Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2512
Peer-review started: October 27, 2015
First decision: November 27, 2015
Revised: December 8, 2015
Accepted: December 19, 2015
Article in press: December 21, 2015
Published online: February 28, 2016
Processing time: 122 Days and 0.3 Hours
AIM: To investigate whether a glucagon-like peptide-1 (GLP-1) analogue inhibits nonalcoholic steatohepatitis (NASH), which is being increasingly recognized in Asia, in non-obese mice.
METHODS: A methionine-choline-deficient diet (MCD) along with exendin-4 (20 μg/kg per day, ip), a GLP-1 analogue, or saline was administered to male db/db mice (non-obese NASH model). Four or eight weeks after commencement of the diet, the mice were sacrificed and their livers were excised. The excised livers were examined by histochemistry for evidence of hepatic steatosis and inflammation. Hepatic triglyceride (TG) and free fatty acid (FFA) content was measured, and the expression of hepatic fat metabolism- and inflammation-related genes was evaluated. Oxidative stress-related parameters and macrophage recruitment were also examined using immunohistochemistry.
RESULTS: Four weeks of MCD feeding induced hepatic steatosis and inflammation and increased the hepatic TG and FFA content. The expression of fatty acid transport protein 4 (FATP4), a hepatic FFA influx-related gene; macrophage recruitment; and the level of malondialdehyde (MDA), an oxidative stress marker, were significantly augmented by a 4-wk MCD. The levels of hepatic sterol regulatory element-binding protein-1c (SREBP-1c) mRNA (lipogenesis-related gene) and acyl-coenzyme A oxidase 1 (ACOX1) mRNA (β-oxidation-related gene) had decreased at 4 wk and further decreased at 8 wk. However, the level of microsomal triglyceride transfer protein mRNA (a lipid excretion-related gene) remained unchanged. The administration of exendin-4 significantly attenuated the MCD-induced increase in hepatic steatosis, hepatic TG and FFA content, and FATP4 expression as well as the MCD-induced augmentation of hepatic inflammation, macrophage recruitment, and MDA levels. Additionally, it further decreased the hepatic SREBP-1c level and alleviated the MCD-mediated inhibition of the ACOX1 mRNA level.
CONCLUSION: These results suggest that GLP-1 inhibits hepatic steatosis and inflammation through the inhibition of hepatic FFA influx and oxidative stress in a non-obese NASH model.
Core tip: Herein, we suggest that a glucagon-like peptide-1 (GLP-1) analogue inhibits nonalcoholic steatohepatitis (NASH), which is being increasingly recognized in Asia, in non-obese mice. A methionine-choline-deficient diet (MCD) was reported to induce steatohepatitis, which is morphologically similar to NASH. Additionally, it increases serum free fatty acid levels and hepatic free fatty acid and triglyceride content in mice. In our study, we showed for the first time that exendin-4, a novel GLP-1 analogue, improved steatohepatitis through the inhibition of hepatic free fatty acid influx and by suppression of macrophage recruitment and oxidative stress in our MCD-fed NASH model.
- Citation: Yamamoto T, Nakade Y, Yamauchi T, Kobayashi Y, Ishii N, Ohashi T, Ito K, Sato K, Fukuzawa Y, Yoneda M. Glucagon-like peptide-1 analogue prevents nonalcoholic steatohepatitis in non-obese mice. World J Gastroenterol 2016; 22(8): 2512-2523
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2512
With the increase in the prevalence of metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), which encompasses a wide range of disorders, has become a major health issue and the most common liver disease worldwide[1]. NAFLD is a term used to describe liver diseases associated with hepatic steatosis without excessive alcohol consumption. Nonalcoholic steatohepatitis (NASH) is characterized by steatosis, necroinflammation, and cytopathic changes. It causes liver cirrhosis and is within the spectrum of NAFLD[2]. Although the pathogenesis of NASH remains to be elucidated, insulin resistance and obesity are considered to play important roles. Recent studies have shown that the prevalence of NAFLD in non-obese patients is considerably high in East Asia[3]. Especially in India, 54% of patients with NAFLD were neither overweight nor had abdominal obesity[4].
Although there is no promising therapy for NASH, several therapeutic approaches have been evaluated in NASH models[5,6]. Glucagon-like peptide-1 (GLP-1) is considered a potential therapeutic agent for the treatment of type 2 diabetes mellitus (DM)[7]. GLP-1 acts on the β-cells in the pancreas leading to their proliferation and the promotion of insulin secretion, which controls the blood glucose level[7,8]. Since GLP-1 is immediately inactivated by dipeptidyl peptidase-4 (DPP-4), a proteolytic enzyme, it has limitations as a therapeutic agent[9]. Exendin-4, a 39-amino acid peptide isolated from the Gila monster (Heloderma suspectum) salivary glands, has approximately 53% homology with mammalian GLP-1[10]. Additionally, it is a long-acting GLP-1 analogue and is resistant to inactivation by DPP-4[10,11].
The pleiotropic actions of GLP-1 have fostered considerable interest in the use of exendin-4 for the treatment of type 2 DM. GLP-1 not only regulates blood glucose levels but also induces satiety and regulates gastrointestinal motor functions[12,13]. Regarding hepatic fat metabolism, a GLP-1 analogue was recently reported to decrease high fat diet-induced hepatic steatosis and inflammation in obese rodents[14-16]. However, it remains to be elucidated whether exendin-4 attenuates steatohepatitis in a non-obese NASH animal model.
A methionine-choline-deficient diet (MCD) has been reported to induce steatohepatitis, which is morphologically similar to NASH except for body weight gain[17]. A recent report indicated that MCD-induced adipose tissue lipolysis resulted in an increased serum free fatty acid level and hepatic triglyceride content in mice[18]. The db/db mice spontaneously developed type 2 diabetes and fatty liver because of a functional defect in the long-form of the leptin receptor[19]. These mice when fed with MCD showed decreased body weight and insulin resistance; however, they developed steatohepatitis and liver fibrosis within just 8 wk. Thus, they could be used as a rodent model to study non-obese NASH[17,20]. In the present study, we examined whether exendin-4 decreased hepatic steatosis and inflammation and affected hepatic fat metabolism in db/db mice. Based on our observations, we suggest that that exendin-4 improves steatohepatitis through the inhibition of hepatic free fatty acid influx and suppression of macrophage recruitment and oxidative stress in an MCD-fed NASH animal model.
Exendin-4, a GLP-1 agonist, was purchased from Sigma Aldrich (St. Louis, MO, United States). The non-obese NASH model mice received MCD, while the control group mice received a methionine-choline-sufficient diet (MCS). Both the diets were purchased in powdered form (Oriental Yeast Co., LTD., Tokyo, Japan). Exendin-4 was diluted in saline to achieve a concentration of 2 μg/mL (as used in a previous study[14]).
Six-week-old male db/db mice were purchased from Japan SLC Inc. (Hamamatsu, Japan). After a 1-wk acclimatization period on a basal diet (Oriental Yeast), 44 mice were divided into 3 groups and received 1 of the following diets: (1) MCS; (2) MCD with saline; or (3) MCD with exendin-4 (20 μg/kg per day, ip) for 4 or 8 wk. The exendin-4 dose was determined according to a previous study[14]. All mice were given free access to water and the experimental diets. Body weight and food consumption of the mice in each group were recorded weekly. Protocols describing the use of mice were approved by the Institutional Animal Care and Use Committee of Aichi Medical University, and were in accordance with the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals.” After 4 or 8 wk of the experimental diet, the mice were euthanized and their livers were rapidly excised and fixed either in buffered formalin (10%) or frozen in liquid nitrogen, and stored at -80 °C. Blood samples were collected from the left ventricle and centrifuged, and the serum samples were stored at -80 °C.
Serum alanine aminotransferase (ALT) and fasting blood glucose levels were determined using commercially available kits (Wako, Osaka, Japan). Serum immunoreactive insulin (IRI) levels were measured using a Mouse Insulin ELISA kit (Funakoshi, Tokyo, Japan), and then the homeostasis model assessment-insulin resistance (HOMA-IR) was calculated.
The stored liver samples (100 mg) were lysed and homogenized in 2 mL of a solution containing 150 mmol/L NaCl, 0.1% TritonX-100, and 10 mmol/L Tris using a polytron homogenizer (NS-310E; MicroTech Nichion, Tokyo, Japan) for 1 min. Hepatic triglyceride (TG) and free fatty acid (FFA) content was measured using the Triglyceride Detection Kit and Free Fatty Acids Detection Kit (Wako), respectively. The levels of hepatic malondialdehyde (MDA), a marker of reactive oxygen species (ROS), were measured using an MDA Assay Kit (Northwest Life Science, Vancouver, WA, United States).
Five-micrometer-thick sections from the liver tissues fixed in formalin and embedded in paraffin were examined in all the experimental groups. Hematoxylin and eosin staining was performed to assess hepatic inflammation. Oil Red O staining was performed using a standard technique to assess hepatic fat deposition. Hepatic inflammation was scored from 0 to 3, based on the histological scoring system for NAFLD edited by Kleiner et al[21], and averaged over 5 fields per slide at 50× magnification. The Oil Red O positive area was quantified in 5 randomly selected fields per section. The percentage of Oil Red O-positive areas was measured using a computerized image analysis system with Image-Pro Plus, version 4.5 (Media Cybernetics, Silver Spring, MD, United States).
For immunohistochemical analysis, endogenous peroxidase activity was blocked with hydrogen peroxide, and nonspecific binding was blocked with 10% normal goat serum in phosphate-buffered saline. After blocking, the liver sections were incubated overnight with anti-F4/80 (rat monoclonal, 1:50 dilution; Abcam, Cambridge, MA, United States), a macrophage marker. Antigen-antibody complexes were detected using the avidin-biotin peroxidase method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, United States). The number of F4/80 positive cells was counted independently by 2 pathologists (Nakade Y and Fukuzawa Y), who were blinded to the source of the specimens, and averaged over 5 fields per slide at 50 × magnification.
The frozen liver specimens were crushed in TRIzol reagent (Life Technologies, Tokyo, Japan). RNA extraction was performed using an RNeasy Mini Kit (Qiagen, Tokyo, Japan). RNA was resuspended in 40 μL of RNase-free water and quantified by spectrophotometry [optical density (OD) 260 and low-mass gel electrophoresis] (Invitrogen, Tokyo, Japan). Total RNA was reverse transcribed to cDNA using a High Capacity cDNA Reverse Transcriptional Kit (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s instructions. Real time quantitative polymerase chain reaction (PCR) was carried out with the ABI Step One Sequence Detection System (Applied Biosystems) using the TaqMan Gene Expression Assays [acyl-coenzyme A oxidase 1 (ACOX1), Mm01246834_m1; microsomal triglyceride transfer protein (MTTP), Mm00435015_m1; sterol regulatory element-binding protein-1c (SREBP-1c), Mm01495763_g1; fatty acid transport protein (FATP) 2, Mm0128768_m1; FATP4, Mm01327413_g1; tumor necrosis factor-α (TNF-α), Mm00443258_m1; monocyte chemotactic protein-1 (MCP-1), Mm00441242_m1; cc-chemokine receptor 2 (CCR-2), Mm99999051_Gh] and the TaqMan Universal PCR Master Mix (Applied Biosystems), according to the manufacturer’s instructions. The detailed protocol for TaqMan PCR was determined based on a previous study[22].
The liver tissue samples (100 mg) were lysed in a sodium dodecylsulphate (SDS) sample buffer, separated on a 10% SDS-acrylamide gel, and electrotransferred to the nitrocellulose membranes. After blocking with 5% nonfat dry milk in TBST buffer [10 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% Tween-20], the membranes were probed with anti-rabbit polyclonal FATP4 antibody (1:400; Abcam), and incubated using HRP-conjugated anti-rabbit or anti-mouse immunoglobulin G secondary antibodies (1:2000; DAKO Japan, Tokyo, Japan). Antibody binding was then visualized using an enhanced chemiluminescence reagent (GE Healthcare, Tokyo, Japan), and the band images detected using the LAS1000 system (Fuji Film, Tokyo, Japan) were densitometrically analyzed using Image Gauge (Fuji Film).
All results are expressed as mean ± SE. Values in 2 groups were compared using the Student’s t-test. Multiple group comparison was performed using analysis of variance (ANOVA) followed by Fisher’s protected least significant difference post-hoc test. A P value < 0.05 was considered statistically significant.
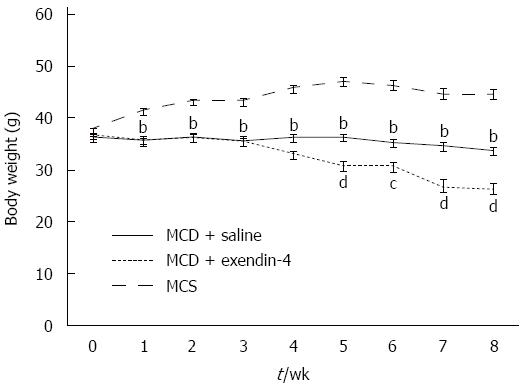
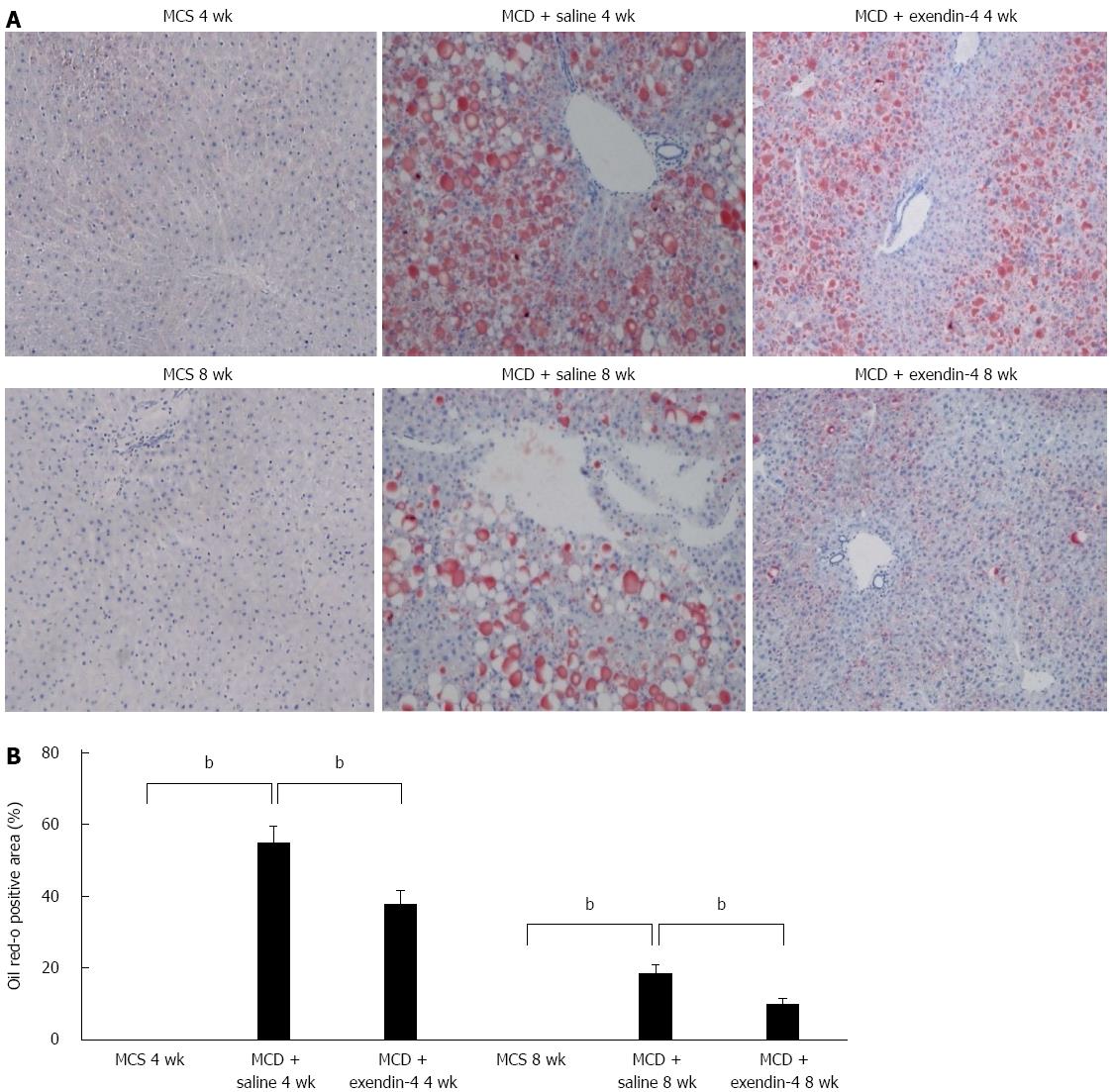
The daily intake of MCS was more than that of MCD in the 4- and 8-wk experiments (Table 1). The MCD-fed mice had lost weight whereas the MCS-fed mice had gained weight over the 8-wk experimental period (Figure 1). MCD resulted in an Oil Red O-positive area at 4 wk, and this area had reduced in size at 8 wk (Figure 2). The hepatic TG and FFA content was significantly higher in the MCD-fed mice than in the MCS-fed mice at both 4 and 8 wk (Table 1).
| Group | n | Daily food consumption (g) | Liver weight (g) | Hepatic TG (mg/g liver) | Hepatic FFA (mg/g liver) | Serum ALT (IU/L) |
| MCS 4 wk | 6 | 6.4 ± 0.5 | 2.3 ± 0.03 | 102 ± 30 | 0.82 ± 0.06 | 30 ± 5.4 |
| MCD + saline 4 wk | 8 | 3.4 ± 0.5b | 1.6 ± 0.02b | 314 ± 17b | 1.24 ± 0.18b | 209 ± 30b |
| MCD + exendin-4 4 wk | 8 | 3.2 ± 0.6 | 1.3 ± 0.04d | 200 ± 40d | 1.05 ± 0.11 | 190 ± 29 |
| MCS 8 wk | 6 | 6.2 ± 0.5 | 2.2 ± 0.03 | 43 ± 9 | 0.64 ± 0.01 | 32 ± 16 |
| MCD + saline 8 wk | 8 | 3.4 ± 0.4a | 1.4 ± 0.04a | 137 ± 31a | 0.87 ± 0.06a | 103 ± 41a |
| MCD + exendin-4 8 wk | 8 | 3.0 ± 0.6 | 1.1 ± 0.03c | 96 ± 19 | 0.69 ± 0.06 | 81 ± 24 |
Administration of exendin-4 did not change the daily intake of MCD until 4 and 8 wk (Table 1). Additionally, it did not change the body weight of the MCD-fed mice until the end of the 4-wk experiment; however, it significantly reduced the body weight of these mice from 5 to 8 wk (Figure 1). Exendin-4 administration also significantly attenuated the MCD-induced Oil Red O-positive area in the MCD + exendin-4 group compared to in the saline group at 4 and 8 wk (Figure 2). Moreover, it significantly reduced the hepatic TG content in MCD-fed mice at both 4 and 8 wk (Table 1). Furthermore, extendin-4 administration showed a tendency to decrease hepatic FFA content in the MCD-fed mice at both 4 and 8 wk (Table 1).
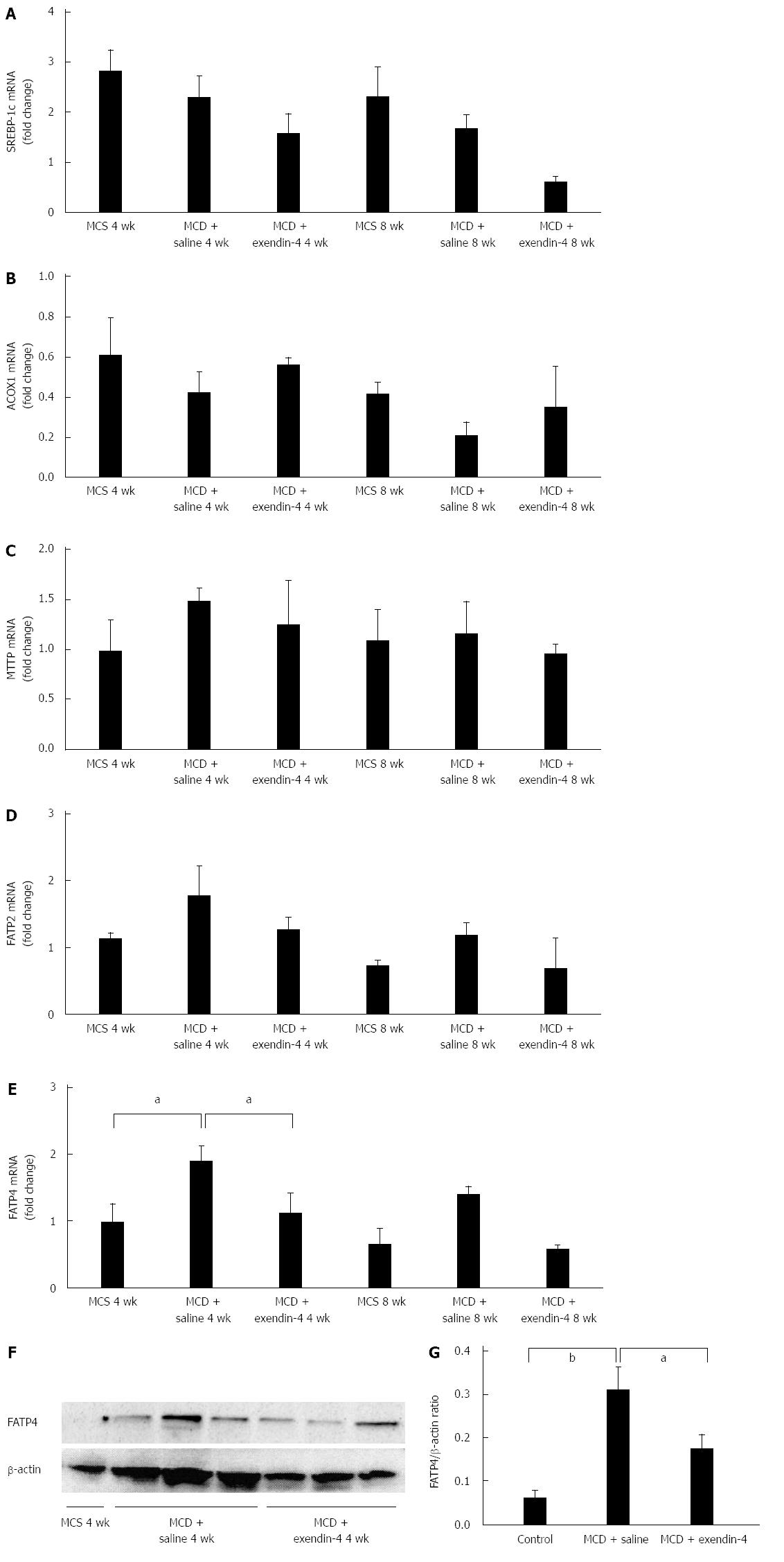
SREBP-1c mRNA (Figure 3A) and ACOX1 mRNA levels (Figure 3B) tended to decrease in the MCD-fed mice at 4 and 8 wk. MTTP mRNA levels were unchanged in the MCD-fed mice at 4 and 8 wk (Figure 3C). FATP2 mRNA levels tended to increase, and FATP4 mRNA levels significantly increased in the MCD-fed mice at 4 wk (Figure 3D and E). FATP2 and FATP4 mRNA levels tended to increase in the MCD-fed mice at 8 wk (Figure 3D and E). FATP4 protein levels were significantly increased in the MCD-fed mice at 4 wk (Figure 3F and G).
Administration of exendin-4 decreased SREBP-1c mRNA levels in MCD-fed mice at both 4 and 8 wk (Figure 3A). Additionally, it tended to reverse the MCD-induced decrease of ACOX1 mRNA levels at both 4 and 8 wk (Figure 3B); however, it did not alter MTTP mRNA levels in MCD-fed mice at 4 and 8 wk (Figure 3C). Exendin-4 administration slightly decreased the FATP2 mRNA levels augmented by 4- and 8-wk MCD, and significantly depressed the FATP4 mRNA levels augmented by 4-wk MCD (Figure 3D and E). The 8-wk exendin-4 administration tended to decrease the FATP2 mRNA levels altered by MCD (Figure 3D and E). Moreover, extendin-4 significantly inhibited the augmentation of FATP4 protein levels altered by the 4-wk MCD (Figure 3F and G).
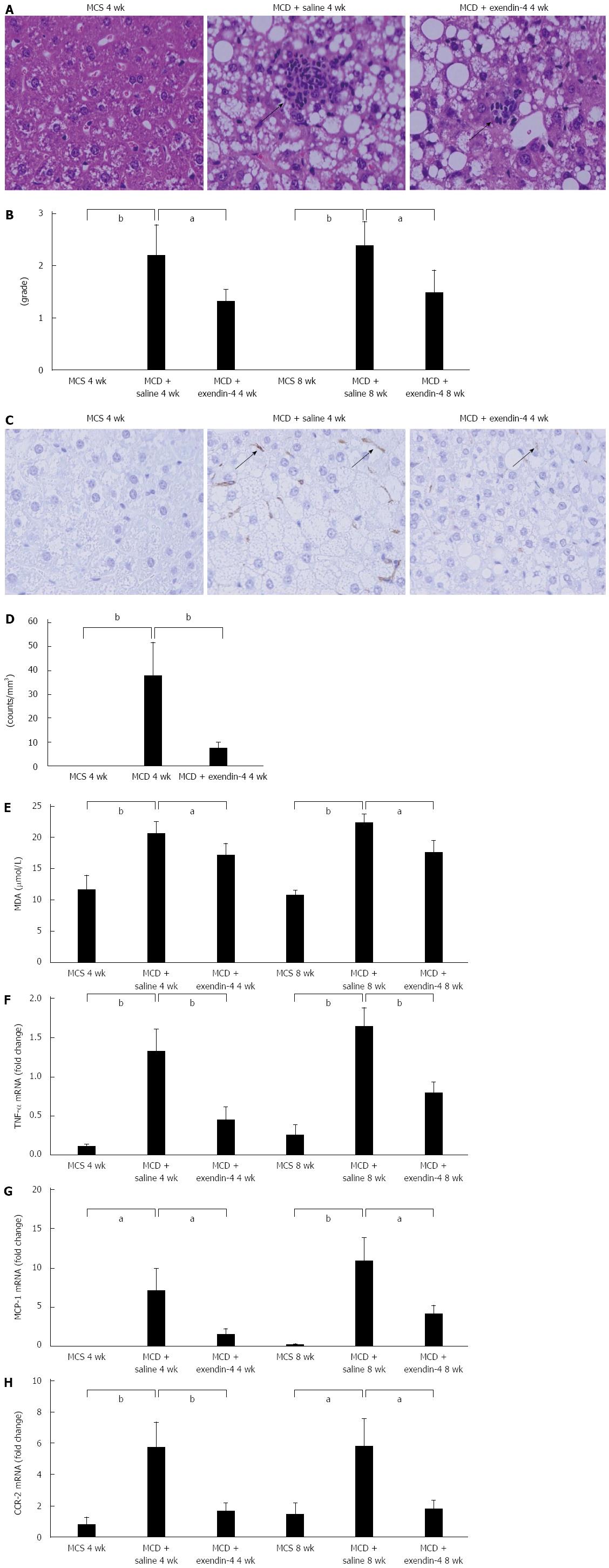
Serum ALT levels were significantly higher in the MCD-fed mice than in the MCS-fed mice at 4 wk (Table 1). Hepatic inflammatory foci appeared in the 4-wk MCD-fed mice (Figure 4A). The hepatic inflammation score was significantly augmented in the 4-wk MCD-fed mice, and was further exacerbated in the 8-wk MCD-fed mice (Figure 4B). F4/80 positive cells in the liver appeared in the 4-wk MCD-fed mice (Figure 4C and D). The hepatic MDA and TNF-α mRNA levels were significantly increased by MCD at both 4 and 8 wk (Figure 4E and F). Hepatic MCP-1 and CCR-2 mRNA levels were significantly increased in MCD-fed mice at both 4 and 8 wk (Figure 4G and H). In contrast, exendin-4 did not significantly alter the serum ALT levels that were increased by MCD at 4 wk; however, it significantly improved these hepatic indices at 8 wk (Table 1). It prevented the MCD-induced augmentation of the hepatic inflammation score at both 4 and 8 wk. Furthermore, it significantly decreased the increased number of F4/80 positive cells due to MCD at 4 wk (Figure 4B and D). Additionally, exendin-4 administration significantly attenuated the hepatic MDA and TNF-α mRNA levels enhanced by MCD at both 4 and 8 wk (Figure 4E and F). Furthermore, it significantly attenuated the MCD-induced increase in hepatic MCP-1 and CCR-2 mRNA levels at both 4 and 8 wk (Figure 4G and H).
The fasting blood glucose, IRI, and HOMA-IR levels were significantly higher in the MCS-fed mice than in the MCD-fed mice at both 4 and 8 wk (Table 2). The administration of exendin-4 tended to further decrease the fasting blood glucose level, and it significantly decreased the IRI level at both 4 and 8 wk (Table 2). The HOMA-IR level in the MCD + exendin-4 group was significantly decreased compared to that in the MCD + saline group (Table 2).
| Group | n | Fasting blood glucose (mg/dL) | IRI (ng/dL) | HOMA-IR |
| MCS 4 wk | 6 | 520 ± 51 | 19.7 ± 5.4 | 26.4 ± 8.1 |
| MCD + saline 4 wk | 8 | 126 ± 13a | 4.3 ± 1.4a | 1.4 ± 0.5a |
| MCD + exendin-4 4 wk | 8 | 109 ± 7 | 2.4 ± 0.9b | 0.7 ± 0.3b |
| MCS 8 wk | 6 | 707 ± 99 | 5.3 ± 3.6 | 9.4 ± 6.9 |
| MCD + saline 8 wk | 8 | 133 ± 12c | 4.2 ± 0.5c | 1.4 ± 0.5c |
| MCD + exendin-4 8 wk | 8 | 94 ± 8.7 | 1.1 ± 0.2d | 0.3 ± 0.04d |
In the current study, we examined whether exendin-4, a long acting GLP-1 analogue, inhibited MCD-induced hepatic steatosis and inflammation in a non-obese NASH model. The administration of exendin-4 improved MCD-induced hepatic steatosis as well as decreased the hepatic TG and FFA content. Furthermore, the MCD-induced increase in the recruitment of macrophages and hepatic inflammation were attenuated by exendin-4.
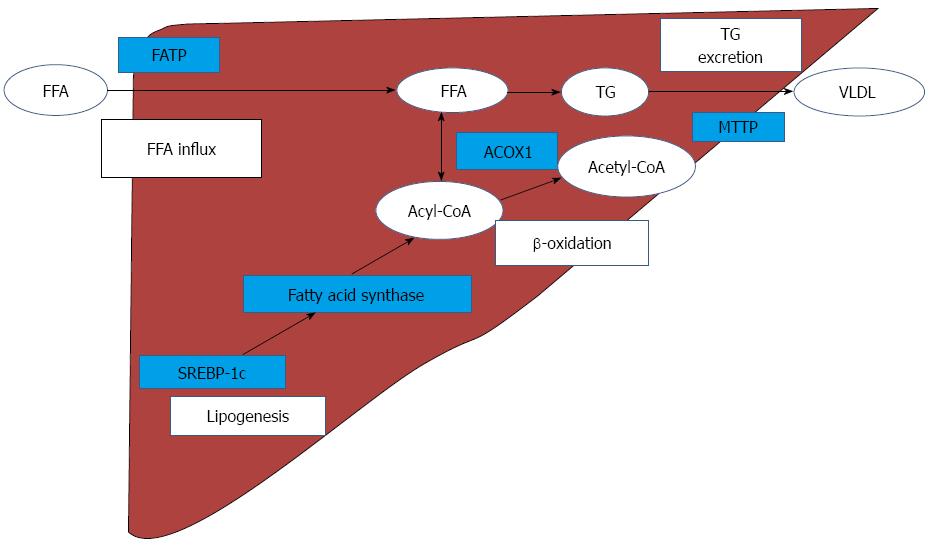
With regard to hepatic fat metabolism, FFA influx and TG excretion, hepatic de novo lipogenesis, and β-oxidation are impaired in NASH models[14,20,23]. The most plausible mechanisms of hepatic lipid metabolism and the related genes are illustrated in Figure 5. Emerging evidence has suggested that FFA is an important source of hepatic TG[24]. In the current study, hepatic FFA levels were significantly increased by MCD; exendin-4 administration tended to decrease the increased levels. The FATP family, which is composed of 6 structurally related members, plays an important role in FFA uptake by the liver. The FATPs, except for FATP1 and FATP6, are found in the liver[25]. FATP4 is considered to play an important role in the enhancement of hepatic fatty acid uptake in MCD NASH models[20]. In the current study, while the FATP2 mRNA levels were only marginally increased, the FATP4 mRNA and protein levels were significantly increased by MCD. Moreover, exendin-4 administration significantly attenuated the increase in MCD-induced FATP4 mRNA and protein levels in the liver. These results suggest that MCD may increase the hepatic FFA influx through FATP4, and exendin-4 administration may contribute to decreasing the influx through the inhibition of FATP4.
With regard to hepatic de novo lipogenesis, a high fat diet increases body weight and insulin resistance and augments the expression of the gene encoding SREBP-1c, an important lipogenic enzyme, in NAFLD mice[14]. Conversely, MCD attenuates SREBP-1c mRNA levels, resulting in the inhibition of de novo lipogenesis in the liver[20,26]. In the present study, we demonstrated that exendin-4 moderately facilitated MCD-induced inhibition of hepatic SREBP-1c mRNA levels, indicating that exendin-4 might have inhibited hepatic de novo lipogenesis in this non-obese NASH model.
A previous study showed that exendin-4 administration enhanced the mRNA levels of ACOX1, which is a rate-limiting enzyme involved in β-oxidation in the liver[14]. We too observed that MCD tended to decrease the ACOX1 mRNA level, and exendin-4 attenuated the MCD-induced decrease in the ACOX1 mRNA level. These results suggest that exendin-4 may stimulate hepatic lipid oxidation in this model animal.
Very low density lipoprotein (VLDL) is reported to play an important role in hepatic TG excretion[5]. The impairment of the synthesis and release of VLDL is thought to be a key factor in the progression of NASH in humans[23]. MTTP, which is an important regulator of hepatic lipid excretion in hepatocytes, is known to be involved in the production of VLDL[27]. In the present study, the MTTP level was not obviously changed by MCD, and exendin-4 did not affect the MTTP mRNA level in this model. These results suggest that the modification of hepatic FFA excretion is not involved in the exendin-4-induced decrease in hepatic steatosis.
Regarding hepatic inflammation, the number of F4/80 positive cells, which reflect the recruitment of macrophages; the hepatic inflammation score; and the TNF-α, MCP-1, and CCR-2 mRNA levels were obviously increased with MCD. Furthermore, MCD significantly increased hepatic MDA, a ROS marker. Exendin-4 counteracted the MCD-induced increase in hepatic inflammation score; F4/80 positive cells; TNF-α, MCP-1, and CCR-2 mRNA levels; and hepatic MDA levels. Altered abundance and composition of fat in the liver was reported to modulate the biological activity of Kupffer cells, which are the recruited macrophages that augment hepatic inflammation in vivo[28]. During the first several weeks of MCD feeding, Kupffer cells were significantly increased in the liver[28]. Increased Kupffer cells release ROS, which enhance the sensitivity of Kupffer cells to hepatotoxin, which in turn stimulates the secretion of pro-inflammatory cytokines and chemokines such as TNF-α and MCP-1[29]. MCP-1 binds to CCR-2, a specific MCP-1 receptor, resulting in the induction of macrophage infiltration into the liver[30,31].
In this study, body weight and the fasting blood glucose and IRI levels were significantly decreased in the mice fed with MCD compared with mice fed with MCS. In a high fat diet diabetic rodent model, exendin-4 improved hepatic glucose homeostasis by promoting insulin signaling through potentiating tyrosine phosphorylation of the insulin receptor substrate-2[32]. Conversely, MCD by itself decreased body weight and improved insulin resistance, and exendin-4 administration did not stimulate insulin secretion in the MCD-fed group. These results indicate that the decrease in the hepatic TG and FFA content by exendin-4 might not be due to an improvement in insulin resistance in MCD-fed animals.
These data suggest that exendin-4, a GLP-1 analogue, attenuates hepatic steatosis through the inhibition of FATP4-related hepatic FFA influx, suppression of SREBP-1c-related hepatic lipogenesis, and stimulation of ACOX1-related β-oxidation. It prevents hepatic inflammation through the suppression of macrophage recruitment and oxidative stress in a non-obese NASH model. Thus, in future, exendin-4 could be used for the treatment of non-obese patients with NASH.
Although there is no promising therapy for nonalcoholic steatohepatitis (NASH), it has been reported that glucagon-like peptide-1 (GLP-1) analogues may decrease hepatic steatosis in high fat diet-induced NASH models. Several studies on the effects of GLP-1 analogues in NASH models have been conducted; however, it remains unclear whether GLP-1 analogues improve hepatic steatosis in non-obese NASH models.
Previous studies have shown that GLP-1 analogues reduce hepatic steatosis in high fat diet-induced NASH models. Additionally, GLP-1 analogues stimulate hepatic lipid oxidation induced by a high fat diet in NASH models.
To our knowledge, this is the first study evaluating the effects of a GLP-1 analogue in a non-obese NASH model. The authors showed that the GLP-1 analogue prevented not only hepatic steatosis but also hepatic inflammation in a methionine choline deficient diet (MCD)-induced non-obese NASH model.
Regarding hepatic inflammation, a previous study has shown that the number of F4/80 positive cells was increased in NASH models. Those data showed that F4/80 positive cells were significantly increased by MCD. The presence of F4/80 positive cells suggests that hepatic steatosis and inflammation induced the recruitment of macrophages in the liver.
A MCD has been reported to induce steatohepatitis, which is morphologically similar to NASH except for body weight gain. MCD-induced adipose tissue lipolysis resulted in an increase in serum free fatty acid levels and hepatic free fatty acid content in rodents.
The authors demonstrated that a GLP-1 analogue, exendin-4, prevented hepatic steatosis and inflammation, and decreased hepatic free fatty acid influx in a non-obese NASH model. The title reflects the topic and contents of the study. The authors clearly state the purpose of the manuscript. The manuscript is well structured and concise. The methods used are appropriate.
P- Reviewer: Gonzalez-Reimers E, Streba LAM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1-16, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, Dhibar T, Bhattacharya B, Bhattacharya D, Manna B. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 5. | Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Kudo H, Yata Y, Takahara T, Kawai K, Nakayama Y, Kanayama M, Oya T, Morita S, Sasahara M, Mann DA. Telmisartan attenuates progression of steatohepatitis in mice: role of hepatic macrophage infiltration and effects on adipose tissue. Liver Int. 2009;29:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Vilsbøll T, Brock B, Perrild H, Levin K, Lervang HH, Kølendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med. 2008;25:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Klinger S, Poussin C, Debril MB, Dolci W, Halban PA, Thorens B. Increasing GLP-1-induced beta-cell proliferation by silencing the negative regulators of signaling cAMP response element modulator-alpha and DUSP14. Diabetes. 2008;57:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Perusicová J. [Incretin strategy in the treatment of type 2 diabetes mellitus--DPPIV]. Vnitr Lek. 2007;53:1005-1009. [PubMed] |
| 10. | Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650-19655. [PubMed] |
| 11. | Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141:1936-1941. [PubMed] |
| 12. | Tang-Christensen M, Cowley MA. GLP-1 analogs: satiety without malaise? Am J Physiol Regul Integr Comp Physiol. 2007;293:R981-R982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 450] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Trevaskis JL, Griffin PS, Wittmer C, Neuschwander-Tetri BA, Brunt EM, Dolman CS, Erickson MR, Napora J, Parkes DG, Roth JD. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G762-G772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 16. | Wang Y, Parlevliet ET, Geerling JJ, van der Tuin SJ, Zhang H, Bieghs V, Jawad AH, Shiri-Sverdlov R, Bot I, de Jager SC. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br J Pharmacol. 2014;171:723-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035-G1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Tanaka N, Takahashi S, Fang ZZ, Matsubara T, Krausz KW, Qu A, Gonzalez FJ. Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta. 2014;1841:1596-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491-495. [PubMed] |
| 20. | Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8245] [Article Influence: 412.3] [Reference Citation Analysis (5)] |
| 22. | Tamaki Y, Nakade Y, Yamauchi T, Makino Y, Yokohama S, Okada M, Aso K, Kanamori H, Ohashi T, Sato K. Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis. J Gastroenterol. 2013;48:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Barter PJ, Nestel PJ. Precursors of plasma triglyceride fatty acids in obesity. Metabolism. 1973;22:779-783. [PubMed] |
| 25. | Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda). 2006;21:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 26. | Jha P, Knopf A, Koefeler H, Mueller M, Lackner C, Hoefler G, Claudel T, Trauner M. Role of adipose tissue in methionine-choline-deficient model of non-alcoholic steatohepatitis (NASH). Biochim Biophys Acta. 2014;1842:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Namikawa C, Shu-Ping Z, Vyselaar JR, Nozaki Y, Nemoto Y, Ono M, Akisawa N, Saibara T, Hiroi M, Enzan H. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | McCuskey RS, Ito Y, Robertson GR, McCuskey MK, Perry M, Farrell GC. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Fox ES, Brower JS, Bellezzo JM, Leingang KA. N-acetylcysteine and alpha-tocopherol reverse the inflammatory response in activated rat Kupffer cells. J Immunol. 1997;158:5418-5423. [PubMed] |
| 31. | Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2088] [Cited by in RCA: 2043] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 32. | Park S, Hong SM, Ahn IS. Exendin-4 and exercise improve hepatic glucose homeostasis by promoting insulin signaling in diabetic rats. Metabolism. 2010;59:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |