new epithelial cells on the ulcerations and a small amount of inflammatory-cell infiltration. Among them, the recovery in the three acupoints group and the medication group was slightly superior to that in the single acupoint and two acupoints groups (Figures 2 and 3).
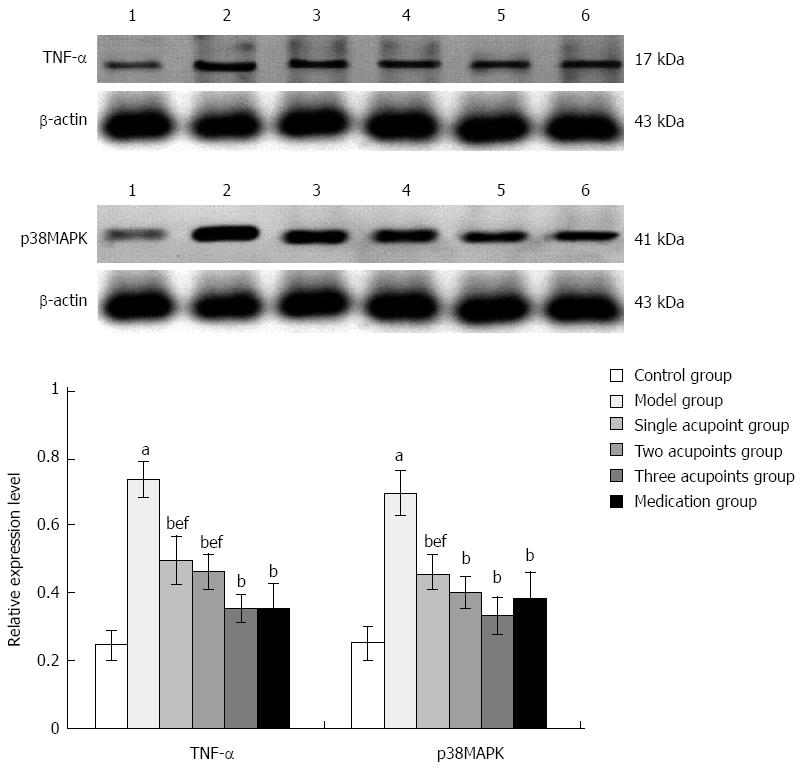
The expression of TNF-α and p38MAPK increased significantly in the model group compared with the control group (Ps < 0.01), and were reduced significantly in each treatment group compared with the model group (all P < 0.01). The expression of TNF-α and p38MAPK was higher in the single acupoint group than in the three acupoints and medication groups, while only TNF-α expression was higher in the two acupoints group (P < 0.01); the difference between the three acupoints group and the medication group was not statistically significant (Figure 4).
The levels of occludin and ZO-1 mRNAs were significantly reduced in the model group compared with the control group (Ps < 0.01), and were increased significantly in each treatment group compared with the model group (all P < 0.01). The levels of occludin and ZO-1 mRNAs were further increased in the three acupoints and medication groups compared with the single and two acupoints groups (all P < 0.01); the difference between the three acupoints group and the medication group was not statistically significant (Figure 5).
Grain-sized moxibustion therapy belongs to the category of direct and small-cone moxibustion. Small-cone moxibustion can alleviate burning pain caused by warm febricity and reduce scarring, which is easily accepted by patients. The point selection for grain-sized moxibustion is simplified; generally, one acupoint is selected, but as many as two or three acupoints can be used. Therefore, this study included three prescriptions: one, two, or three acupoints. In traditional Chinese medicine, UC is mainly characterized by spleen-stomach weakness and damp-heat accumulation. Among them, spleen-stomach weakness is the most common clinical syndrome. Therefore, warming and nourishing the spleen-stomach is the main therapeutic principle during treatment. CV12, an influential point of the abdomen, has the function of fortifying the spleen-stomach, which is the key to treatment of UC. CV 4, a front-mu point of the small intestine, has the function of reinforcing healthy qi and the vital essence, which is the root to treatment of chronic disease. ST 36, a he-sea point of stomach meridian, has the function of treatment for all gastrointestinal digestive diseases.
TNF-α, a cytokine with multiple biologic activities, mediates the inflammatory response, which is closely related with the pathogenesis of UC[17,18]. TNF-α can induce vascular endothelial cells to express adherence factors and adhere to white blood cells, leading to the accumulation of white blood cells at sites of inflammation and an aggravation of local inflammatory responses[19,20]. TNF-α can also stimulate monocyte-macrophages and other types of cells to produce cytokines, further augmenting inflammation[21]. Under a variety of extracellular stimuli, such as stress, cytokines, and G protein-coupled receptors, tyrosine and threonine residues in p38MAPK are phosphorylated, subsequently increasing the expression of lipopolysaccharide, TNF, interleukin-1, and platelet-activating factor[22-25] and further aggravating the inflammatory response. The results of this study showed that grain-sized moxibustion reduced the expression of TNF-α and p38MAPK in colonic tissue of rats with UC, and the therapeutic effect in the three acupoints group was superior to those in the single and two acupoints groups, indicating a mechanism by which this treatment may alleviate the inflammatory response in colonic tissues of UC rats.
The integrity of the intestinal barrier relies on different elements, including robust innate immune responses, epithelial paracellular permeability, epithelial cell integrity, and the production of mucus[26]. Occludin, a type II transmembrane protein, connects adjacent epithelial cells and blocks the gap between them, forming the basic structure of tight junctions with ZO-1 in the cytoplasm. Its adhesion is proportional to its expression. ZO-1 is a peripheral membrane protein, and its C terminus can combine with occludin, actin, stress fibers, and others, thus forming a stable connection between occludin and the actin cytoskeleton to potentially prevent the entrance of harmful substances and pathogens[27]. A previous study showed that moxibustion combined with acupuncture could repair intestinal epithelial barrier lesions and relieve inflammation by upregulating the expression of tight junction proteins in Crohn’s disease patients[28]. Another experimental study indicated that moxibustion could reduce apoptosis of colonic epithelial cells, repair tight junctions, and enhance colonic epithelial barrier function in rats with Crohn’s disease[29]. In this study, the results showed that grain-sized moxibustion increased the levels of occludin and ZO-1 mRNAs in colonic tissue of rats with UC, with a superior effect achieved with three acupoints. Thus, grain-sized moxibustion therapy may increase the expression of the tight junction proteins occludin and ZO-1 to restore intestinal mucosal barrier function, so as to protect the intestinal mucosa and to treat UC.
SASP (at a dosage of 3 g/d) is commonly used in the treatment of patients with UC, and can significantly alleviate the clinical symptoms and the degree of inflammation of the intestinal mucosa[30,31]. In this study, the therapeutic effect in the three acupoints group was similar to that in the medication group, but with avoidance of the adverse reactions from Western medicines. Therefore, it may be a reasonable acupoint prescription in treatment of UC, and can be used as a specific therapy for UC. The therapeutic effect in the three acupoints group was superior to those in the single and two acupoints groups, which is consistent with the study by Yan et al[32,33] showing that the combination of acupoints had a better efficacy. However, it does not mean that the more acupoints are selected, the better the efficacy will be. Huang[34] selected four or five acupoints for the treatment of a stiff neck, but the therapeutic effect was not good, whereas treatment at one point (Xuánzhōng; GB 39), achieved a better efficacy. Other studies have shown that the combination of different acupoints might have synergistic or antagonistic effects[35,36]. Thus, further study on the best acupoint combination for grain-sized moxibustion in treatment of UC is needed. Although the therapeutic effect in the two acupoints group was inferior to that in the three acupoints group, the burning sensation from two acupoints group is minor. Morever, the selection of acupoints in the abdomen avoids the development of moxibustion scars on the limbs. Importantly, there was a certain therapeutic effect in treatment of UC in the two acupoints group. Therefore, it can be used as a complementary therapy for UC. Concerning the combination of grain-sized moxibustion at two acupoints and SASP in treatment of UC, further study is needed.
Ulcerative colitis (UC) is a common disease in the clinic. Many reports have shown that moxibustion is safe and effective in treating UC. Further studies showed that the mechanisms of moxibustion mainly included the regulation of immune function, apoptosis, and expression of protein in acupoint areas. However, the relationship between acupoint combination and efficacy is still unclear. Tumor necrosis factor (TNF)-α, a cytokine with multiple biologic activities, mediates the inflammatory response, which is closely related with the pathogenesis of UC. Activated p38 mitogen-activated protein kinase (p38MAPK) can increase the expression of TNF-α and further aggravate the inflammatory response. Occludin and zonula occludens-1 (ZO-1) are important substances in the integrity of the intestinal barrier. Occludin and ZO-1 form the basic structure of tight junctions to prevent the spread of potential pathogens throughout the body.
Inflammatory mediators and the mucosal barrier of the colon play important roles in UC. A previous study showed that moxibustion combined with acupuncture could repair intestinal epithelial barrier lesions and relieve inflammation by upregulating the expression of tight junction protein expression in Crohn’s disease patients.
In this study, the authors observed the efficacy of different acupoint combinations and explored the mechanism underlying improvement of UC in clinic. They associated the effects of moxibustion therapy with inflammatory responses and function of the mucosal barrier by observing protein levels of TNF-α and p38MAPK, and mRNA expression of occludin and ZO-1. The results showed reductions of TNF-α and p38MAPK, and increases of occludin and ZO-1 mRNAs in colonic tissue in the moxibustion groups suggests that the improved function of the mucosal barrier in the colon and the decreased invasion of inflammatory factors are potential mechanisms for grain-sized moxibustion in repairing the intestinal mucosal tissue. The combined therapeutic effect of moxibustion with grain-sized moxa at CV 12, CV 4, and ST 36 is superior to that from one or two acupoints.
This study provides a reasonable acupoint prescription for grain-sized moxibustion in the treatment of UC, and clarifies part of the scientific evidence for its therapeutic effects for UC.
Moxibustion is a therapy that treats and prevents diseases using moxa floss. The combustion of the moxa floss permits transmission of heat to the acupoints or other parts of the body that have various pathologic changes. Direct moxibustion is one of its treatment methods to. Grain-sized moxibustion therapy is a form of direct moxibustion, where a seed-shaped moxa cone is burned directly on the skin. It has the advantages of a short treatment duration, with only a mild burning sensation and slight scarring of the skin, which is easily accepted by patients.
It is a very good research article about grain-sized moxibustion at different acupoints regulating inflammatory mediators and mucosal barrier of colonic tissue in rats with ulcerative colitis. It demonstrated that, in the groups treated with one, two, or three acupoints, the expression levels of inflammatory biomarkers TNF-α and p38MAPK were reduced, while occludin and ZO-1 mRNAs were increased in colonic tissue of rats with UC.
P- Reviewer: Munoz M, Vetvicka V S- Editor: Yu J L- Editor: Filopodia E- Editor: Zhang DN
| 1. | Zhang Z, Kennedy H. Ulcerative colitis: current medical therapy and strategies for improving medication adherence. Eur J Gastroenterol Hepatol. 2009;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Clark M, Colombel JF, Feagan BC, Fedorak RN, Hanauer SB, Kamm MA, Mayer L, Regueiro C, Rutgeerts P, Sandborn WJ. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21-23, 2006. Gastroenterology. 2007;133:312-339. [PubMed] |
| 3. | Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, Cui Y, Wu H. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Wu HG, Zhou LB, Shi DR, Liu SM, Liu HR, Zhang BM, Chen HP, Zhang LS. Morphological study on colonic pathology in ulcerative colitis treated by moxibustion. World J Gastroenterol. 2000;6:861-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich SN, Hahn EG, Brinkhaus B. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Mu JP, Wu HG, Zhang ZQ, Liu HR, Zhu Y, Shi Z, Wang XM. [Meta-analysis on acupuncture and moxibustion for treatment of ulcerative colitis]. Zhongguo Zhen Jiu. 2007;27:687-690. [PubMed] |
| 7. | Wang XM, Lu Y, Wu LY, Yu SG, Zhao BX, Hu HY, Wu HG, Bao CH, Liu HR, Wang JH. Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis. World J Gastroenterol. 2012;18:6819-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Han Y, Ma TM, Lu ML, Ren L, Ma XD, Bai ZH. Role of moxibustion in inflammatory responses during treatment of rat ulcerative colitis. World J Gastroenterol. 2014;20:11297-11304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Wu HG, Liu HR, Tan LY, Gong YJ, Shi Y, Zhao TP, Yi Y, Yang Y. Electroacupuncture and moxibustion promote neutrophil apoptosis and improve ulcerative colitis in rats. Dig Dis Sci. 2007;52:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Wu HG, Huang Z, Liu HR, Zhang W, Shi Y, Zhu Y, Cui YH, Liu SM. [Experimental study on influence of acupuncture and moxibustion therapy on apoptosis of colonic epithelial cells in rats of ulcerative colitis]. Zhongguo Zhen Jiu. 2005;25:119-122. [PubMed] |
| 11. | Zhou EH, Liu HR, Wu HG, Shi Z, Zhang W, Zhu Y, Shi DR, Zhou S. Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig Dis Sci. 2009;54:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Zhao TP, Kou ST, Ma XP, Liu HR, Wang JH, Wu HG. Orthogonally designed study of the effect of moxibustion on acupoint area temperature and Cx43 expression in ulcerative colitis rats. Shanghai Zhengjiu Zazhi. 2010;29:335-338. |
| 13. | Lu Y, Bao CH, Wu LY, Zhang C, Dou CZ, Zhu YF. Clinical and experimental research of moxibustion in treatment of ulcerative colitis. Zhongguo Zhongxiyi Jiehe Zazhi. 2013;6:1261-1264. |
| 14. | Russell WMS, Burch RL. The Principles of Humane Experimental Technique. Available from: http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc. |
| 15. | Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013;7:1341-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Suzuki H, Kaneko T, Mizokami Y, Narasaka T, Endo S, Matsui H, Yanaka A, Hirayama A, Hyodo I. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J Gastroenterol. 2013;19:2718-2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Stallhofer J, Friedrich M, Konrad-Zerna A, Wetzke M, Lohse P, Glas J, Tillack-Schreiber C, Schnitzler F, Beigel F, Brand S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm Bowel Dis. 2015;21:2327-2340. [PubMed] |
| 18. | Mosli MH, Al-Harbi O, Feagan BG, Almadi MA. A Saudi Gastroenterology association position statement on the use of tumor necrosis factor-alfa antagonists for the treatment of inflammatory bowel disease. Saudi J Gastroenterol. 2015;21:185-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Guo CY, Ma XJ, Liu Q, Yin HJ, Shi DZ. [Effect of Chinese herbal drug-containing serum for activating blood, activating blood and dispelling toxin on TNF-alpha-induced adherence between endothelial cells and neutrophils and the expression of MAPK pathway]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2015;35:204-209. [PubMed] |
| 20. | Prisco AR, Prisco MR, Carlson BE, Greene AS. TNF-α increases endothelial progenitor cell adhesion to the endothelium by increasing bond expression and affinity. Am J Physiol Heart Circ Physiol. 2015;308:H1368-H1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Burger D, Dayer JM. Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Ann N Y Acad Sci. 2002;966:464-473. [PubMed] |
| 22. | Zhang X, Li C, Li J, Xu Y, Guan S, Zhao M. Protective effects of protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-κB signal pathways. Int Immunopharmacol. 2015;26:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kim DH, Kim ME, Lee JS. Inhibitory effects of extract from G. lanceolata on LPS-induced production of nitric oxide and IL-1β via down-regulation of MAPK in macrophages. Appl Biochem Biotechnol. 2015;175:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kimura H, Mikami D, Kamiyama K, Sugimoto H, Kasuno K, Takahashi N, Yoshida H, Iwano M. Telmisartan, a possible PPAR-δ agonist, reduces TNF-α-stimulated VEGF-C production by inhibiting the p38MAPK/HSP27 pathway in human proximal renal tubular cells. Biochem Biophys Res Commun. 2014;454:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Liu X, Yan Y, Bao L, Chen B, Zhao Y, Qi R. Ginkgolide B inhibits platelet release by blocking Syk and p38 MAPK phosphorylation in thrombin-stimulated platelets. Thromb Res. 2014;134:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 27. | Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467-G475. [PubMed] |
| 28. | Shang HX, Wang AQ, Bao CH, Wu HG, Chen WF, Wu LY, Ji R, Zhao JM, Shi Y. Moxibustion combined with acupuncture increases tight junction protein expression in Crohn’s disease patients. World J Gastroenterol. 2015;21:4986-4996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 29. | Bao CH, Wu LY, Shi Y, Wu HG, Liu HR, Zhang R, Yu LQ, Wang JH. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn’s disease. World J Gastroenterol. 2011;17:4960-4970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Furfaro F, Bezzio C, Ardizzone S, Massari A, de Franchis R, Maconi G. Overview of biological therapy in ulcerative colitis: current and future directions. J Gastrointestin Liver Dis. 2015;24:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Feagan BG. Maintenance therapy for inflammatory bowel disease. Am J Gastroenterol. 2003;98:S6-S17. [PubMed] |
| 32. | Araújo S, Lobato J, Reis Ede M, Souza DO, Gonçalves MA, Costa AV, Goulart LR, Goulart IM. Unveiling healthy carriers and subclinical infections among household contacts of leprosy patients who play potential roles in the disease chain of transmission. Mem Inst Oswaldo Cruz. 2012;107 Suppl 1:55-59. [PubMed] |
| 33. | Ji LX, Yan LP, Wang HJ, Wang B, Zhang XY, Zhang TS, Jin XF. [Selection of basic acupoints for composing “gastric-disorder-formula” for electroacupuncture prevention of acute gastric mucosal lesion in rats]. Zhen Ci Yan Jiu. 2008;33:296-300, 325. [PubMed] |
| 34. | Huang DJ. Clinical application of grain-sized moxibustion combined with tapping and cupping. J Sichuan Tradit Chin Med. 2002;20:74-76. |
| 35. | Yu WJ, Lu MX, Cao X, Feng H, Yu Z, Xu B. Effect of electroacupuncture Tianshu and combination regulation in different states of rat gastric motility. Zhongguo Zhongyiyao Xinxi Zazhi. 2013;20:49-52. |
| 36. | Yu Z, Xia YB, Lu MX, Lin J, Yu WJ, Xu B. [Influence of electroacupuncture stimulation of “tianshu” (ST 25), “quchi” (LI 11) and “shangjuxu” (ST 37) and their pairs on gastric motility in the rat]. Zhen Ci Yan Jiu. 2013;38:40-47. [PubMed] |