Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2460
Peer-review started: September 25, 2015
First decision: October 14, 2015
Revised: November 6, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: February 28, 2016
Processing time: 155 Days and 20.3 Hours
Currently, gastric cancer (GC) is one of the most frequently diagnosed neoplasms, with a global burden of 723000 deaths in 2012. It is the third leading cause of cancer-related death worldwide. There are numerous possible factors that stimulate the pro-carcinogenic activity of important genes. These factors include genetic susceptibility expressed in a single-nucleotide polymorphism, various acquired mutations (chromosomal instability, microsatellite instability, somatic gene mutations, epigenetic alterations) and environmental circumstances (e.g., Helicobcter pylori infection, EBV infection, diet, and smoking). Most of the aforementioned pathways overlap, and authors agree that a clear-cut pathway for GC may not exist. Thus, the categorization of carcinogenic events is complicated. Lately, it has been claimed that research on early-onset gastric carcinoma (EOGC) and hereditary GC may contribute towards unravelling some part of the mystery of the GC molecular pattern because young patients are less exposed to environmental carcinogens and because carcinogenesis in this setting may be more dependent on genetic factors. The comparison of various aspects that differ and coexist in EOGCs and conventional GCs might enable scientists to: distinguish which features in the pathway of gastric carcinogenesis are modifiable, discover specific GC markers and identify a specific target. This review provides a summary of the data published thus far concerning the molecular characteristics of GC and highlights the outstanding features of EOGC.
Core tip: There are numerous factors that may trigger gastric carcinogenesis. They include genetic susceptibility, acquired mutations and favourable environmental circumstances, which combine and multiply within the lifetime. Therefore, the incidence of gastric cancer is the highest among the elderly. Conversely, young patients are exposed to environmental carcinogens for a short period, so they are a reliable subgroup in which to study primary genetic alterations. This review provides a summary of the data published thus far concerning the molecular characteristics of gastric cancer and highlights the outstanding features of early-onset gastric cancer.
- Citation: Skierucha M, Milne AN, Offerhaus GJA, Polkowski WP, Maciejewski R, Sitarz R. Molecular alterations in gastric cancer with special reference to the early-onset subtype. World J Gastroenterol 2016; 22(8): 2460-2474
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2460.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2460
Currently, gastric cancer (GC) is one of the most frequently diagnosed neoplasms worldwide.
Its incidence rate in 2012 reached approximately 140000 new cases in Europe and approximately 952000 worldwide. In Europe, GC is responsible for approximately 107000 deaths annually, placing it as the fourth most common cause of cancer-related death. Globally, GC caused 723000 deaths in 2012, making it the third leading cause of cancer-related death worldwide[1,2]. Fortunately, the global incidence of GC has been decreasing since the Second World War[3].
The most common classification used, the Lauren classification, differentiates between intestinal and diffuse types of GCs[4]. These two types of GCs vary not only in morphology but also in epidemiology, progression pattern, genetics and clinical picture. Recently, it has been suggested that tumour location also matters because there appears to be a difference between proximal and distal non-diffuse GCs due to their distinct gene expression levels[5,6].
Despite the scientific tendency to consider the intestinal and diffuse GC types as separate entities, clinically, all of them are treated similarly. For the time being, slow but satisfactory effects[7-9] have resulted in decreasing the overall incidence of GC. However, there are supporters of the theory that more individualized treatment would be more beneficial[5].
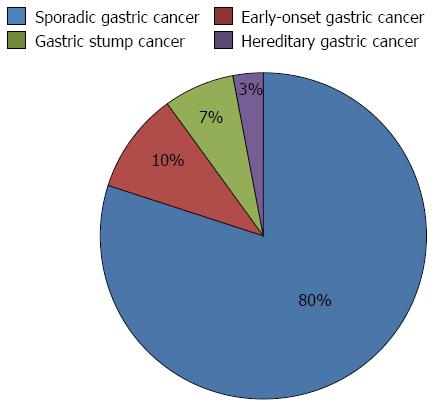
Alternatively, GCs can be divided into early-onset gastric carcinoma (EOGC)-occurring in patients at the age of 45 years or younger[10]-and conventional GCs, which liberally encompass the remaining group of patients. Sometimes, there are also special subgroups that are distinguished: patients with hereditary diffuse GC and patients with gastric stump cancer; however, these two types can overlap with both EOGC and conventional gastric cancer[11] (Figure 1).
There are many possible alterations that eventually stimulate the pro-carcinogenic activity of genes. Most of these pathways overlap, and authors agree that a clear-cut pattern of mutations in GCs does not exist[10]; thus, the categorization of carcinogenic events is highly complicated. The current scientific challenge is to recognize which alterations of GC are crucial, what are the relationships between these alterations and how to prevent their incidence.
Recently, it has been claimed that research on EOGC and hereditary GCs may contribute towards unravelling some part of the mystery of the GC molecular pattern because younger patients are less exposed to environmental carcinogens, and their neoplasms rely more on genetic and molecular factors[10].
The comparison of various aspects that differ and coexist in EOGCs and conventional GCs might enable scientists to distinguish which features in the pathway of the gastric carcinogenesis are modifiable, discover specific GC markers and identify a target for specifically directed treatment.
This review summarizes the data published thus far regarding the molecular characteristics of GC and highlights the outstanding features of EOGC.
EOGC, as mentioned earlier, may pave the way for elucidating the primary alterations that initiate the gastric malignant process. The occurrence of gastric cancer in young patients could be explained in at least a few ways. Younger patients are exposed to the same environmental factors as the rest of the population; however because of some unknown reasons, they are more prone to develop gastric tumours at an earlier age. First, their molecular susceptibility to gastric carcinogenesis is to blame[12], probably with a hereditary component[13]. There are reports that an early diagnosis is associated with a higher GC risk for other family members[14] and that a paternal history of GC correlates with an earlier diagnosis than in the general population[15]. The limitations of the hypothesis concerning the EOGC hereditary background are environmental risk factors shared by members of one family[16].
From another point of view, the early occurrence of GC may not be a fault of the host but of a specific tumour that is very aggressive, skips the consecutive steps of traditional neoplastic development and does not stay latent for years but, instead, progresses rapidly after the first alterations. The latter hypothesis would be supported by a poorer prognosis in younger patients[17]. However, others have claimed that prognosis, similar to that in older patients, depends on an early diagnosis and curative resection[18,19].
Nevertheless, Kwak et al[15] suggested that there is a third, pragmatic reason for the diagnosis of EOGC likely concerning patients with a family history of GC. These patients undergo screening earlier, or, unlike the general population (screening standards depend on the country). Consequently, their tumours are recognized at an early stage; however, under common circumstances, these tumours would be found later, at an older age, when the cancer has caused symptoms.
In approximately 30%-40% of cases of hereditary diffuse gastric cancer (HDGC), an E-cadherin (CDH1) germline mutation is detectable[20]. CDH1 is the gene that encodes E-cadherin, the protein that is essential in cell-cell adhesion[21]. A high percentage (approximately 80%) of CDH1 mutation carriers generate premature termination codons, which induce nonsense-mediated decay (NMD), resulting in impaired transcript loss. This predisposition can then be the cause of the early onset of GC in CDH1-mutation carriers[22]. It has been proven that heterozygous germline mutations of CDH1 causes an autosomal dominant condition that is associated with HDGC[23-25]. The mutation may be caused by several mechanisms, including deletion, frameshift mutation, missense mutation and splice-site mutation. Moreover, the mutation in HDGC may affect any part of the CDH1 gene length[26], including the untranslated regions[24], which distinguish HDGC from sporadic diffuse GC where mutations are observed in exons 7-9 of the E-cadherin gene[26].
It has been observed that the penetration of mutations is high, between 70%-80%[27]. The remaining allele is deactivated by mutation and loss of function by various mechanisms. The most frequent method is methylation[28-31]. However, as long as the remaining allele works properly, the gastric mucosa remains normal. Arguably, the second hit could occur simultaneously in multiple cells in cooperation with micro-environmental cofactors[32,33], possibly explaining the multifocal growth pattern of the tumour[34].
The loss of E-cadherin function together with overexpression of epidermal growth factor receptor (EGFR) is the most common alteration in diffuse-type GC. Mutant E-cadherin binds EGFR poorly, or the bound complex is less stable. This may enhance EGFR surface motility and facilitate its activation[35].
Two-thirds of the families susceptible to HGC lack the CDH1 mutation, and their predisposition remains genetically unexplained. It is likely caused by alterations in other genes. Oliviera et al[36] suggested that there may be a need to screen these families for a TP53 mutation. Majewski et al[37] identified a mutation in the CTNNA1 gene encoding the α-E-catenin protein, which functions in the same complex as E-cadherin. However, this alternative mutation has not reoccurred in other studies, likely because of the founder effect or other unrecognized factors, such as geographical influences[38].
The role of CDH1 mutation needs further investigation. It was reported that the absence of E-cadherin in a transgenic mouse model did not cause gastric malignancy. The authors suggested that the loss of E-cadherin induces possible pre-cancerous lesions in the gastric mucosa but may not be sufficient for its malignant conversion[39]. It is possible that environmental influences modify the disease risk in susceptible individuals[33].
Another example of HGC occurs in Lynch syndrome (hereditary nonpolyposis colon cancer, HNPCC). The essence of this disease is a mutation within mismatch repair genes (MSH2, MSH6, PMS2 or MLH1), leading to an increased mutation rate in oncogenes and tumour suppressor genes and the development of a neoplasm. Frequent extracolonic locations of tumours in HNPCC are the stomach and uterus[40]. According to some reports, HNPCC increases the lifetime risk of gastric cancer up to 7%[41].
Other rarely occurring mutations connected with HGC are: TP53 mutation in Li-Fraumeni syndrome[42,43], STK11 mutation in Peutz-Jeghers syndrome[44,45], APC mutation in familial adenomatous polyposis[46,47] and BRCA2 mutation[48].
The cause of GC is multifactorial and includes: (1) genetic susceptibility expressed in a single-nucleotide polymorphism (SNP); (2) various acquired mutations [e.g. chromosomal instability (CIN), microsatellite instability (MSI), somatic gene mutations, epigenetic alterations] that are heterogeneous intra- and interpatient[38]; and (3) favourable environmental circumstances [e.g., diet, Helicobacter pylori (H. pylori) infection, EBV infection, and smoking][49,50].
Nishimura[51] assessed the number of genomic alterations that can start malignant gastric processes to be 4.18, based on the frequencies of the major genome alterations, which represent the expected value of the occurrence.
Single-nucleotide polymorphism: One in 100-300 nucleotides in the human genome varies. These widely known polymorphisms, known as SNPs, are responsible for 90% of genetic variability[52]. Genetic resemblance suggests ethnic kinship. Some variations, together with environmental triggers, make the carrier more prone to develop a range of diseases, including GC. Moreover, the coexistence of some SNPs even accumulates the risk of GC[50,53]. This is a reasonable explanation of the high incidence of GC in the Japanese population, which, unlike the European population, has a low incidence of H. pylori colonization. However, > 60% of the Japanese population carry at least one high-risk GC-associated SNP[54].
The candidate SNPs in GC concern genes involved in: (1) the inflammatory response [interleukin (IL)-1[55-60], IL-17[61-64], tumor necrosis factor (TNF) α[65,66], toll-like receptors (TLRs)[67,68]]; (2) protection against invading pathogens (MUC1)[69,70]; (3) cell-to-cell adhesion (CDH1)[71-73]; (4) the repair of DNA damage related to H. pylori (XPA, XPC, ERCC2)[32,74-76]; (5) the metabolism of foliate (methylenetetrahydrofolate reductase)[77,78]; (6) the metabolism of polycyclic aromatic hydrocarbons (GSTT1, SULT1A1, NAT2, EPHX1)[79,80]; (7) the metabolism of oestrogen and androgen[81]; (8) the metabolism of xenobiotics (Cyp2e1)[82]; and (9) other functions that are not fully understood, for example PSCA[83].
It has been reported that IL-1β-31*C, IL-1β-511*T and IL-1RN*2/*2 are variations of the IL-1 gene cluster that have the greatest importance in GC susceptibility in various ethnic populations[55,56], particularly among the Caucasian population[57-60]. However, there are also studies that undermine the role of these variations in GC development[84-86] and pertain to Irish[87], Swedish[88], German[89] and Japanese populations[90]. On the other hand, Sitarz et al[91] showed that the IL-1β-31*C allele promoter polymorphism is significantly associated with gastric stump cancer, whereas it does not influence the occurrence of any type of sporadic GC. The authors emphasize that the differences between the studies may be due to many factors, such as heterogeneous patient groups, different populations, sample sizes, different clinical characteristics, controls drawn from high-risk areas for chronic gastritis, confounding factors from other environmental cofactors, interactions with other genes regulating inflammatory responses and others[87]. Therefore, the issue needs further investigation and a wider comparable analysis.
The IL-17 187G>A polymorphism is associated with a higher risk of developing GC based on H. pylori colonization[61-64].
Gorouhi et al[65] reported that the TNFα-308AA genotype was associated with a statistically significant increased risk of GC, whereas TNFa-857TT raised attention and required more studies. These results were supported by the parallel meta-analysis of Zhang et al[66] and seemed to concern the Caucasian population in particular.
TLR polymorphisms are linked to gastrointestinal malignancies[67]. TLR4 may increase the risk of non-cardia cancer[68].
Mucins are a family of proteins that maintain the integrity of the mucus layer and protect it from environmental invaders. Due to their vast role in regulating cell homeostasis and their role in several cancers, they have been categorized as oncoproteins[92-95]. The rs4072037(G>A) polymorphism plays a role in increasing the risk of gastric malignancy. The G allele version seems to be protective, It causes MUC1 under-expression[70], resulting in better conditions for H. pylori to invade and cause extensive inflammation. However, it seems that alterations of MUC regions do not cause clinical progression in patients with a premalignant phenotype[96].
Autosomal-dominant mutations of CDH1 are the cause of HDGC. However, it seems that the CDH1 polymorphism is also significant in sporadic GC. It has been reported that the promoter polymorphism at position -160 C/A of CDH1 importantly increases the risk of GC in Europeans, whereas Asians seem to be tolerant to this polymorphism[71]. Jenab et al[72] showed that three CDH1 polymorphisms within the CDH1-160C/A haplotype block the increased risk of GC in smokers but not in never-smokers.
Other SNPs concern those of methylenetetrahydrofolate reductase, which has demonstrated 281 polymorphic variants. MTHFR 677C>T and MTHFR 1298A>C were shown to be associated with GCs in East Asians[77,78]. Another SNP, CYP2E1*2 (C2), was reported to enhance the GC risk in the Asian population[82].
Similarly, a polymorphism in exon 1 of PSCA was shown to increase the risk of diffuse GC and to distinguish it from the intestinal subtype[83,97-101]. It is likely that PSCA protein regulates gastric epithelial cell proliferation; therefore, the down-regulation of PSCA may lead to pathological cell division. The SNPs concern the alleles rs2976392 and rs2294008[83]. However, other studies have reported conflicting data, hindering the interpretation. The issue remains open to further research.
Chromosomal instability: The term chromosomal instability comprises altered DNA copy number (aneuploidy) and various changes in chromosome regions, such as translocation, amplification, deletion or the loss of one allele in a pair [loss of heterozygosity (LOH)][102,103]. Altogether, CIN results in the loss or gain of function of some genes, including oncogenes and tumour suppressor genes.
CIN is an inherent part of carcinogenesis that occurs at each stage of the oncologic diseases[103]. It is not permanent, differs within geographical regions[104] and increases with disease progression[102,105]. Therefore, recognizing frequent CIN patterns in GC can result in improving early diagnosis, staging and treatment.
It was reported that intestinal GC correlates with the gain of copy number at 8q, 17q and 20q[105-109] and with amplification and overexpression of EGF and c-ErbB2, which are the molecules involved in self-sufficient growth[110,111]. Diffuse GC is characterized by a gain of copy number at 12q and 13q[105-109] and with amplification of FGFR[112,113]. Both subtypes display overexpression of HGF and c-myc[112,114,115] and amplification of the HER2 gene (ERBB2). The latter feature is of particular clinical interest because HER2 can be therapeutically blocked by monoclonal antibodies[116,117]. GC patients treated with a humanized antibody against HER2 (trastuzumab) benefit with a 2.5-mo longer survival than the group treated with standard chemotherapy[118]. However, thereafter, the disease progresses, and resistance develops, raising doubt about the usefulness of this agent[50].
Other changes that promote uncontrolled cell growth are inversions causing the generation of the SLC1A2-CD44 fusion protein[119] and the ROS1 gene rearrangement. However, the latter alteration rarely occurs in GCs (< 1%) and differentiates the subgroup of patients who hypothetically may be treated with kinase inhibitors[119,120].
LOH is a common event in GC. The frequently occurring LOH in the genes APC, TP53 and NME1 play a possible role in evaluation of a patient’s clinical status[121,122]. Gains at chromosomes 17q, 19q and 20q are distinctive for GCs in young patients[10,123].
Microsatellite instability: MSI is defined as the presence of small deletions or expansions in a tumour’s DNA within short tandem repeats (microsatellite regions) and do not match normal DNA.
MSI is not only present in HNPCC but occurs in up to every second sporadic GC[50,124]. In GCs, MSI is mostly caused by the epigenetic alterations in the mismatch repair genes (MMRs)[125,126]. Consequently, the impaired mismatch repair system fails to fulfil its task, resulting in multiple mutations within cell growth-regulating genes (TGF-βRII, IGFIIR,RIZ, TCF4, DP2), apoptosis genes (BAX, BCL10, FAS, CASPASE5, APAF1) and DNA repair genes (hMSH6, hMSH3, MED1, RAD50, BLM, ATR, MRE11)[125,127-130]. However, the inactivation of mismatch repair genes, by itself, is thought to be insufficient to induce carcinogenesis but might be a coexistent factor[126].
The high incidence of microsatellite instability in GCs (MSI-H GC) is more likely to occur at an antral location, in the intestinal type, in the expanding type, and with H. pylori seropositivity, and correlates with a lower prevalence of lymph-node metastases[131-133]. Moreover, MSI correlates with a lower incidence of TP53 mutations[133] and is characterized by a better survival rate than with tumours with low levels of MSI[134,135]. It is possible that high levels of MSI indirectly cause nonspecific immunological reactions in the hosts, resulting in tumour cell elimination[136].
MSI seems to be a promising tool to identify patients with genetic instability and patients with precancerous lesions because it occurs in both gastric adenoma and intestinal metaplasia[126].
Epigenetic alterations: Epigenetic alterations are responsible for the diversity in the expression of a gene and are not caused by changes in DNA sequences but by modifications outside DNA, such as DNA CpG island hypermethylation [CpG island methylator phenotype, (CIMP)], hypomethylation, histone modification, chromatin remodelling or miRNA changes. The literature dedicated to GC highlights the role of CpG island methylation and miRNA.
In GCs, CpG island methylation involves primarily the promoters of the CDH1, CDKN2A, CDKN2B and hMLH1 genes and results in the down-expression of their products (E-cadherin, p16, p15, MLH1)[137,138]. CpG island methylation seems to frequently occur in GC cells, regardless of their stem cell origin and independent of one another. Possibly, CpG island methylation carries the carcinogenic process a step further. This hypothesis would be consistent with the observation that promoter hypermethylation is accelerated with histopathological progression of malignancy, from chronic gastritis, intestinal metaplasia and adenoma to carcinoma[138-140].
miRNAs are short stable RNA segments that, despite noncoding characteristics, play a vast role in the regulation of gene expression. They attain this goal by binding to DNA or by inhibiting or degrading mRNA that is ready for translation. Altogether, they regulate approximately 60% of the coding genes; therefore, their role in GC seems to be significant[141].
miRNAs can act as oncogenes (oncomiRNAs), tumour suppressors (tsmiRNAs) or cellular pathway modulators, such as metastasis regulators (metastamiRNAs). Research over the last decade has identified numerous miRNAs that have varied roles in GC development.
Questions for the future include the following: are miRNA alterations necessary for tumour progression, can they be used as diagnostic and/or prognostic markers[141-145], can they be targeted pharmacologically[146] and can they influence the individual response to chemotherapy[147-149]?
Somatic gene mutations: In recent research[150], which is a part of The Cancer Genome Atlas Project, the authors suggested that both the rate of somatic mutations and their singularity should not be disregarded in the GC classification. In fact, they provided a roadmap for patient stratification and trials of targeted therapies. The authors of the study identified many mutations that are repeated in each subtype of GC but with different frequencies. Examples of the most common mutations occur in the genes TP53, CDH1, SMAD4, PIK3CA, RHOA, ARID1A, KRAS, MUC3, APC, ERBB1, PTEN, HLAB, and B2M.
Some of these alterations were investigated separately in earlier studies. Zang et al[151] reported that somatic inactivation of FAT4 and ARID1A may be the key to malignant events in GCs. Wang et al[152] suggested that ARID1A seems to be a good prognostic indicator because its alterations were clinically associated with better prognosis in a stage-independent manner. Other studies[153] proved that RHOA mutations occur specifically in diffuse GCs, so they are a potential therapeutic target for this poor-prognosis subtype of GC.
EBV is an infectious agent that occurs in epithelial cells of 9% of GCs[154]. However, the distribution of EBV-positive GCs varies globally[3]. EBV-positive tumours are associated with an extreme CIMP[150,155], and differ from the MSI subtype[156]. In Bass et al[150], all EBV-positive tumours lacked MLH1 alterations, characteristic of MSI[157]; however, they displayed promoter hypermethylation within the CDKN2A (p16INK4A) region, and most of them had mutations in diverse locations within the PIK3C1 gene, confirming previous reports[158,159]. This particular feature separates EBV-positive tumours from other GCs that display PIK3C1 mutations in 3%-42% but are localized in the kinase domain, exon 20[150].
Gastritis is the single most common cause of GC, and H. pylori, a class I carcinogen according to WHO classification[160], is the most common cause of gastritis[161,162]. Therefore, H. pylori plays a role in the environmental trigger that creates a favourable background for GC through several mechanisms. One of them is depleting the mucosa’s antioxidant competences[163], as shown in mouse models[164]. H. pylori was also reported to initiate the down-regulation of sonic hedgehog (Shh) expression, paving the way for early premalignant changes[165]. Shh is a protein that plays a role in cellular differentiation in gastric mucosa. Under expression of Shh promotes an intestinal phenotype by the upregulation of Cdx2, MUC2 and villin, which are intestine-related genes[32,166]. It seems that the levels of Shh expression fluctuate during the beginning of metaplasia to advanced cancer, and it is associated with tumour stage[167].
Moreover, H. pylori can promote intestinal transformation by the interaction of CagA (bacterial virulence factor) with E-cadherin[168]. It was also reported that decreased levels of E-cadherin may occur in relation to H. pylori infection[169].
According to the currently accepted hypothesis, GC develops from cancer stem cells (CSCs)[32]. However, under chronic inflammation, this role might be carried out by bone marrow-derived cells (BMDCs)[170-172].
Chronic inflammation alters the secretion of gastrin in gastric mucosa. Hypergastrinaemia and hypogastrinaemia are both suspected of being involved in the development of GC[168,173,174].
A proper inflammatory response is highly dependent on the condition of the immune system, which is also involved in GC. For example, it was reported that the CTLA-4 polymorphism attenuates the T-cell response and increases the risk of gastric cardia cancer[175]. The accumulation of regulatory T cells (Tregs), which are associated with CCL17 and CCL22 chemokines[176], reflects the clinical status because it correlates with regional lymph node metastasis and patient survival[177].
The role of elevated eosinophil levels remains uncertain. In low-risk areas, eosinophils are recruited by Th2 lymphocytes and act to prevent GC; however, in high-risk areas, they are attracted by Th1 lymphocytes and favour the spread of the lesions[178].
COX-2 overexpression is known to be an important mechanism in GC development. It occurs commonly, but remains uncertain why. Suggested mediators include the C/EBP-β transcription factor[115,179,180] and Wnt/β-catenin signalling pathway[181]. COX-2 overexpression particularly concerns adenocarcinomas[182], appears at early stage of carcinogenesis and is detected even in precursor lesions[115,183,184]. Silencing COX-2 by promoter hypermethylation or FOXP3[185] seems to protect against GC progression because it is correlated with longer remission and improved survival[186]. Therefore, COX-2 could be used as a prognostic indicator[187,188].
The COX-2 genotype also matters because the 1195AA COX-2 genotype was reported to increase the risk of GC more than twice, and, with coexistent H. pylori infection or smoking, even enhances malignant progression[189].
Non-steroidal anti-inflammatory drugs may disrupt the pathway of carcinogenesis dependent on COX-2 related processes. Their long-term use turns out to show a reduced risk of GC[190-192]. This group of drugs might be used in lymph node metastasis prophylaxis[193]. However, Sitarz et al[194] found that a reduction of COX-2 using nonsteroidal anti-inflammatory drugs in GC chemoprevention may be relevant only for older patients.
To consider EOGC as an independent oncologic problem, scientists must precisely differentiate it from sporadic GC. In 2007, Milne et al[10] summarized the distinctive features of EOGCs, compared with conventional GCs, as including female predominance, common multifocal growth and a diffuse phenotype without intestinal metaplasia. The molecular profile included the lack of MSI, infrequent loss of heterozygosity, infrequent loss of TFF1 expression, no loss of RUNX3, gains at chromosomes 17q, 19q and 20q and more frequent expression of low-molecular-weight isoforms of cyclin E.
Newer characteristics of EOGCs have been identified in recent reports. Clinical studies include the observation of Karim[195] that male predominance occurs among EOGC patients but decreases with age. Takatsu et al[196] reported a tendency to present lymph node metastases, a finding that was indirectly supported by studies of CDH1 variants[197]. Molecular alterations also include a new marker that is a genetic variant of rs10052016 at 5p15[198]. Moreover, Bacani et al[124] showed that MSI, in at least one marker, was found in 30% of EOGCs. They assessed that approximately 1% of EOGC is caused by germline MMR mutations. Carvalho et al[199] excluded RUNX3 as having a tumour suppressor function in EOGC, but other authors were less convinced that this is the case[10]. Sugimoto et al[200] was the first to describe that a de novo large genomic deletion of CDH1 was associated with EOGC.
GC is a heterogenic and complex problem (Tables 1, 2 and 3). The number of factors that influence its beginning and course is already overwhelming, and, in the light of modern technological possibilities, that number could increase exponentially. Moreover, various molecular alterations seem to overlap[126,201,202], additionally complicating the problem. However, it seems to be reasonable to consider that there are some early triggers that impair genome stability and predispose to a further avalanche of carcinogenic events[137].
| Factors1 | Sporadic gastric cancer | Ref. |
| SNP | IL-1, IL-17, TNFα, TLRs (inflammatory response) | [55-68] |
| MUC1 (protection against invaders) | [69,70] | |
| CDH1 (cell-to-cell adhesion) | [71-73] | |
| XPA, XPC, ERCC2 (repair of DNA damage related to H. pylori infection) | [32,74-76] | |
| MTHFR (metabolism of foliate) | [77,78] | |
| GSTT1, SULT1A1, NAT2, EPHX1 (metabolism of polycyclic aromatic hydrocarbons) | [79,80] | |
| Cyp2e1 (metabolism of xenobiotics) | [82] | |
| PSCA | [83] | |
| CIN | gain of copy number at 8q, 17q, 12q, 13q and 20q | [105-109] |
| amplification of EGF and c-ErbB2 | [110,111] | |
| amplification of FGFR | [112,113] | |
| amplification of ERBB2 | [116,117] | |
| overexpression of HGF and c-myc | [112,114,115] | |
| SLC1A2-CD44 fusion | [119] | |
| ROS1 rearrangement | [120] | |
| LOH | APC, TP53, NME1 | [121,122] |
| MSI | TGFβRII, IGFIIR,RIZ, TCF4, DP2 (cell growth-regulating genes) | [125,127-136] |
| BAX, BCL10, FAS, CASPASE5, APAF1 (apoptosis genes) | ||
| hMSH6, hMSH3, MED1, RAD50, BLM, ATR, MRE11 (DNA repair genes) | ||
| Somatic gene mutations | TP53, CDH1, SMAD4, PIK3CA, RHOA, ARID1A, KRAS, MUC3, APC, ERBB1, PTEN, HLAB, B2M, FAT4 | [150-153] |
| Epigenetic alterations | CpG island methylation of the promoters of CDH1, CDKN2A, CDKN2B and hMLH1 | [137,138] |
| miRNA variations | [141] | |
| Environment | Diet | [200] |
| H. pylori infection | [163,165,168] | |
| EBV infection | [150,154-156] | |
| Hyper/hypogastrinaemia | [168,173,174] | |
| Smoking | [200] | |
| Others | COX-2 overexpression | [182-189] |
| Factors | Early-onset gastric cancer | Ref. |
| SNP | rs10052016 at 5p15 | [198] |
| CIN | Gain of copy number at 17q, 19q, and 20q | [10] |
| No loss of RUNX3 | ||
| Infrequent loss of TFF1 expression | ||
| More frequent expression of low-molecular-weight isoforms of cyclin E | ||
| LOH | Infrequent LOH | [10] |
| MSI | Lack of MSI | [10] |
| vs 30% incidence | [124] | |
| Others | Low COX-2 expression | [10] |
| Male predominance | [195] | |
| Tendency to metastases | [196] |
| Factors | Hereditary gastric cancer | Ref. |
| Germline mutations | CDH1 | [23-34] |
| TP53 (Li-Fraumeni syndrome) | [36,42,43] | |
| CTNNA1 | [37] | |
| MSH2, MSH6, PMS2, MLH1 (Lynch syndrome) | [40,41] | |
| APC (Familial adenomatous polyposis) | [46,47] | |
| STK11 (Peutz-Jeghers syndrome) | [44,45] | |
| BRCA2 | [48] |
In our research, we focused on the early steps of GC development. Therefore, we favour the classification of GC that differentiates EOGC. Patients with this type of tumour are automatically deprived of many risk factors and molecular changes that appear with the passage of a patient’s and tumour’s life. Therefore, young patients present a relatively pure model of gastric carcinogenesis.
From the review of the latest literature, we conclude that defining characteristic factors of early-onset GC is in progress, and the issue needs further clarification.
P- Reviewer: Li Y, Liang H, Park WS, Tang SY S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
| 1. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3657] [Article Influence: 304.8] [Reference Citation Analysis (2)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20512] [Article Influence: 2051.2] [Reference Citation Analysis (20)] |
| 3. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 4. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 5. | Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437-447. [PubMed] |
| 7. | Power DG, Kelsen DP, Shah MA. Advanced gastric cancer--slow but steady progress. Cancer Treat Rev. 2010;36:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 9. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1291] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 10. | Milne AN, Sitarz R, Carvalho R, Carneiro F, Offerhaus GJ. Early onset gastric cancer: on the road to unraveling gastric carcinogenesis. Curr Mol Med. 2007;7:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Sitarz R, Kolasinska-Bzoma M, Polkowski W, Oferhaus GJA, Maciejewski R. Gastric cancer-topical problem. Zdr Publ. 2010;120:311-315. |
| 12. | Shanks AM, El-Omar EM. Helicobacter pylori infection, host genetics and gastric cancer. J Dig Dis. 2009;10:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Lindor NM, Rabe KG, Petersen GM, Chen H, Bapat B, Hopper J, Young J, Jenkins M, Potter J, Newcomb P. Parent of origin effects on age at colorectal cancer diagnosis. Int J Cancer. 2010;127:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Shin CM, Kim N, Yang HJ, Cho SI, Lee HS, Kim JS, Jung HC, Song IS. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44:e34-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Kwak HW, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Joo J, Ryu KW, Kim YW. Individual having a parent with early-onset gastric cancer may need screening at younger age. World J Gastroenterol. 2015;21:4592-4598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Theuer CP, de Virgilio C, Keese G, French S, Arnell T, Tolmos J, Klein S, Powers W, Oh T, Stabile BE. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg. 1996;172:473-476; discussion 476-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ramos-De la Medina A, Salgado-Nesme N, Torres-Villalobos G, Medina-Franco H. Clinicopathologic characteristics of gastric cancer in a young patient population. J Gastrointest Surg. 2004;8:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Medina-Franco H, Heslin MJ, Cortes-Gonzalez R. Clinicopathological characteristics of gastric carcinoma in young and elderly patients: a comparative study. Ann Surg Oncol. 2000;7:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Oliveira C, Seruca R, Carneiro F. Genetics, pathology, and clinics of familial gastric cancer. Int J Surg Pathol. 2006;14:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Grunwald GB. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993;5:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 201] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Karam R, Carvalho J, Bruno I, Graziadio C, Senz J, Huntsman D, Carneiro F, Seruca R, Wilkinson MF, Oliveira C. The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene. 2008;27:4255-4260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Carneiro F. Hereditary gastric cancer. Pathologe. 2012;33 Suppl 2:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. E-cadherin alterations in hereditary disorders with emphasis on hereditary diffuse gastric cancer. Prog Mol Biol Transl Sci. 2013;116:337-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Carneiro F, Oliveira C, Suriano G, Seruca R. Molecular pathology of familial gastric cancer, with an emphasis on hereditary diffuse gastric cancer. J Clin Pathol. 2008;61:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Berx G, Becker KF, Höfler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226-237. [PubMed] |
| 27. | Pharoah PD, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 28. | Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 327] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Oliveira C, de Bruin J, Nabais S, Ligtenberg M, Moutinho C, Nagengast FM, Seruca R, van Krieken H, Carneiro F. Intragenic deletion of CDH1 as the inactivating mechanism of the wild-type allele in an HDGC tumour. Oncogene. 2004;23:2236-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Oliveira C, Sousa S, Pinheiro H, Karam R, Bordeira-Carriço R, Senz J, Kaurah P, Carvalho J, Pereira R, Gusmão L. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology. 2009;136:2137-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Barber M, Murrell A, Ito Y, Maia AT, Hyland S, Oliveira C, Save V, Carneiro F, Paterson AL, Grehan N. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J Pathol. 2008;216:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 33. | McColl KE, El-Omar E. E-cadherin germline mutations and risk of gastric cancer. Gastroenterology. 2002;123:1406; author reply 1406-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Carneiro F, Huntsman DG, Smyrk TC, Owen DA, Seruca R, Pharoah P, Caldas C, Sobrinho-Simões M. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol. 2004;203:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Bremm A, Walch A, Fuchs M, Mages J, Duyster J, Keller G, Hermannstädter C, Becker KF, Rauser S, Langer R. Enhanced activation of epidermal growth factor receptor caused by tumor-derived E-cadherin mutations. Cancer Res. 2008;68:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Oliveira C, Ferreira P, Nabais S, Campos L, Ferreira A, Cirnes L, Alves CC, Veiga I, Fragoso M, Regateiro F. E-Cadherin (CDH1) and p53 rather than SMAD4 and Caspase-10 germline mutations contribute to genetic predisposition in Portuguese gastric cancer patients. Eur J Cancer. 2004;40:1897-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Majewski IJ, Kluijt I, Cats A, Scerri TS, de Jong D, Kluin RJ, Hansford S, Hogervorst FB, Bosma AJ, Hofland I. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol. 2013;229:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Schuetz JM, Leach S, Kaurah P, Jeyes J, Butterfield Y, Huntsman D, Brooks-Wilson AR. Catenin family genes are not commonly mutated in hereditary diffuse gastric cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2272-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Mimata A, Fukamachi H, Eishi Y, Yuasa Y. Loss of E-cadherin in mouse gastric epithelial cells induces signet ring-like cells, a possible precursor lesion of diffuse gastric cancer. Cancer Sci. 2011;102:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Lynch HT, Smyrk T, Lynch JF. Overview of natural history, pathology, molecular genetics and management of HNPCC (Lynch Syndrome). Int J Cancer. 1996;69:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, Myrhøj T, Sunde L, Wijnen JT, Lynch HT. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 42. | Masciari S, Dewanwala A, Stoffel EM, Lauwers GY, Zheng H, Achatz MI, Riegert-Johnson D, Foretova L, Silva EM, Digianni L. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Yamada H, Shinmura K, Okudela K, Goto M, Suzuki M, Kuriki K, Tsuneyoshi T, Sugimura H. Identification and characterization of a novel germ line p53 mutation in familial gastric cancer in the Japanese population. Carcinogenesis. 2007;28:2013-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 508] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 45. | van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258-164; author reply 1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 46. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 47. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 48. | Jakubowska A, Nej K, Huzarski T, Scott RJ, Lubiński J. BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br J Cancer. 2002;87:888-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47134] [Article Influence: 3366.7] [Reference Citation Analysis (5)] |
| 50. | McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 51. | Nishimura T. Total number of genome alterations in sporadic gastrointestinal cancer inferred from pooled analyses in the literature. Tumour Biol. 2008;29:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Collins FS, Brooks LD, Chakravarti A. A DNA polymorphism discovery resource for research on human genetic variation. Genome Res. 1998;8:1229-1231. [PubMed] |
| 53. | Saeki N, Saito A, Choi IJ, Matsuo K, Ohnami S, Totsuka H, Chiku S, Kuchiba A, Lee YS, Yoon KA. A functional single nucleotide polymorphism in mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology. 2011;140:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Saeki N, Ono H, Sakamoto H, Yoshida T. Genetic factors related to gastric cancer susceptibility identified using a genome-wide association study. Cancer Sci. 2013;104:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Kimang’a AN. IL-1B-511 Allele T and IL-1RN-L/L Play a Pathological Role in Helicobacter Pylori (H. Pylori) Disease Outcome in the African Population. Ethiop J Health Sci. 2012;22:163-169. [PubMed] |
| 56. | Zhao JD, Geng PL, Li ZQ, Cui S, Zhao JH, Wang LJ, Li JZ, Ji FX, Li GY, Shen GS. Associations between interleukin-1 polymorphisms and gastric cancers among three ethnicities. World J Gastroenterol. 2012;18:7093-7099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Camargo MC, Mera R, Correa P, Peek RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 58. | Vincenzi B, Patti G, Galluzzo S, Pantano F, Venditti O, Santini D, Ruzzo A, Schiavon G, Caraglia M, Marra M. Interleukin 1beta-511T gene (IL1beta) polymorphism is correlated with gastric cancer in the Caucasian population: results from a meta-analysis. Oncol Rep. 2008;20:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Loh M, Koh KX, Yeo BH, Song CM, Chia KS, Zhu F, Yeoh KG, Hill J, Iacopetta B, Soong R. Meta-analysis of genetic polymorphisms and gastric cancer risk: variability in associations according to race. Eur J Cancer. 2009;45:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1604-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Qinghai Z, Yanying W, Yunfang C, Xukui Z, Xiaoqiao Z. Effect of interleukin-17A and interleukin-17F gene polymorphisms on the risk of gastric cancer in a Chinese population. Gene. 2014;537:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Zhang X, Zheng L, Sun Y, Zhang X. Analysis of the association of interleukin-17 gene polymorphisms with gastric cancer risk and interaction with Helicobacter pylori infection in a Chinese population. Tumour Biol. 2014;35:1575-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Rafiei A, Hosseini V, Janbabai G, Ghorbani A, Ajami A, Farzmandfar T, Azizi MD, Gilbreath JJ, Merrell DS. Polymorphism in the interleukin-17A promoter contributes to gastric cancer. World J Gastroenterol. 2013;19:5693-5699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Shibata T, Tahara T, Hirata I, Arisawa T. Genetic polymorphism of interleukin-17A and -17F genes in gastric carcinogenesis. Hum Immunol. 2009;70:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Gorouhi F, Islami F, Bahrami H, Kamangar F. Tumour-necrosis factor-A polymorphisms and gastric cancer risk: a meta-analysis. Br J Cancer. 2008;98:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Zhang J, Dou C, Song Y, Ji C, Gu S, Xie Y, Mao Y. Polymorphisms of tumor necrosis factor-alpha are associated with increased susceptibility to gastric cancer: a meta-analysis. J Hum Genet. 2008;53:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 68. | Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, Vaughan TL, McColl KE, Lissowska J, Zatonski W. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 69. | Zheng L, Zhu C, Gu J, Xi P, Du J, Jin G. Functional polymorphism rs4072037 in MUC1 gene contributes to the susceptibility to gastric cancer: evidence from pooled 6,580 cases and 10,324 controls. Mol Biol Rep. 2013;40:5791-5796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Xu Q, Yuan Y, Sun LP, Gong YH, Xu Y, Yu XW, Dong NN, Lin GD, Smith PN, Li RW. Risk of gastric cancer is associated with the MUC1 568 A/G polymorphism. Int J Oncol. 2009;35:1313-1320. [PubMed] |
| 71. | Wang GY, Lu CQ, Zhang RM, Hu XH, Luo ZW. The E-cadherin gene polymorphism 160C-& gt; A and cancer risk: A HuGE review and meta-analysis of 26 case-control studies. Am J Epidemiol. 2008;167:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Jenab M, McKay JD, Ferrari P, Biessy C, Laing S, Munar GM, Sala N, Peña S, Crusius JB, Overvad K. CDH1 gene polymorphisms, smoking, Helicobacter pylori infection and the risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Eur J Cancer. 2008;44:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Zhang B, Pan K, Liu Z, Zhou J, Gu L, Ji J, Ma J, You WC, Deng D. Genetic polymorphisms of the E-cadherin promoter and risk of sporadic gastric carcinoma in Chinese populations. Cancer Epidemiol Biomarkers Prev. 2008;17:2402-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Dong Z, Guo W, Zhou R, Wan L, Li Y, Wang N, Kuang G, Wang S. Polymorphisms of the DNA repair gene XPA and XPC and its correlation with gastric cardiac adenocarcinoma in a high incidence population in North China. J Clin Gastroenterol. 2008;42:910-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Capellá G, Pera G, Sala N, Agudo A, Rico F, Del Giudicce G, Plebani M, Palli D, Boeing H, Bueno-de-Mesquita HB. DNA repair polymorphisms and the risk of stomach adenocarcinoma and severe chronic gastritis in the EPIC-EURGAST study. Int J Epidemiol. 2008;37:1316-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Li WQ, Zhang L, Ma JL, Zhang Y, Li JY, Pan KF, You WC. Association between genetic polymorphisms of DNA base excision repair genes and evolution of precancerous gastric lesions in a Chinese population. Carcinogenesis. 2009;30:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Zintzaras E. Association of methylenetetrahydrofolate reductase (MTHFR) polymorphisms with genetic susceptibility to gastric cancer: a meta-analysis. J Hum Genet. 2006;51:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, Peters U. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA. 2008;299:2423-2436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 79. | Boccia S, Sayed-Tabatabaei FA, Persiani R, Gianfagna F, Rausei S, Arzani D, La Greca A, D’Ugo D, La Torre G, van Duijn CM. Polymorphisms in metabolic genes, their combination and interaction with tobacco smoke and alcohol consumption and risk of gastric cancer: a case-control study in an Italian population. BMC Cancer. 2007;7:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Agudo A, Sala N, Pera G, Capellá G, Berenguer A, García N, Palli D, Boeing H, Del Giudice G, Saieva C. Polymorphisms in metabolic genes related to tobacco smoke and the risk of gastric cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:2427-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Freedman ND, Ahn J, Hou L, Lissowska J, Zatonski W, Yeager M, Chanock SJ, Chow WH, Abnet CC. Polymorphisms in estrogen- and androgen-metabolizing genes and the risk of gastric cancer. Carcinogenesis. 2009;30:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Boccia S, De Lauretis A, Gianfagna F, van Duijn CM, Ricciardi G. CYP2E1PstI/RsaI polymorphism and interaction with tobacco, alcohol and GSTs in gastric cancer susceptibility: A meta-analysis of the literature. Carcinogenesis. 2007;28:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, Kato S. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 84. | Kamangar F, Cheng C, Abnet CC, Rabkin CS. Interleukin-1B polymorphisms and gastric cancer risk--a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | García-González MA, Lanas A, Quintero E, Nicolás D, Parra-Blanco A, Strunk M, Benito R, Angel Simón M, Santolaria S, Sopeña F. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol. 2007;102:1878-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | He B, Zhang Y, Pan Y, Xu Y, Gu L, Chen L, Wang S. Interleukin 1 beta (IL1B) promoter polymorphism and cancer risk: evidence from 47 published studies. Mutagenesis. 2011;26:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Murphy G, Thornton J, McManus R, Swan N, Ryan B, Hughes DJ, O’Morain CA, O’Sullivan M. Association of gastric disease with polymorphisms in the inflammatory-related genes IL-1B, IL-1RN, IL-10, TNF and TLR4. Eur J Gastroenterol Hepatol. 2009;21:630-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Persson C, Engstrand L, Nyrén O, Hansson LE, Enroth H, Ekström AM, Ye W. Interleukin 1-beta gene polymorphisms and risk of gastric cancer in Sweden. Scand J Gastroenterol. 2009;44:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Wex T, Leodolter A, Bornschein J, Kuester D, Kähne T, Kropf S, Albrecht C, Naumann M, Roessner A, Malfertheiner P. Interleukin 1 beta (IL1B) gene polymorphisms are not associated with gastric carcinogenesis in Germany. Anticancer Res. 2010;30:505-511. [PubMed] |
| 90. | Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol. 2001;36:696-699. [PubMed] |
| 91. | Sitarz R, de Leng WW, Polak M, Morsink FH, Bakker O, Polkowski WP, Maciejewski R, Offerhaus GJ, Milne AN. IL-1B -31T& gt; C promoter polymorphism is associated with gastric stump cancer but not with early onset or conventional gastric cancers. Virchows Arch. 2008;453:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1118] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 93. | Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Boltin D, Niv Y. Mucins in Gastric Cancer - An Update. J Gastrointest Dig Syst. 2013;3:15519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 96. | Marín F, Bonet C, Muñoz X, García N, Pardo ML, Ruiz-Liso JM, Alonso P, Capellà G, Sanz-Anquela JM, González CA. Genetic variation in MUC1, MUC2 and MUC6 genes and evolution of gastric cancer precursor lesions in a long-term follow-up in a high-risk area in Spain. Carcinogenesis. 2012;33:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Qiao L, Feng Y. Genetic variations of prostate stem cell antigen (PSCA) contribute to the risk of gastric cancer for Eastern Asians: a meta-analysis based on 16792 individuals. Gene. 2012;493:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Shi D, Wang S, Gu D, Wu D, Wang M, Chu H, Tong N, Ma L, Zhong D, Zhang Z. The PSCA polymorphisms derived from genome-wide association study are associated with risk of gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Wang T, Zhang L, Li H, Wang B, Chen K. Prostate stem cell antigen polymorphisms and susceptibility to gastric cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Zhang T, Chen YN, Wang Z, Chen JQ, Huang S. Effect of PSCA gene polymorphisms on gastric cancer risk and survival prediction: A meta-analysis. Exp Ther Med. 2012;4:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Gu X, Zhang W, Xu L, Cai D. Quantitative assessment of the influence of prostate stem cell antigen polymorphisms on gastric cancer risk. Tumour Biol. 2014;35:2167-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Hudler P. Genetic aspects of gastric cancer instability. ScientificWorldJournal. 2012;2012:761909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 103. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 2818] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 104. | Buffart TE, Louw M, van Grieken NC, Tijssen M, Carvalho B, Ylstra B, Grabsch H, Mulder CJ, van de Velde CJ, van der Merwe SW. Gastric cancers of Western European and African patients show different patterns of genomic instability. BMC Med Genomics. 2011;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Suzuki K, Ohnami S, Tanabe C, Sasaki H, Yasuda J, Katai H, Yoshimura K, Terada M, Perucho M, Yoshida T. The genomic damage estimated by arbitrarily primed PCR DNA fingerprinting is useful for the prognosis of gastric cancer. Gastroenterology. 2003;125:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Weiss MM, Kuipers EJ, Postma C, Snijders AM, Pinkel D, Meuwissen SG, Albertson D, Meijer GA. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell Oncol. 2004;26:307-317. [PubMed] |
| 107. | Tsukamoto Y, Uchida T, Karnan S, Noguchi T, Nguyen LT, Tanigawa M, Takeuchi I, Matsuura K, Hijiya N, Nakada C. Genome-wide analysis of DNA copy number alterations and gene expression in gastric cancer. J Pathol. 2008;216:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Tomioka N, Morita K, Kobayashi N, Tada M, Itoh T, Saitoh S, Kondo M, Takahashi N, Kataoka A, Nakanishi K. Array comparative genomic hybridization analysis revealed four genomic prognostic biomarkers for primary gastric cancers. Cancer Genet Cytogenet. 2010;201:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Grabsch HI, Tan P. Gastric cancer pathology and underlying molecular mechanisms. Dig Surg. 2013;30:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 110. | Park JB, Rhim JS, Park SC, Kimm SW, Kraus MH. Amplification, overexpression, and rearrangement of the erbB-2 protooncogene in primary human stomach carcinomas. Cancer Res. 1989;49:6605-6609. [PubMed] |
| 111. | Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283-287. [PubMed] |
| 112. | Hara T, Ooi A, Kobayashi M, Mai M, Yanagihara K, Nakanishi I. Amplification of c-myc, K-sam, and c-met in gastric cancers: detection by fluorescence in situ hybridization. Lab Invest. 1998;78:1143-1153. [PubMed] |
| 113. | Hattori Y, Odagiri H, Nakatani H, Miyagawa K, Naito K, Sakamoto H, Katoh O, Yoshida T, Sugimura T, Terada M. K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc Natl Acad Sci USA. 1990;87:5983-5987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | Lee JH, Han SU, Cho H, Jennings B, Gerrard B, Dean M, Schmidt L, Zbar B, Vande Woude GF. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947-4953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 115. | Milne AN, Carvalho R, Morsink FM, Musler AR, de Leng WW, Ristimäki A, Offerhaus GJ. Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Mod Pathol. 2006;19:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Okines AF, Cunningham D. Trastuzumab: a novel standard option for patients with HER-2-positive advanced gastric or gastro-oesophageal junction cancer. Therap Adv Gastroenterol. 2012;5:301-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Gunturu KS, Woo Y, Beaubier N, Remotti HE, Saif MW. Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Ther Adv Med Oncol. 2013;5:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 118. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5324] [Article Influence: 354.9] [Reference Citation Analysis (3)] |
| 119. | Tao J, Deng NT, Ramnarayanan K, Huang B, Oh HK, Leong SH, Lim SS, Tan IB, Ooi CH, Wu J. CD44-SLC1A2 gene fusions in gastric cancer. Sci Transl Med. 2011;3:77ra30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 120. | Lee J, Lee SE, Kang SY, Do IG, Lee S, Ha SY, Cho J, Kang WK, Jang J, Ou SH. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 121. | Gazvoda B, Juvan R, Zupanic-Pajnic I, Repse S, Ferlan-Marolt K, Balazic J, Komel R. Genetic changes in Slovenian patients with gastric adenocarcinoma evaluated in terms of microsatellite DNA. Eur J Gastroenterol Hepatol. 2007;19:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Karim S, Mirza Z, Naseer MI, Al-Qahtani MH, Ali A. Clinicopathological characteristics and chronology of p53 expression in the development of gastric cancer. Hepatogastroenterology. 2013;60:2113-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Varis A, van Rees B, Weterman M, Ristimäki A, Offerhaus J, Knuutila S. DNA copy number changes in young gastric cancer patients with special reference to chromosome 19. Br J Cancer. 2003;88:1914-1919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Bacani J, Zwingerman R, Di Nicola N, Spencer S, Wegrynowski T, Mitchell K, Hay K, Redston M, Holowaty E, Huntsman D. Tumor microsatellite instability in early onset gastric cancer. J Mol Diagn. 2005;7:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Yamamoto H, Imai K, Perucho M. Gastrointestinal cancer of the microsatellite mutator phenotype pathway. J Gastroenterol. 2002;37:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Ottini L, Falchetti M, Lupi R, Rizzolo P, Agnese V, Colucci G, Bazan V, Russo A. Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann Oncol. 2006;17 Suppl 7:vii97-vi102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 127. | Ottini L, Falchetti M, Saieva C, De Marco M, Masala G, Zanna I, Paglierani M, Giannini G, Gulino A, Nesi G. MRE11 expression is impaired in gastric cancer with microsatellite instability. Carcinogenesis. 2004;25:2337-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Ottini L, Falchetti M, D’Amico C, Amorosi A, Saieva C, Masala G, Frati L, Cama A, Palli D, Mariani-Costantini R. Mutations at coding mononucleotide repeats in gastric cancer with the microsatellite mutator phenotype. Oncogene. 1998;16:2767-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Menoyo A, Alazzouzi H, Espín E, Armengol M, Yamamoto H, Schwartz S. Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 2001;61:7727-7730. [PubMed] |
| 130. | Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res. 2002;62:2447-2454. [PubMed] |
| 131. | Simpson AJ, Caballero OL, Pena SD. Microsatellite instability as a tool for the classification of gastric cancer. Trends Mol Med. 2001;7:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 132. | Chung YJ, Song JM, Lee JY, Jung YT, Seo EJ, Choi SW, Rhyu MG. Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res. 1996;56:4662-4665. [PubMed] |
| 133. | Yamamoto H, Perez-Piteira J, Yoshida T, Terada M, Itoh F, Imai K, Perucho M. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 1999;116:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 134. | Fang WL, Chang SC, Lan YT, Huang KH, Chen JH, Lo SS, Hsieh MC, Li AF, Wu CW, Chiou SH. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36:2131-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 135. | Choi YY, Bae JM, An JY, Kwon IG, Cho I, Shin HB, Eiji T, Aburahmah M, Kim HI, Cheong JH. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014;110:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 136. | di Pietro M, Marra G, Cejka P, Stojic L, Menigatti M, Cattaruzza MS, Jiricny J. Mismatch repair-dependent transcriptome changes in human cells treated with the methylating agent N-methyl-n’-nitro-N-nitrosoguanidine. Cancer Res. 2003;63:8158-8166. [PubMed] |
| 137. | Zheng L, Wang L, Ajani J, Xie K. Molecular basis of gastric cancer development and progression. Gastric Cancer. 2004;7:61-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 138. | An C, Choi IS, Yao JC, Worah S, Xie K, Mansfield PF, Ajani JA, Rashid A, Hamilton SR, Wu TT. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11:656-663. [PubMed] |
| 139. | Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C, Juhng SW, Rashid A, Hamilton SR, Wu TT. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646-4654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 140. | Oue N, Mitani Y, Motoshita J, Matsumura S, Yoshida K, Kuniyasu H, Nakayama H, Yasui W. Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer. 2006;106:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 141. | Song S, Ajani JA. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2013;10:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 142. | Link A, Kupcinskas J, Wex T, Malfertheiner P. Macro-role of microRNA in gastric cancer. Dig Dis. 2012;30:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 143. | Li X, Zhang Y, Zhang Y, Ding J, Wu K, Fan D. Survival prediction of gastric cancer by a seven-microRNA signature. Gut. 2010;59:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 144. | Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 676] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 145. | Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ. MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark. 2012;11:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 146. | Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1160] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 147. | Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 561] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 148. | Hummel R, Hussey DJ, Haier J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer. 2010;46:298-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 149. | Wu H, Huang M, Lu M, Zhu W, Shu Y, Cao P, Liu P. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother Pharmacol. 2013;71:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 150. | Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C, Shen H. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4850] [Article Influence: 440.9] [Reference Citation Analysis (2)] |
| 151. | Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 152. | Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 630] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 153. | Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 420] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 154. | Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 155. | Matsusaka K, Kaneda A, Nagae G, Ushiku T, Kikuchi Y, Hino R, Uozaki H, Seto Y, Takada K, Aburatani H. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187-7197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 156. | Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, Baylin SB, Issa JP. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999;59:5438-5442. [PubMed] |
| 157. | Geddert H, Zur Hausen A, Gabbert HE, Sarbia M. EBV-infection in cardiac and non-cardiac gastric adenocarcinomas is associated with promoter methylation of p16, p14 and APC, but not hMLH1. Anal Cell Pathol (Amst). 2010;33:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Lee J, van Hummelen P, Go C, Palescandolo E, Jang J, Park HY, Kang SY, Park JO, Kang WK, MacConaill L. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS One. 2012;7:e38892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 159. | Sukawa Y, Yamamoto H, Nosho K, Kunimoto H, Suzuki H, Adachi Y, Nakazawa M, Nobuoka T, Kawayama M, Mikami M. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol. 2012;18:6577-6586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 160. | Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177-240. [PubMed] |
| 161. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 162. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 924] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 163. | Sobala GM, Schorah CJ, Shires S, Lynch DA, Gallacher B, Dixon MF, Axon AT. Effect of eradication of Helicobacter pylori on gastric juice ascorbic acid concentrations. Gut. 1993;34:1038-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 164. | Touati E, Michel V, Thiberge JM, Wuscher N, Huerre M, Labigne A. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology. 2003;124:1408-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 165. | Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006;6:936-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 166. | Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 167. | Wang LH, Choi YL, Hua XY, Shin YK, Song YJ, Youn SJ, Yun HY, Park SM, Kim WJ, Kim HJ. Increased expression of sonic hedgehog and altered methylation of its promoter region in gastric cancer and its related lesions. Mod Pathol. 2006;19:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 168. | Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Azuma T. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 362] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 169. | Terrés AM, Pajares JM, O’Toole D, Ahern S, Kelleher D. H pylori infection is associated with downregulation of E-cadherin, a molecule involved in epithelial cell adhesion and proliferation control. J Clin Pathol. 1998;51:410-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 170. | Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 171. | Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492-8495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 172. | Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 173. | Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36-47. [PubMed] |
| 174. | Waldum HL, Hauso Ø, Sørdal ØF, Fossmark R. Gastrin May Mediate the Carcinogenic Effect of Helicobacter pylori Infection of the Stomach. Dig Dis Sci. 2015;60:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 175. | Sun T, Zhou Y, Yang M, Hu Z, Tan W, Han X, Shi Y, Yao J, Guo Y, Yu D. Functional genetic variations in cytotoxic T-lymphocyte antigen 4 and susceptibility to multiple types of cancer. Cancer Res. 2008;68:7025-7034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 176. | Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 177. | Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 178. | Piazuelo MB, Camargo MC, Mera RM, Delgado AG, Peek RM, Correa H, Schneider BG, Sicinschi LA, Mora Y, Bravo LE. Eosinophils and mast cells in chronic gastritis: possible implications in carcinogenesis. Hum Pathol. 2008;39:1360-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 179. | Sankpal NV, Moskaluk CA, Hampton GM, Powell SM. Overexpression of CEBPbeta correlates with decreased TFF1 in gastric cancer. Oncogene. 2006;25:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 180. | Regalo G, Canedo P, Suriano G, Resende C, Campos ML, Oliveira MJ, Figueiredo C, Rodrigues-Pereira P, Blin N, Seruca R. C/EBPbeta is over-expressed in gastric carcinogenesis and is associated with COX-2 expression. J Pathol. 2006;210:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 181. | Nuñez F, Bravo S, Cruzat F, Montecino M, De Ferrari GV. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS One. 2011;6:e18562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 182. | Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276-1280. [PubMed] |
| 183. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 184. | Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923-1931. [PubMed] |
| 185. | Hao Q, Zhang C, Gao Y, Wang S, Li J, Li M, Xue X, Li W, Zhang W, Zhang Y. FOXP3 inhibits NF-κB activity and hence COX2 expression in gastric cancer cells. Cell Signal. 2014;26:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 186. | de Maat MF, van de Velde CJ, Umetani N, de Heer P, Putter H, van Hoesel AQ, Meijer GA, van Grieken NC, Kuppen PJ, Bilchik AJ. Epigenetic silencing of cyclooxygenase-2 affects clinical outcome in gastric cancer. J Clin Oncol. 2007;25:4887-4894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 187. | Park ES, Do IG, Park CK, Kang WK, Noh JH, Sohn TS, Kim S, Kim MJ, Kim KM. Cyclooxygenase-2 is an independent prognostic factor in gastric carcinoma patients receiving adjuvant chemotherapy and is not associated with EBV infection. Clin Cancer Res. 2009;15:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 188. | Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, Nordling S, Ristimäki A, Haglund C. Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res. 2005;11:7362-7368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 189. | Liu F, Pan K, Zhang X, Zhang Y, Zhang L, Ma J, Dong C, Shen L, Li J, Deng D. Genetic variants in cyclooxygenase-2: Expression and risk of gastric cancer and its precursors in a Chinese population. Gastroenterology. 2006;130:1975-1984. [PubMed] |
| 190. | Hu PJ, Yu J, Zeng ZR, Leung WK, Lin HL, Tang BD, Bai AH, Sung JJ. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 191. | Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320:1642-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 309] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 192. | Akre K, Ekström AM, Signorello LB, Hansson LE, Nyrén O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer. 2001;84:965-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 193. | Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181-10189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 194. | Sitarz R, Leguit RJ, de Leng WW, Morsink FH, Polkowski WP, Maciejewski R, Offerhaus GJ, Milne AN. Cyclooxygenase-2 mediated regulation of E-cadherin occurs in conventional but not early-onset gastric cancer cell lines. Cell Oncol. 2009;31:475-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 195. | Karim S. Clinicopathological and p53 gene alteration comparison between young and older patients with gastric cancer. Asian Pac J Cancer Prev. 2014;15:1375-1379. [PubMed] |
| 196. | Takatsu Y, Hiki N, Nunobe S, Ohashi M, Honda M, Yamaguchi T, Nakajima T, Sano T. Clinicopathological features of gastric cancer in young patients. Gastric Cancer. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 197. | Yanjun X, Wenming C, Lisha Y, Qi X, Jianmin G, Xinbao W, Xiangdong C, Jieer Y. Detection of CDH1 gene variants in early-onset diffuse gastric cancer in Chinese patients. Clin Lab. 2014;60:1823-1830. [PubMed] |
| 198. | Du J, Xu Y, Dai J, Ren C, Zhu C, Dai N, Ma H, Shi Y, Hu Z, Lin D. Genetic variants at 5p15 are associated with risk and early onset of gastric cancer in Chinese populations. Carcinogenesis. 2013;34:2539-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 199. | Carvalho R, Milne AN, Polak M, Corver WE, Offerhaus GJ, Weterman MA. Exclusion of RUNX3 as a tumour-suppressor gene in early-onset gastric carcinomas. Oncogene. 2005;24:8252-8258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 200. | Sugimoto S, Yamada H, Takahashi M, Morohoshi Y, Yamaguchi N, Tsunoda Y, Hayashi H, Sugimura H, Komatsu H. Early-onset diffuse gastric cancer associated with a de novo large genomic deletion of CDH1 gene. Gastric Cancer. 2014;17:745-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 201. | Hiyama T, Tanaka S, Yoshihara M, Sasao S, Kose K, Shima H, Tuncel H, Ueno Y, Ito M, Kitadai Y. Chromosomal and microsatellite instability in sporadic gastric cancer. J Gastroenterol Hepatol. 2004;19:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 202. | Bevilacqua RA, Simpson AJ. Methylation of the hMLH1 promoter but no hMLH1 mutations in sporadic gastric carcinomas with high-level microsatellite instability. Int J Cancer. 2000;87:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |