Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2441
Peer-review started: September 29, 2015
First decision: November 27, 2015
Revised: December 17, 2015
Accepted: January 9, 2016
Article in press: January 11, 2016
Published online: February 28, 2016
Processing time: 150 Days and 3.1 Hours
Pancreatic ductal adenocarcinoma (PDAC) is the fourth cause of cancer death with an overall survival of 5% at five years. The development of PDAC is characteristically associated to the accumulation of distinctive genetic mutations and is preceded by the exposure to several risk factors. Epidemiology has demonstrated that PDAC risk factors may be non-modifiable risks (sex, age, presence of genetic mutations, ethnicity) and modifiable and co-morbidity factors related to the specific habits and lifestyle. Recently it has become evident that obesity and diabetes are two important modifiable risk factors for PDAC. Obesity and diabetes are complex systemic and intertwined diseases and, over the years, experimental evidence indicate that insulin-resistance, alteration of adipokines, especially leptin and adiponectin, oxidative stress and inflammation may play a role in PDAC. Peroxisome proliferator activated receptor-γ (PPARγ) is a nuclear receptor transcription factor that is implicated in the regulation of metabolism, differentiation and inflammation. PPARγ is a key regulator of adipocytes differentiation, regulates insulin and adipokines production and secretion, may modulate inflammation, and it is implicated in PDAC. PPARγ agonists are used in the treatment of diabetes and oxidative stress-associated diseases and have been evaluated for the treatment of PDAC. PPARγ is at the cross-road of diabetes, obesity, and PDAC and it is an interesting target to pharmacologically prevent PDAC in obese and diabetic patients.
Core tip: Pancreatic cancer has a dismal prognosis with an overall five years survival less than 5%. Obesity and diabetes are risk factors of pancreatic ductal adenocarcinoma (PDAC) increasing the likelihood of its development. Agonists of one specific nuclear receptor transcription factor, the peroxisome proliferator-activated receptor γ (PPARγ), are currently used or evaluated for the treatment of these diseases. PPARγ is a well-known protein implicated in the regulation of metabolism, inflammation, and differentiation; standing at the cross-road of these diseases it may be a key factor linking PDAC to diabetes and obesity, a master regulator whose modulation could be the key for PDAC treatment.
- Citation: Polvani S, Tarocchi M, Tempesti S, Bencini L, Galli A. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J Gastroenterol 2016; 22(8): 2441-2459
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2441.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2441
The nuclear receptor transcription factors (NR) are a group of evolutionary conserved proteins that double as receptors and regulators of gene transcription. NR regulate the mRNA transcription binding to specific sequences of DNA, called hormone-response element or HRE[1-3].
NR are ligand activated transcription factors sharing a high degree of structural homology and are classified according to the sequence homology in six different families. The first NR was identified by a group guided by Prof. Evans in 1985[4] and was described as a glucocorticoid receptor possessing a DNA binding domain (DBD) and a ligand binding domain (LBD); the two regions, as their names evidently suggest, are necessary for the recognition of HRE and for the binding to the ligands, respectively. Interestingly, these two distinguishing features of a NR, are present in all but two receptors, DAX-1 and SHP, who lack the DBD and are classified in the newly introduced NR0 seventh family of NR[1].
Besides this homology classification, two parallel systems are used to aggregates NR: one is based on the functional status and localization before the binding to the ligand and one on the existence of ligands. NR are classified in type I, II, III, or IV depending if they act as homodimers (I and III), heterodimers (II) or monomers (IV); type III receptors differ from type I for the cell localization before the ligand binding: type I are cytosolic whereas type III are nuclear[1].
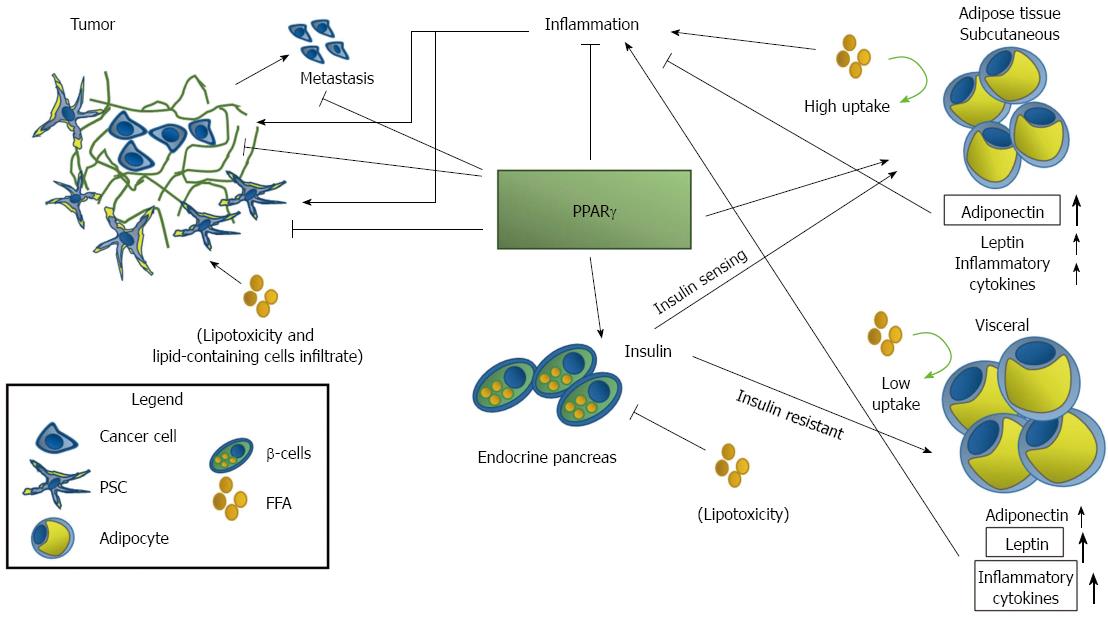
Of the 48 NR identified, for only half there is a known ligand, whereas the other half is indicated as being “orphan”. The number of orphan receptors has changed over time due to identification for some of them of a specific natural ligand: orphan nuclear receptors with an identified ligand are classified as “adopted”. NR are implicated in almost every physiological and pathological condition and several nuclear receptors are associated to neoplasm in several organs and in PDAC[5-9]. Of the numerous NR we will focus our attention on the peroxisome proliferator-activated receptor (PPAR)-γ, a member of the PPAR group of nuclear receptors, strongly tied to diabetes, obesity and pancreatic cancer (PC) (Figure 1).
Peroxisomes are cellular organelles identified in the late 1960 in rat liver[10,11]; single-membrane bound, they are involved in cellular functions ranging from fatty acid metabolism and transport to reactive oxygen species (ROS) detoxification[11]. Clofibrate, a hypo-lipidemic agent, was the first of a series of peroxisome proliferators, molecules capable of inducing the “proliferation” of peroxisomes. The first receptor of these peroxisome proliferators, generically named PPAR, was discovered by Issemann I in the 1990[12]; in 1992, the number of identified PPARs increases to three after the characterization in the frog Xenopus of three novel receptors activated by peroxisome proliferators and by agents causing carcinogenesis in rodent liver; these receptors acted as transcriptional activators of the acyl coenzyme A oxidase gene, which encodes the key enzyme of peroxisomal fatty acid β-oxidation[13].
PPARs belong to the thyroid-like NR1C group of nuclear receptors transcription factors[3]; so far the group comprises three genes, localized in different chromosomes encoding for different transcripts and proteins: PPARγ (NR1C3), PPARα (NR1C1) and PPARβ/δ (NR1C2). PPARs are common finding in human cells; indeed whereas PPARγ and PPARβ/δ are ubiquitous, PPARα is more expressed in the liver, hearth and kidney where β-oxidation is higher[14,15]. The PPARs share the classical features of the nuclear receptor transcription factor prototyped by the Steroid receptor, with some exceptions[1,5,16]: they possess a LBD at the C-terminal and a DBD towards the N-terminal, separated by a hinge region. Interestingly, only PPARγ is expressed as multiple proteins transcribed starting from several distinct transcripts[16-18]. Overall the three NR show an 80% homology, and are more divergent in the LBD, a finding that explain the different response to ligands[15,16,19]. Differently from steroid hormones receptors, that are “Type I” NR, PPARs are type II NR meaning that they works in tandem as obligatory heterodimers with retinoid-X-receptors (RXR-α/β/γ, NR2B1/2/3)[3,20-22]: when the NR bind a ligand the dimers, localized in the cell nuclei, undergo a transition of three dimensional configuration and recognize specific HRE, named PPAR response elements (PPRE), in the promoters of target genes resulting in their activation or repression. PPRE sequences are direct repeats of two six nucleotide-long core sequences (AGGTCA) separated by a single nucleotide (DR1): this motif was identified by study on DNA sequences bound by PPAR and may not represent the exact response element[23,24]. Furthermore additional features of the consensus sequence have been described such as the presence of a specific six nucleotide long sequence upstream of the PPRE and it has been demonstrated that PPAR may bind also to imperfect PPRE motif[23]. Nonetheless, in the ligand-activated PPAR:RXR heterodimers, PPAR bind the 5’ end of the DR1, whereas RXR bind to the other half; this peculiarity, in association with the asymmetric nature of the DR1, determines that the dimers act only unidirectionally on one DNA strand, although the same complex may bind the other DNA strand too.
PPAR mechanisms of action are not limited to activation/repression of target genes, but other functions have been described (see later for details). Interestingly, PPARs transcriptional activity is not only modulated by PPAR ligands, but by ligands of RXR too, such as retinoic acid[15,19].
Finally, the ligand-binding, that is associated to the above mentioned change of the three-dimensional configuration of the receptors, determines also the attachment of co-activators and detachment of co-repressors proteins, adding an additional layer of complexity to the regulation of PPARs functions (see[25] for details). One of the more studied PPAR co-activators is PGC-1 that is apparently implicated in adiponectin signaling[26,27], but other co-activators are steroid receptor co-activator 1, p300, and CREB binding protein[27,28]; all of them are tightly involved in regulation of metabolism and cancer development. Usually PPAR are packed with RXR and co-repressor molecules in PPRE and the co-repressors detach after ligand binding: in this way PPARs exert their activation and repression role. Interestingly, PPARs may regulate gene expression and molecular pathway by other mechanisms such as transrepression, an inhibitory action that is independent from DNA binding: these mechanisms are usually associated to secondary modifications of the receptors and are relevant for regulation of inflammation[5,15,19,29,30].
PPARG is encoded by a single gene located on chromosome 3p24, spanning a chromosomal region more than 150 kb. The gene is composed by 9 exons that give rise to at least 7 transcripts according to[16-18] although a survey on ensembl.org suggests the existence of up to 14 possible transcripts, 10 of them possibly protein-coding. Excepting three recently discovered transcripts[16,17], all the transcripts share the last 6 exons of the gene (called exons 1-6) and are produced by use of alternative promoters and different 5’ exons (called, from the 5’ end of the gene, A1, A2 and B). Nonetheless, for a long time only two proteins were believed to be produced by PPAR: PPARγ1 and PPARγ2.
PPARγ2 differs from PPARγ1 for the addition of 28 AA at the N-terminus and for the tissue expression and overall activity: whereas PPARγ2 is localized only in the adipose tissue and is believed to be more adipogenic, PPARγ1 is more broadly expressed[18]. Recently, three new PPARγ transcripts were isolated (γ2ORF4, γ3ORF4, and more recently γ1ORF4)[16,17].
These new transcripts have different 5’-UTR but share exons 1-4, and harbor a readthrough in intron 4, while lacking the exon 5-6; the resulting protein (γORF4) misses the LBD and acts as dominant negative NR. γ2ORF4 and γ3ORF4 transcripts were identified in sporadic colorectal cancer where γORF4, conversely to PPARγ, does not restrain cell growth but instead stimulates it[17]; 1ORF4 was later identified in adipocytes[16]. The existence of this dominant negative isoform suggests that PPARγ might possess a sort of self-regulation. Interestingly, the three transcripts may differentially contribute to adipose differentiation being γ3ORF4 constantly expressed along adipocyte differentiation but not in mature cells while γ1ORF4 and γ2ORF4 exhibit stage-specific expression[16].
PPARγ was classified as an orphan NR until the discovery of its natural endogenous ligands, the prostaglandin 15-deoxy-delta (12, 14)-prostaglandin J(2) (15d-PGJ2) and oxidize lipids[19,31]. Rosiglitazone and pioglitazone are Thiazolidinediones (TZD), synthetic ligands of PPARγ: they are used for the treatment of diabetes and hyperlipidemia and have been investigated, together with other PPARγ ligands, in clinical trials for cancer and oxidative stress-related diseases treatment[5,32]. Besides PPARγ-dependent effects, TZD may act independently of PPARγ activation (i.e., the same effect mediated by TZD can't be obtained by PPARγ overexpression or the use of specific non TZD ligands) and PPARγ-dependent and PPARγ-independent effects may co-exist[33-36].
PPARγ is implicated in important biological processes ranging from regulation of metabolism to inflammation and differentiation; as such, PPARγ has been linked to several diseases including cancer[5,15,19,37,38].
PPARγ mechanisms of function comprises the standard arrays of activation/repression mechanisms of regulation of gene expression and transrepression. Interestingly, PPARγ being an ubiquitinase may regulate the function of different pathway, especially inflammatory, modulating the proteasome-mediated degradation of proteins[39,40].
PPARγ may be modified by post-translational modifications, such as phosphorylations operated by the MAPK p38 and JNKs, that may cause inhibition of transcriptional activity, alteration of localization and degradation[41-43]. These secondary modifications are important for PPARγ function: for example, phosphorylation of PPARγ at Ser273 mediated by CDK5 in adipose tissue does not impair its adipogenic capacity but induces insulin resistance and is associated with a reduction of adiponectin secretion[44,45].
The tumors of the pancreas are classified according to their origin in tumors of exocrine and neuroendocrine pancreas.
PCs originating from the neuro-endocrine compartment (pancreatic neuroendocrine tumor, PNET) contribute to 5% of the tumors and may (functional PNET) or may not (nonfunctional PNET) overproduce hormones[46,47]. About 50% of PNET are non-functioning tumors that, conversely to functional PNET, are difficult to detect and are usually more malignant, with the reported percentage of malignant tumors as high as 90%[48].
Ninety five per cent of PCs are of exocrine origin and the most common form of all PCs is pancreatic ductal adenocarcinoma (PDAC) (see below for details). Others less frequent exocrine malignancies are the acinar cell carcinoma, the intraductal papillary mucinous neoplasm (IPMN) and the mucinous cystic neoplasms (MCN)[5,49,50]. Acinar cell carcinoma is a rare malignancy and differs significantly from PDAC, IPMN, and MCN, lacking their distinctive molecular alterations and presenting genomic alterations of its own[49-53]. Conversely, IPMN and MCN are considered benign cystic lesions that may develop to PDAC.
MCN is almost exclusively diagnosed in women, with a peak incidence in their 50s, as a single cystic lesion in the tail of the organ; the lesion is characterized by mucin-producing epithelial and show an ovarian-like stroma[50]. IPMN arise from ductal epithelium, appear as papillary projections in the main duct or in the branch ducts, and are almost equally distributed in men and women in their late 60s[49].
PDAC alone contributes to about 90% of all PCs[54,55] which explain why PC and PDAC are commonly used as synonymous (as we will do henceforth). PDAC is characterized by the presence of several distinctive molecular alterations, the most frequent being activating mutations in the small GTPase Kirsten RAS (Kras)[56] but overall, at least 60 mutations in 13 different molecular pathway have been described[53].
The progression model of PDAC points to a ductal origin of the disease with precursor lesions identified in the pancreatic intraductal neoplasia (PanIN). PanINs development is characterized by the early accumulation of genetic alterations found on PDAC, such as the Kras mutations and inactivation of the tumor suppressor p16; noteworthy, MCN and IPMN, although tumors on their own, may transform in PDAC and are considered among its potential precursors[5,57,58]. Quite recently, it has been proposed that PDAC may arise from acinar cells undergoing a transdifferentiation to ductal cells, in a process called acinar to ductal metaplasia (ADM)[59-61]. This idea is supported by the findings that in some animal models of PDAC, acinar cells are the focal point of PDAC growth acquiring the features of ductal cells and expressing ductal markers[60,61]; furthermore, Kras activation in mature acinar cells induces PanIN lesions and coactivation of Notch/Kras promotes ADM phenotype, with Notch regulating both initiation and dysplastic progression of acinar-derived PanINs[62].
The PC is not among the 10 most frequent cancers neither in the US nor Worldwide and accounts for only a fraction of the totaling cancers[63,64]. However, with its incidence substantially equaling the mortality, PDAC is one of the deadliest cancer and the fourth cause of cancer death, which explains its importance in cancer research[63,64].
The high mortality rate is the consequence of essentially a combination of two factors: the delay in the diagnosis and the absence of an effective therapy.
PDAC is a subtle disease: it is generally asymptomatic or shows itself with aspecific symptoms that make difficult the diagnosis[65,66]. On one hand, malignant lesions possess clinical and imaging features similar to benign alterations of the pancreas: actually, cystic lesions are easily detectable but their presence is not suggestive of PDAC and the finding it not considered sufficient for surgical procedure due to morbidity and cost consideration; on the other hand the available biomarkers are plagued by low specificity and/or low sensibility[66-68]. Typical biomarkers for the detection and surveillance of PDAC are carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA-19-9)[66-68]; both however are not exclusively expressed in PDAC and their use is limited to the surveillance of recurrence after surgical resection (CA-19-9) or as predictor of survival (CEA)[66-68]. Evaluation of new and improved biomarkers with prognostic and diagnostic value are currently ongoing in case-control studies[66]. The lack of specific biomarkers and efficient diagnostic systems for early detection is even more problematic for patient management because, conversely to other malignancies, PC has a tendency to dissemination even before tumor formation, as suggested by Rhim A in 2012[69]. Consequently, when a patient is diagnosed for PDAC, the disease may be already at an advanced stage, with local or distant metastasis, that precludes a successful surgical resection, the only effective therapeutic intervention. Even for patient eligible for resection the 5-years survival is around 20% whereas the 5-years overall survival for all patient is less than 5%[63,64].
From 1996, the year of its approval, the treatment of PC is still preferentially based on the nucleotide analogue gemcitabine or its combination with other drugs, with modest results and important side-effects[70]. Median overall survival in patients treated with gemcitabine is 5 mo and phase III clinical trials have failed to improve this data[66,71]. In fact, until very recently, only a combined therapy with gemcitabine and Erlotinib, an EGFR inhibitor, demonstrated a modest increase in survival compared to gemcitabine monotherapy[72]. More recently, promising improvements have been made in the management of PDAC: two different phase III trials have demonstrated that treatment of metastatic patients with Folfirinox or with nab-Paclitaxel plus gemcitabine significantly increases the survival, although folfirinox was associated to important toxic side effects[66,71,73-75].
An essential feature of PDAC is the presence of a “desmoplastic” reaction, i.e., the formation of a stiff fibrotic tissue around the tumor, consequential to the activation of pancreatic stellate cells (PSC)[76]. PSC, in response to TGF-β, PDGF, and other factors, proliferate and transform in myofibroblast-like cells expressing α-smooth muscle actin (α-sma); activated PSC produce an excess of extracellular matrix components creating the stiff stroma around the tumor[76-78]. The desmoplastic reaction is believed to be an important factor for tumor chemoresistance; supporting this notion, in 2009 Olive et al[79] demonstrated in mouse models that the inhibition of the Hedgehog (Hh) pathway had beneficial effect on delivery and susceptibility to gemcitabine treatment. This seminal work was followed by the clinical trial IPI-926-03; designed to test the effect of the Hh inhibitor Saridegib on PDAC in association with gemcitabine, the trial was blocked after an interim analysis when it became evident that patients treated with gemcitabine and the placebo lived longer. Despite this failure, novel Hh inhibitors, like Sonidegib[80], have demonstrated to be effective in the treatment of cancers other than PDAC and are currently tested for PDAC; these efforts clearly suggest that the identification of an effective drug or a cocktail of drugs is today believed the best solution for PDAC treatment as a “systemic disease”.
PDAC development has been associated to several risk and co-morbidity factors. PDAC risk factors can be classified in modifiable and non modifiable risks: non modifiable risks are sex, age, presence of genetic mutations, ethnicity, whereas modifiable and co-morbidity factors are related to the specific habits and lifestyle as well to the co-presence of other diseases[5,81-84]. PDAC is more frequent in men than in women, in elderly, and the presence of genetic mutations for examples in BRCA1, APC, or ATM genes increases the likelihood of PDAC development and is in agreement with the finding of the numerous genetic alterations characterizing the PDAC[53,81]. Of the modifiable risks, the more important is smoking, an habit that may also explain the increased risks associated to sex and ethnicity[81,82]. Recently it has become evident that obesity and diabetes are two important modifiable risk factors for PDAC, which we will discuss in details.
Glucose levels in the body are controlled by insulin and the insulin signaling pathway. Insulin is secreted by pancreatic β-cell located in the islet of Langerhans in response to increased nutrients levels in the blood; insulin then determines the uptake of glucose (mainly) but also of free fatty acids (FFA) by muscle cells, hepatocytes, and adipocytes, for storage as glycogen (skeletal muscle and liver), lipids (adipocytes) and proteins[14,85].
Insulin acts on target cells mainly through insulin receptor (IR), a tyrosine kinase receptor that may also interact with insulin growth factor (IGF)[14,85]. IR is composed by two subunits (α and β) linked by di-sulphide bonds: bind of insulin to the α-subunit leads to the β-subunit tyrosine autophosphorylation and to the phosphorylation of proteins belonging to the insulin receptor substrate (IRS) family and to SHC[86]. IRS interacts and activates the PI3K pathway and AKT which in turn phosphorylates and inhibits glycogen synthase kinase 3 whose main function in insulin pathway is to block the glycogen synthase, the enzyme that catalyze the final step in glycogen synthesis. Through SHC proteins insulin activates the p21RAS and the ERK signaling acting as a mitogen factor and through AKT insulin may activate mTOR[14,85,87]. Moreover, insulin regulates glucose uptake influencing the cellular localization of the Glucose transporter 4 (GLUT-4)[88,89]. In resting condition, around 5% of GLUT-4 is localized at the plasma membrane, while the majority is stored in the trans-Golgi compartment, in the so-called GLUT-4 storing vesicles (GSV)[88,90,91]. After insulin signaling, adipose and muscle cells respond increasing the uptake of glucose through GSV exocytosis, mediated by PI3K-dependent and PI3K independent mechanisms[90,92].
Diabetes mellitus (DM) is a chronic disease that arises when the body is unable to regulate the levels of glucose. If not correctly treated, sustained increased glycemic levels are associated with renal failure, retinal damage, and cardiovascular diseases. In term of etiology, DM is distinguished in type 1 and type 2 diabetes.
Type 1 diabetes is an autoimmune disease characterized by the destruction of β-cells as a consequence of adaptive immunity and innate inflammation and it is associated with a lack of insulin[93-95]; it is diagnosed in early childhood and may be treated with a life-long administration of insulin. Type 1 diabetes is often associated with other diseases such as autoimmune thyroiditis and celiac disease[93,95]. An increased number of evidence suggest that innate immunity mediated by Toll-like receptor (TLR) is implicated in the onset of Type 1 and Type 2 diabetes[96,97].
Type 2 diabetes is by far the most common form of DM: ninety percent of diagnosis of diabetes are of type 2. DM type 2 is associated to insulin-resistance in a condition of normal or even higher levels of plasma insulin concentration[98]. Mechanisms behind insulin resistance vary from reduction of glucose transporter and IR expression to alteration of the insulin downstream signaling pathways and may be associated to genetic abnormalities, obesity and inflammation[99].
Other forms of diabetes are gestational diabetes, which may occur during pregnancy, and disease-associated diabetes like diabetes occurring in pancreatic diseases. Indeed DM is a common finding in acute and chronic pancreatitis and in PC[100,101]. Chronic pancreatitis is characterized by a constant inflammatory reaction, activation of PSC and deposition of stromal fibers that usually evolves in pancreatic fibrosis and results in the destruction of Langerhans islets; interestingly, chronic pancreatic inflammation is associated to an elevated risk of PDAC[100,101], but it seems to be more important in the development of precursor lesions and in the initial stages of PDAC, given that anti-inflammatory drugs have not benefit in PDAC[102]. PDAC too is characterized by a stromal reaction and activation of stellate cells and diabetes is often a secondary effect of PDAC: nearly half of the patients with PDAC suffer from diabetes and its new on-set, especially if transient, might be suggestive of the neoplasm presence[103-106].
DM, especially the type 2, is a risk factor for several cancers and for PDAC[107]. The odds ratio to develop PDAC is 1.82 in diabetic patients and individuals with a recent diagnosis of DM have a 50% greater risk of the malignancy compared with individuals who had diabetes for more than five years[107]. These findings are corroborated by the demonstration that pancreatitis and diabetes increase the proliferation of pancreatic duct glands (PDG) and the production of PC-specific proteins in PDG[108].
Diabetes may facilitate tumor progression and growth increasing the availability of nutrients, e.g., glucose and FFA, increasing inflammation, or through the altered insulin signaling in hyperinsulinemia[103,105,106,109].
One of the key feature of tumor cells is the ability to switch the metabolic machinery towards the aerobic glycolysis (the Warburg effect), where pyruvate is preferentially metabolized by lactate dehydrogenase to lactate[110]. The Warburg effect might be an adaptation of pancreatic tumor cells to the relative hypoxia and metabolic constraints caused by the desmoplastic stromal reaction and it is also a key feature of the stromal compartment of the tumor[110]. On the other hand insulin resistance increases the circulating levels of lipids, especially FFA. FFA might have multiple effects: they constitute the building blocks of cell membrane and hence might favor tumor growth, they may be used as energy sources but may also cause oxidative stress, as much like as excessive glucose availability. The end products of oxidative stress are ROS and peroxidized lipids; although generally detrimental, ROS are important as signaling molecules in inflammation, immune cell response and invasion/migration and are critical in Kras driven transformation in PDAC[111]. Finally FFA may induce inflammation activating the TLR signaling cascade[112].
Experimental evidence point to a major role of insulin in the progression of several tumors and PDAC, independently from its effect on energy homeostasis[113,114]. PC cells express IR and the receptors for the insulin-growth factor (IGF)[109,115,116]. Conversely to insulin, IGF exerts mostly pro-mitogenic and pro-survival effects through the ERK pathway, but insulin signaling may pass through IGFR and IGF may relay signals through IR[109,110,114,117] binding to IR-A, a fetal form of insulin receptor that is expressed in several cancers, including PDAC[109,118]. The interplay between insulin and IGF pathways is also suggested by the fact that IR and IGFR form hybrid receptors with affinity for insulin and IGF[85,109]. Moreover, high insulin levels may reduce the production of IGF binding protein by the liver increasing the bio-availability of IGF[119].
The role of diabetes and insulin has been recently demonstrated in a mouse model of hyperinsulinemia associated to DM type 2[108,120] where tumor growth is increased compared to control mice, in presence or absence of pancreatic inflammation induced by cerulein; in this model tumor growth was reduced by the anti-diabetic metformin[120], who as it turns out is also able to reduce the expression of cancer stem markers and to increase the expression of miRNAs that are typically lost in PC[121],in agreement with the finding that insulin could induce differentiation. Interestingly, in diabetic mice the inflammatory infiltrate is reduced compared to lean mice independently to cerulein treatment without difference in the overall inflammatory condition. These results suggest that tumor growth is mainly driven by hyperinsulinemia and not hyperglycemia, in agreement with models of DM type 1[122].
Mammalian body may store energy in the form of lipid droplets in the adipose tissue. Adipose tissue is mainly composed by adipocytes, cells specialized in fat accumulation, and by a small fraction of immune and stromal cells as well as pre-adipocytes. Traditionally we recognize two type of adipose tissue: the brown and the white adipose tissue. Brown tissue is localized in the cervical area, uses lipids to produce heat by thermogenesis, and decreases with aging[123]; white adipose tissue is implicated in obesity and is the main storing facility of lipids. When there is an unbalance between fat accumulation and fat mobilization the individual may become obese. The body mass index (BMI), that is the ratio of the body weight and the square of the height (in cm), is used to categorize individual in underweight, normal weight, overweight, and obese; when BMI is higher than 30 the individual is considered obese. Noteworthy, an important limitation of the BMI for the identification of overweight and obese individuals is that it does not account for the difference in the visceral fat and subcutaneous adipose tissues that are known to have different effects on health[124-127].
Obesity may arise from a plethora of causes but two are recognized as more important: overfeeding and low or even lack of physical activity. One of the co-morbidity of obesity is DM type 2, that in association with metabolic changes induced by fat accumulation (e.g., abdominal obesity, high triglycerides content), constitutes a clinical condition known as metabolic syndrome.
One of the major problem of obesity, besides the induction of DM type 2 and increased risk of cardiovascular diseases, is its proved association with several cancers, including pancreatic[94,113]. These associations are worrying given that obesity is expanding in the developed and developing countries, reaching levels as high as 40% of the population (70% if counting overweight too), and its diffusion in childhood[94,128,129]. Obese individuals have an increased risk to develop PC, a higher chance to have a metastatic disease at the time of diagnosis, and a lower chance of survival, with a hazard ratio of death positively correlated with the duration of obesity[81].
The mechanisms underlying the association of obesity and PC are not well understood. It is postulated that obesity might increase the risk of PC altering the production of adipokines, inducing diabetes, altering the intestinal flora, and maintaining a sustained inflammation increasing the production of inflammatory cytokines[85,94].
Conversely to the common sense which bring to think to adipose tissue as a simple reservoir of energy in the form of fat, it has become evident over the years that the adipose tissue is actively involved in the regulation of the entire body metabolism. Indeed the adipose tissue acts as an endocrine organ secreting “adipokines”, molecules that may signal to metabolic active organs and to the central nervous system. Leptin and adiponectin are probably the better known adipokines but besides these two factors, adipose tissue may also produce resistin and other inflammatory citokines, an evidence that as brought many researchers to prefer the term “adipocytokines” when referring to the factors produced by the adipose tissue. Probably, it is the imbalance of these adipocytokines that increases the risks of the cancer of the pancreas in obese patients, as we will detail in the ensuing paragraphs, focusing our attention on leptin and adiponectin.
Leptin is secreted by adipocytes and regulates appetite and energy metabolism acting mainly on the central nervous system and at the peripheral levels on cells expressing the leptin receptor (OBR). In the central nervous system leptin induces satiety and reduces hunger, whereas the peripheral effects vary among target cells. In pancreatic β-cells for examples, leptin inhibits insulin secretion and synthesis in several ways (e.g., by reducing the expression of glucose-induced uncoupling-protein 2, by modifying PI3k pathway and cAMP)[130]; in turns, insulin induces leptin secretion and synthesis in adipose tissue[131]. Distinct OBR has been described: all are tyrosine kinase receptors and are similar in the extracellular domain but differ in the intracellular domain, and the OBRb is the longest isoform[132]. OBR are expressed in cancer cells and activate the downstream MAPK/ERK and STAT3 pathways. Expression of leptin is correlated with the quantity of visceral adipose tissue and it is often associated to some sort of leptin resistance that causes hyperinsulinemia[131,133].
PC cells express OBRb and the shorter OBRa[134,135] and the overexpression of leptin in vivo is associated with increased tumor growth and metastatization[135]. In vitro, leptin increases migration through the up-regulation of MMP13 mediated by JAK2/STAT3, and through the PI3K/AKT pathways. The role of leptin as an invasive modulator is also confirmed by the finding that OBRb expression correlates with MMP13 and lymph node metastasis in primary tumors[135]. OBRb expression in PDAC cells in vitro is regulated by the hypoxia inducible factor 1α (HIF-1α) that bind to the OBRb promoter; this finding is corroborated by the demonstration that HIF-1α expression correlates with lymph node metastasis and OBRb in primary samples[136].
One of the feature of PDAC and other cancers is the induction of cachexia, a syndrome characterized by progressive loss of weight, an effect potentially mediated by leptin. However, Brown et al[137] demonstrated that there was no association between patients with increased plasma leptin and weight loss or anorexia and a more recent metabolomic study suggested that PDAC patients with cachexia have lower leptin and higher interleukin (IL)-6 and TNF-α compared to PDAC patients without cachexia[138]. These results are in agreement with the finding that newly diagnosed PC patients have a decreased concentration of leptin compared to control or DM type 2 independently from age, BMI, and waist circumference[132,139]. On the contrary, the ratio adiponectin/leptin and the level of resistin, another adipokine, are increased in PDAC patient[132,139].
It is tempting to speculate that leptin role in PDAC is primary associated to the induction of metastatization and may be linked to its ability to function as a pro-inflammatory cytokine. Indeed leptin is structurally similar to IL-6, also produced by the adipose tissue; IL-6 is a pro-inflammatory cytokine that increases invasion and migration and epithelial to mesenchymal transition (EMT) of PC cells, and synergies with leptin acting on STAT3[8,140,141]. Moreover the hypothesized role of leptin as a pro-metastatic agent is demonstrated also by its ability to induce cell invasion in ovarian, prostate, gastric, and breast cancers, and to induce EMT in A549 lung cancer cells[142-147].
Adiponectin is another adipokine produced primarily by adipocytes which resembles the C1q, a protein of the complement system[148,149]. Adiponectin may also be secreted by other cells, like lymphocytes and skeletal muscle cells; however it is believed that their contribution to circulating adiponectin is limited[124,150,151]. Adiponectin undergoes to several secondary modifications that influence secretion, multimerization, and clearance, ranging from glycosylation, succination, and thiol modifications[152]. Adiponectin is secreted by adipocytes as a high molecular weight protein oligomers (HMW), low molecular weight oligomers (LMW), medium weight multimers and monomers[152] and may be cleaved by the leukocytes elastase to generate the globular adiponectin[152].
Two adiponectin receptors (AdipoR1 and AdipoR2) have been described: they show different affinity for HMW, LMW and monomers, and different tissue distribution. AdipoR1 has high affinity for the globular adiponectin, whereas the more metabolically active HMW binds preferentially to AdipoR2. Activation of the receptors may results in different effects mediated by AMPK and the p38 MAPK[153]. Noteworthy, AdipoR2 induces the activation of the nuclear receptors PPARγ and PPARα[153,154]. Activation of the adiponectin pathway has several effects: reduces liver gluconeogenesis but increases liver utilization of FFA by β-oxidation, increases adipocytes uptake of FFA, and increases insulin sensitivity acting at many levels. Adiponectin has also anti-inflammatory effects and an inflammatory condition reduces this adipokine[126,151,153]; the mechanisms underlying these associations are complex and may be linked to the regulation of PPARγ.
Adiponectin elicits opposite effects compared to leptin and its levels are inversely correlated to BMI and to the presence of diabetes[155].
Interestingly, activation of the downstream adiponectin pathway results in inhibition of leptin signaling blocking the Jak and STAT3 phosphorylation[113,156]; besides the metabolic effects, this interplay may be important in the formation of the PDAC desmoplastic reaction following PSC activation (see later for details).
In cancer, whereas leptin is usually pro-angiogenic, pro-proliferative, and pro-invasive, adiponectin reduces proliferation, invasion and angiogenesis[124,144,145,147,153]. Indeed, low adiponectin levels are a common finding in tumors and correlate with tumor stage; this happens for breast, prostate and liver cancer and has been reported for PDAC. However, the association PDAC-adiponectin is not clear: both low and high adiponectin has been linked to an increased cancer risk[132,155,157-160].
In two case-control studies higher adiponectin and lower leptin levels were associated to the presence of PDAC, before and after controlling for co-factors such as BMI and smoke; moreover PDAC patients have high adiponectin compared to chronic pancreatitis and control[157,158]; interestingly, AdipoR1 and AdipoR2 are also high expressed in PDAC and the adiponectin/leptin ratio is higher in cancer patient compared to healthy and DM type 2 patients[132,157]. Conversely, a prospective study demonstrated that low pre-diagnostic levels of this adipokine were associated to an elevated risk to develop PDAC[159]. Another perspective study concluded that pre-diagnostic levels of adiponectin were not predictive of PDAC development in the general population but lower levels in never smokers were associated to increased risk of PDAC[155]; finally a study in Finnish male smokers supports these previous cited works showing that higher adiponectin concentration was inversely associated to PDAC[160].
There are not clear reasons behind these different findings. It is possible to speculate that they may be caused by differences in the studied population but another possible explanation may be that these studies are inherently different being prospective and case-control. Indeed low levels of adiponectin are associated to obesity and DM type 2 that predispose to PDAC and the high adiponectin detected in case-control studies might be a compensatory mechanisms to insulin resistance and cachexia in more advanced stages. Furthermore, it is also conceivable that the differences are associated to genetic variants of adiponectin gene ADIPOQ and not to its expression levels. Two independent works published in the last two years have demonstrated that specific single nucleotide polymorphisms (SNP) might predispose or protect from PDAC: the two groups analyzed the expression and association of the DM type 2 associated SNP rs1501299 of the ADIPOQ gene showing that the CC and AC genotypes are associated to a higher risk compared to the AA genotype[161,162].
On the other hand it has been demonstrated that adiponectin protects β-cells of the Islet of Langerhans from apoptosis activating the ERK and AKT pathways, without altering the AMPK pathway, and independently from AdipoR signaling; furthermore, adiponectin induces insulin synthesis and secretion[163].
Association of adiponectin levels to PDAC has been extensively studied in vitro and in vivo. One of the most used animal model in PC research is the KrasG12D/Pdx-1-Cre mouse model. In this mouse, the Cre recombinase, under the control of the Pdx-1 promoter, induces the expression of the constitutively active KrasG12D as early as 8.5 d.p.c. in a common precursor of pancreatic exocrine and endocrine cells. The KrasG12D/Pdx-1-Cre mouse develops ductal lesions identical to human PanIN lesions and the pancreas shows extensive desmoplastic and inflammatory reaction; within 1 year the mouse lesions progress to PDAC[164]. In KrasG12D/Pdx-1-Cre mice, chronic or intermittent calorie restriction delays tumor progression but increases the expression of Sirt1 and serum adiponectin while reducing leptin[165]. These results are in agreement to similar findings on cancer cells of different origins and are confirmed by the demonstration that adiponectin treatment of murine Pan02 PC cells in vitro modestly reduces cell proliferation and increases apoptosis, and that orthotopic transplantation of the same cells in knockout adiponectin mice results in increased tumor growth[166].
Nonetheless, Huang et al[26] have suggested that adiponectin conversely promotes cancer progression. Using a similar mouse models but different cell lines, the Authors demonstrated in vivo that adiponectin deficiency markedly reduced tumor growth and that adiponectin reduced apoptosis throughout AdipoR1 but not AdipoR2. The downstream surviving pathway involves the phosphorylation of AMPK and increased expression of Sirt1 which in turn deacetylates proliferator-activated receptor gamma co-activator 1-a (PGC1a) altering the expression of mitochondrial genes[26,27].
These opposite results may be associated to differences in the experimental animal models: indeed whereas Kato et al[166] used an orthotopic model, Haung et al[26] performed subcutaneous tumor engraftment; it might also be possible that in the tested cell lines adiponectin signaling may be transmitted preferentially by one of the two AdipoR who we know activate different signaling cascades. Anyway, these in vivo discrepancies reflect the uncertainty in the epidemiological data and certainly point out that more work need to be done to ascertain the role of adiponectin in PDAC development.
The research of a causative association of the NR PPARγ to PDAC has been the subject of intense research. These efforts are mainly driven by the tempting idea to apply TZD or other PPARγ-targeted drugs to the therapy of this disease (for recent reviews see[5,167,168]) and it is based on the assumption that PPARγ is a key metabolic regulator and play an anti-inflammatory role inhibiting the pro-inflammatory NF-κB, STAT and AP-1 pathways[18,19].
In vitro results are promising and point out to an inhibitory role of PPARγ. Treatment of PC cells with PPARγ agonists induces apoptosis, ductal differentiation and arrest cells in G0/G1 phase by PPARγ -dependent and -independent mechanisms[33-35]. PPARγ may also reduce cell motility and invasion altering the expression levels of component of the urokinase plasminogen activator (uPA) system, the catenin p120 localization and suppressing Cdc42 and RAC1[169,170]. UPA system is implicated in the remodeling of the extracellular matrix and casually involved in multiple steps of cancer development[171]; in PDAC, PPARγ agonists alter the total urokinase activity by increasing the expression of uPA inhibitor-1 and decreasing uPA[170].
These results are confirmed by in vivo experiments where PPARγ reduces tumor growth and enhances the gemcitabine effects possibly modifying the Notch and NF-κB- dependent inflammatory environment and the desmoplastic reaction[5,172,173]. In rectal xenograft models pioglitazone-mediated suppression of tumor spreading was paralleled by IL-8 and Cox-2 mRNA reduction in vitro[174]; although the effects of Cox-2 in PDAC cancer are highly associated to its expression levels, this inducible enzyme is regulated by PPARγ and may metabolize arachidonic acid to produce precursors of PPARγ ligands creating a positive feedback loop[19]; interestingly, PPARγ agonists and Cox-2 inhibitors may synergize and may be exploited for PDAC treatment[175,176].
Other synergisms and interactions with pathways involved in PDAC has been described in the literature: for examples, PPARγ activation contemporary to the inhibition of IFN-β or the administration of RXR agonists increases the anti-proliferative effects on PDAC cells[5] or, moving away from PDAC, PPARγ may interact with NRF2 to ameliorate and respond to ROS stress (for a recent review see[19]).
Alteration of E3 ubiquitin ligases is associated to the development of several diseases and pancreatic neoplasms[39,40,177]. Interestingly, PPARγ is a NR but is also an E3 ubiquitin ligase; as ubiquitin ligase PPARγ may play an important role in the regulation of PDAC development: it has been demonstrated that PPARγ may terminate the pro-inflammatory response mediated by NF-κB, targeting p65 for nuclear export and degradation[39], and may induce the proteasome-dependent degradation of the MUC1-C oncoprotein, one of several mucins involved in cell proliferation and invasion[40].
Besides in vitro and in vivo data, analysis of primary tumors clearly show that the NR is expressed at high levels in the neoplasms[33,178-180]. The expression of PPARγ is inversely correlated to the overall survival, the higher the expression the shorter the survival, especially in patients who received chemotherapy after surgery; conversely, PPARγ expression directly correlate with TNM stage[33,178-180], suggesting that PPARγ is a critical target for PDAC treatment.
PDAC is a subtle and difficult to treat systemic disease. We have shown that diabetes and obesity, and by extension systemic metabolic disease, are important risks factors for the neoplasm. Finding a way to tackle the risks factors to prevent the PDAC is an obvious therapeutic strategy; even more interesting would be find a target, or possibly targets, shared by these risks and PDAC: PPARγ is a possibility, and we will show you why.
The metabolic syndrome comprises a spectrum of factors and may cause damage and promote PDAC in several ways, including lipid toxicity and increased inflammatory response, linked to insulin resistance and obesity. The benefit of TZD treatment and activation of PPARγ range from improved insulin-sensitivity, alteration of lipid metabolism and adipocytes differentiation, and reduction of inflammation as a direct consequence of the modification of adipose tissue metabolism.
The PPARγ agonists TZD have been widely used in the treatment of DM type 2 to ameliorate the insulin-resistance. As we previously mentioned, increased insulin production comes with several side effects and it is a risk of PDAC, associated with increased availability of glucose and oxidative stress, not to mention that insulin may act as a mimetic mitogen. PPARγ and insulin are tightly related and activation of PPARγ results in increased insulin sensitivity. When compared to metformin, the NR agonists have several advantages modulating insulin signaling cascade at different levels.
First of all, it has been demonstrated that PPARγ regulates insulin production and synthesis in β-cells. PPARγ indirectly regulates the insulin transcription mediated by PDX-1. PDX-1 is a master regulator of pancreas development and differentiation and in β-cells it regulates the expression of insulin gene. Interestingly, PDX-1 is a target gene of PPARγ[38,181] and treatment of rat insulinoma cells Ins-1 with TZD increases the expression of Nkx6.1, another transcription factor implicated in insulin transcription[38].
Insulin secretion by β-cells is mainly dependent on the activation of ATP-sensitive potassium channel (K-ATP): building up of glucose in the β-cells increases the ATP/ADP ratio and brings up to the closure of K-ATP channels followed by membrane depolarization and influx of Ca2+ from ER and extracellular space: the end result of this process is the fusion of insulin-containing vesicles with the plasma membrane and insulin secretion. PPARγ increases insulin secretion in β-cells regulating the expression of G-protein-coupled transmembrane receptor 40, a FFA receptor (for a review of FFA role on insulin secretion see[182]), and possibly through the up-regulation of the cholesterol transporter ATP-binding cassette transporter A1 whose expression is increased after TZD treatment. On the other hand PPARγ also induces the expression of GLUT-2 and GLUT-4 and of other glycolytic enzymes that influence the ATP/ADP ratio[183-185].
Moreover PPARγ may ameliorates DM type 2 increasing the insulin sensitivity in the peripheral tissues. PPARγ increases the uptake of glucose in the skeletal and adipose tissue by regulation of glucose transporters and might increases insulin signaling modulating AMPK, much as metformin[87]. Activation of AMPK increases muscle glucose uptake, reduces liver gluconeogenesis and hepatic glucose output as well as increasing fatty acid oxidation, generally promoting catabolic events to produce ATP[14,87]. mTORC inhibition and p53 activation follow AMPK activation and interestingly this is a key mechanism used by adiponectin to oppose leptin effects[156].
Nonetheless it is supposed that the main effect of TZD and PPARγ is primarily due to the increased insulin secretion by β-cells and activation of lipid and glucose metabolism in adipocytes and only secondary to the metabolic effects on liver and skeletal muscle, a hypothesis that bridges DM type 2 to obesity.
PPARγ is a master regulator of adipocytes differentiation. Pre-adipocytes are maintained in un-differentiated state by the Wnt/β-catenin pathways that activates the expression of COUP-TFII NR which recruits the SMRT corepressor complex to repress PPARG1 and PPARG2 gene[186]. Interestingly, a regulatory network linking activation of β-catenin to PPARγ has been hypothesized to participate in the resistance to oxidative stress[19].
Obesity is characterized by accumulation of visceral adipose tissue that produces high quantities of inflammatory cytokines, mainly leptin but also IL-1β, TNF-α, IL-8, IL-6[187]. In adipocytes, PPARγ activation has been associated to the up-regulation of IRS-2 and CAP components of insulin pathway and hence to increased insulin-sensitivity[188,189].
Activation of PPARγ in pre-adipocytes leads to the novo differentiation of subcutaneous adipocytes accompanied by apoptosis of older visceral adipocytes[28,190]. Despite the overall risk of increased body weight in patients treated with TZD, redistribution of lipid and fat from visceral to subcutaneous depots has advantages in the long term: the new adipocytes are smaller and more insulin-sensitive. Formation of new adipocytes also reduces the concentration of circulating FFA and brings to a concomitant reduction of lipotoxicity both in β-cells and in pancreatic tissues whose infiltration by lipid-containing cells is associated to increased risks of developing PanIN lesion[191]. Moreover the new adipose tissue produces higher amounts of anti-inflammatory adiponectin and lower of leptin. Production of both adipokines is under direct and indirect control of PPARγ: leptin expression is indirectly reduced by PPARγ by antagonism on leptin promoter with C/EBP whereas adiponectin is under direct control of the NR that also induces the secretion of HMW adiponectin from adipocytes[192].
Moreover, in mice PPARγ and C/EBP synergize to induce adipocyte-specific expression of resistin, another adipokine associated with inflammation and DM type 2[193]. Noteworthy, in human the main source of resistin are macrophages and PPARγ inhibits resistin synthesis[194]. Macrophages can acquire distinct phenotypes depending on the stimuli they receive from the environment: the M1 is considered an inflammatory phenotype whereas the M2 macrophages are considered anti-inflammatory[195,196]; interestingly, adipose tissue in obese and DM type 2 patients is characteristically rich in M1 macrophages. PPARγ primes primary human monocytes into M2 differentiation but does not influence M2 marker expression in resting or M1 macrophages, and regulates the expression of CD36, a scavenger receptor that recognize oxidized lipids and is an hallmark of M2 phenotype[19,196].
Apart from regulating levels of the classic adipokines adiponectin and leptin, PPARγ may suppress the production of the pro-inflammatory cytokines TNF-α, IL-6, PAI-1, in adipose tissue. Suppression of these cytokines is associated with the repression of NF-κb signaling cascade. It is well known that PPARγ and NF-κB interact in several ways. PPARγ transrepresses NF-κB acting as a transcriptional co-repressor of NF-κB-target genes or by direct binding with NF-κB[19,197,198]; moreover the nuclear receptor may reduce NF-κB activation and even induce its degradation[39]. PPARγ inhibits NF-κB signaling also in immune cells, an inhibition that results in a substantial anti-inflammatory response. Conversely, NF-κB negatively regulates PPARγ transcriptional activity through histone deacetylase 3[19].
We may hypothesize that these systemic beneficial alterations are accompanied by a protective and anti-tumorigenic effects on PDAC. Indeed, as we previously described, experimental evidence suggest that PPARγ might be protective against PDAC, reducing tumor growth and metastatization and inducing apoptosis, and patients treated with TZD have a lower risks to develop cancer[113]. As we cited previously, PPARγ activation results in modulation of AMPK and interestingly in cancer cells AMPK activation suppresses and reverses EMT by modulating the AKT-MDM2-Foxo3 signaling pathway and hence blocking the metastatization of cancer cells[199]; this evidence suggests that PPARγ-direct inhibition of metastatization might be reinforced indirectly by other genes.
The inhibition of inflammation and the reduction of ROS and oxidative stress is also important in tumor progression impairing given that production of ROS is necessary in Kras mediated tumorigenesis[111]. We have previously noted that PSC activation is a typical feature of PDAC and the ensuing desmoplastic reaction constitutes a bivalent cellular habitat. Indeed, Apte et al[76] proposed that in the earliest stages of carcinogenesis PSC activation could represent an attempt to initially restrict tumor growth while later on PDAC development cancer cells subvert PSC into cancer-permissive cells protecting tumor growth. PPARγ is important in maintaining PSC in their “quiescent” vitamin A phenotype and hence activation of PPARγ could theoretically impair PSC activation and the formation of a permissive cancer habitat. This effect could be strengthened by the alteration of adipokines: indeed leptin signaling is pro-fibrogenic and sustains the proliferation of liver stellate cells facilitating the M phase as well as altering the levels of TIMP factors; conversely, adiponectin in stellate cells induces apoptosis and reduces the expression of collagen 1α1[200].
Although the mechanisms linking obesity and diabetes to PDAC are not fully understood, we have described strong experimental evidence showing that the adopted nuclear receptor PPARγ is implicated in the three process. Being at the cross-road of these interlinked diseases PPARγ is a strong candidate for a combined therapeutic approach devoted to the prevention and to the treatment of PDAC in obese and diabetic patients.
As a concluding remark, PPARγ agonists may be and are used to treat insulin-resistance with great effect, technically and theoretically they may be used to ameliorate the obesity and obesity-associated inflammation and the desmoplastic reaction, they have proven to be effective both in vitro and in vivo in the treatment of PDAC. And so, may we conclude that PPARγ is the perfect choice and we have a cure for these diseases? Unfortunately, not now, not yet.
What is still missing is a precise understanding of the association of these diseases and more importantly a safer and effective PPARγ agonist to be used for the treatment of metabolic syndrome, inflammation, and PDAC. Clinical use of PPARγ agonists for PDAC treatment never passed clinical trials although TZD are used for diabetes[28,113].
As it stands out, PPARγ if not the “perfect choice” is as far as it gets a “good choice” that could become “perfect” if and when a drug with a safer profile and no PPARγ-independent effects will be discovered.
At least, PPARγ is a start.
P- Reviewer: Peng Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 476] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Chen T. Nuclear receptor drug discovery. Curr Opin Chem Biol. 2008;12:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 831] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635-641. [PubMed] |
| 5. | Polvani S, Tarocchi M, Tempesti S, Galli A. Nuclear receptors and pathogenesis of pancreatic cancer. World J Gastroenterol. 2014;20:12062-12081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Polvani S, Tarocchi M, Tempesti S, Mello T, Ceni E, Buccoliero F, D’Amico M, Boddi V, Farsi M, Nesi S. COUP-TFII in pancreatic adenocarcinoma: clinical implication for patient survival and tumor progression. Int J Cancer. 2014;134:1648-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol. 2006;21:533-540. [PubMed] |
| 8. | Guan J, Zhang H, Wen Z, Gu Y, Cheng Y, Sun Y, Zhang T, Jia C, Lu Z, Chen J. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014;345:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Safe S, Jin UH, Hedrick E, Reeder A, Lee SO. Minireview: role of orphan nuclear receptors in cancer and potential as drug targets. Mol Endocrinol. 2014;28:157-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | de Duve C. The peroxisome: a new cytoplasmic organelle. Proc R Soc Lond B Biol Sci. 1969;173:71-83. [PubMed] |
| 11. | Gabaldón T. Peroxisome diversity and evolution. Philos Trans R Soc Lond B Biol Sci. 2010;365:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2546] [Cited by in RCA: 2520] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 13. | Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879-887. [PubMed] |
| 14. | Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009;2009:818945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Mello T, Polvani S, Galli A. Peroxisome proliferator-activated receptor and retinoic x receptor in alcoholic liver disease. PPAR Res. 2009;2009:748174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Aprile M, Ambrosio MR, D’Esposito V, Beguinot F, Formisano P, Costa V, Ciccodicola A. PPARG in Human Adipogenesis: Differential Contribution of Canonical Transcripts and Dominant Negative Isoforms. PPAR Res. 2014;2014:537865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Sabatino L, Casamassimi A, Peluso G, Barone MV, Capaccio D, Migliore C, Bonelli P, Pedicini A, Febbraro A, Ciccodicola A. A novel peroxisome proliferator-activated receptor gamma isoform with dominant negative activity generated by alternative splicing. J Biol Chem. 2005;280:26517-26525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 662] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 19. | Polvani S, Tarocchi M, Galli A. PPARγ and Oxidative Stress: Con(β) Catenating NRF2 and FOXO. PPAR Res. 2012;2012:641087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58:760-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 392] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Kojetin DJ, Matta-Camacho E, Hughes TS, Srinivasan S, Nwachukwu JC, Cavett V, Nowak J, Chalmers MJ, Marciano DP, Kamenecka TM. Structural mechanism for signal transduction in RXR nuclear receptor heterodimers. Nat Commun. 2015;6:8013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Lefebvre B, Benomar Y, Guédin A, Langlois A, Hennuyer N, Dumont J, Bouchaert E, Dacquet C, Pénicaud L, Casteilla L. Proteasomal degradation of retinoid X receptor alpha reprograms transcriptional activity of PPARgamma in obese mice and humans. J Clin Invest. 2010;120:1454-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 819] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 24. | Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1312] [Cited by in RCA: 1330] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 25. | Viswakarma N, Jia Y, Bai L, Vluggens A, Borensztajn J, Xu J, Reddy JK. Coactivators in PPAR-Regulated Gene Expression. PPAR Res. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Huang B, Cheng X, Wang D, Peng M, Xue Z, Da Y, Zhang N, Yao Z, Li M, Xu A. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1α signaling. Oncotarget. 2014;5:4732-4745. [PubMed] |
| 27. | Martínez-Redondo V, Pettersson AT, Ruas JL. The hitchhiker’s guide to PGC-1α isoform structure and biological functions. Diabetologia. 2015;58:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 28. | Monsalve FA, Pyarasani RD, Delgado-Lopez F, Moore-Carrasco R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediators Inflamm. 2013;2013:549627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 29. | Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1016] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 30. | Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 479] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 31. | Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803-812. [PubMed] |
| 32. | Shu L, Huang R, Wu S, Chen Z, Sun K, Jiang Y, Gong P, Cai X. PPARγ and Its Ligands, Potential Anti-Tumor Agents in the Digestive System. Curr Stem Cell Res Ther. 2015;Epub ahead of print. [PubMed] |
| 33. | Ceni E, Mello T, Tarocchi M, Crabb DW, Caldini A, Invernizzi P, Surrenti C, Milani S, Galli A. Antidiabetic thiazolidinediones induce ductal differentiation but not apoptosis in pancreatic cancer cells. World J Gastroenterol. 2005;11:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Kitamura S, Miyazaki Y, Hiraoka S, Nagasawa Y, Toyota M, Takakura R, Kiyohara T, Shinomura Y, Matsuzawa Y. PPARgamma agonists inhibit cell growth and suppress the expression of cyclin D1 and EGF-like growth factors in ras-transformed rat intestinal epithelial cells. Int J Cancer. 2001;94:335-342. [PubMed] |
| 35. | Hong J, Samudio I, Liu S, Abdelrahim M, Safe S. Peroxisome proliferator-activated receptor gamma-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology. 2004;145:5774-5785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Galli A, Ceni E, Mello T, Polvani S, Tarocchi M, Buccoliero F, Lisi F, Cioni L, Ottanelli B, Foresta V. Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis B virus-transgenic mice by peroxisome proliferator-activated receptor gamma-independent regulation of nucleophosmin. Hepatology. 2010;52:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Sugii S, Evans RM. Epigenetic codes of PPARγ in metabolic disease. FEBS Lett. 2011;585:2121-2128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:1036-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Hou Y, Moreau F, Chadee K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat Commun. 2012;3:1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 40. | Hou Y, Gao J, Xu H, Xu Y, Zhang Z, Xu Q, Zhang C. PPARγ E3 ubiquitin ligase regulates MUC1-C oncoprotein stability. Oncogene. 2014;33:5619-5625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Ceni E, Crabb DW, Foschi M, Mello T, Tarocchi M, Patussi V, Moraldi L, Moretti R, Milani S, Surrenti C. Acetaldehyde inhibits PPARgamma via H2O2-mediated c-Abl activation in human hepatic stellate cells. Gastroenterology. 2006;131:1235-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | von Knethen A, Tzieply N, Jennewein C, Brüne B. Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J Cell Sci. 2010;123:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 44. | Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 721] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 45. | Choi JH, Choi SS, Kim ES, Jedrychowski MP, Yang YR, Jang HJ, Suh PG, Banks AS, Gygi SP, Spiegelman BM. Thrap3 docks on phosphoserine 273 of PPARγ and controls diabetic gene programming. Genes Dev. 2014;28:2361-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Kondo NI, Ikeda Y. Practical management and treatment of pancreatic neuroendocrine tumors. Gland Surg. 2014;3:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 47. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 48. | Fendrich V, Waldmann J, Bartsch DK, Langer P. Surgical management of pancreatic endocrine tumors. Nat Rev Clin Oncol. 2009;6:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Sahora K, Fernández-del Castillo C. Intraductal papillary mucinous neoplasms. Curr Opin Gastroenterol. 2015;31:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Fukushima N, Fukayama M. Mucinous cystic neoplasms of the pancreas: pathology and molecular genetics. J Hepatobiliary Pancreat Surg. 2007;14:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, Brennan M, Cameron JL, Klimstra DS. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953-962. [PubMed] |
| 52. | Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF, O’Reilly EM. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 54. | Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1647] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 55. | Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91:1410-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Collins MA, Pasca di Magliano M. Kras as a key oncogene and therapeutic target in pancreatic cancer. Front Physiol. 2013;4:407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Yonezawa S, Higashi M, Yamada N, Goto M. Precursor lesions of pancreatic cancer. Gut Liver. 2008;2:137-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Distler M, Aust D, Weitz J, Pilarsky C, Grützmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int. 2014;2014:474905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 59. | Pin CL, Ryan JF, Mehmood R. Acinar cell reprogramming: a clinically important target in pancreatic disease. Epigenomics. 2015;7:267-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Wagner M, Lührs H, Klöppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254-1262. [PubMed] |
| 61. | Wagner M, Greten FR, Weber CK, Koschnick S, Mattfeldt T, Deppert W, Kern H, Adler G, Schmid RM. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | De La O JP, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci USA. 2008;105:18907-18912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 63. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 64. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 65. | Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007;34:284-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol. 2015;21:9297-9316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 67. | Reitz D, Gerger A, Seidel J, Kornprat P, Samonigg H, Stotz M, Szkandera J, Pichler M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J Clin Pathol. 2015;68:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, Kim BR. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1667] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 70. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 71. | Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 72. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2776] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 73. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5637] [Article Influence: 402.6] [Reference Citation Analysis (1)] |
| 74. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4887] [Article Influence: 407.3] [Reference Citation Analysis (0)] |
| 75. | Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 76. | Apte M, Pirola RC, Wilson JS. Pancreatic stellate cell: physiologic role, role in fibrosis and cancer. Curr Opin Gastroenterol. 2015;31:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924-1940. [PubMed] |
| 78. | Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1455] [Cited by in RCA: 1616] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 79. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2541] [Article Influence: 158.8] [Reference Citation Analysis (0)] |
| 80. | Burness CB. Sonidegib: First Global Approval. Drugs. 2015;75:1559-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Wörmann SM, Algül H. Risk factors and therapeutic targets in pancreatic cancer. Front Oncol. 2013;3:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Vrieling A, Bueno-de-Mesquita HB, Boshuizen HC, Michaud DS, Severinsen MT, Overvad K, Olsen A, Tjønneland A, Clavel-Chapelon F, Boutron-Ruault MC. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126:2394-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Michaud DS, Vrieling A, Jiao L, Mendelsohn JB, Steplowski E, Lynch SM, Wactawski-Wende J, Arslan AA, Bas Bueno-de-Mesquita H, Fuchs CS. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan). Cancer Causes Control. 2010;21:1213-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Cade JE, Burley VJ, Norat T. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:2536-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 86. | Sasaoka T, Kobayashi M. The functional significance of Shc in insulin signaling as a substrate of the insulin receptor. Endocr J. 2000;47:373-381. [PubMed] |
| 87. | Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 655] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 88. | Govers R. Cellular regulation of glucose uptake by glucose transporter GLUT4. Adv Clin Chem. 2014;66:173-240. [PubMed] |
| 89. | Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 329] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 90. | Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 608] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 91. | Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123-135. [PubMed] |
| 92. | Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 93. | Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun Rev. 2015;14:781-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 94. | Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 95. | Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes. 2015;6:707-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 96. | Santoni M, Andrikou K, Sotte V, Bittoni A, Lanese A, Pellei C, Piva F, Conti A, Nabissi M, Santoni G. Toll like receptors and pancreatic diseases: From a pathogenetic mechanism to a therapeutic target. Cancer Treat Rev. 2015;41:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Prajapati B, Jena PK, Rajput P, Purandhar K, Seshadri S. Understanding and modulating the Toll like Receptors (TLRs) and NOD like Receptors (NLRs) cross talk in type 2 diabetes. Curr Diabetes Rev. 2014;10:190-200. [PubMed] |
| 98. | Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95:727-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 541] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 99. | Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1596] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 100. | Andersen DK, Andren-Sandberg Å, Duell EJ, Goggins M, Korc M, Petersen GM, Smith JP, Whitcomb DC. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013;42:1227-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 101. | Andersen DK. Diabetes and cancer: placing the association in perspective. Curr Opin Endocrinol Diabetes Obes. 2013;20:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Funahashi H, Satake M, Dawson D, Huynh NA, Reber HA, Hines OJ, Eibl G. Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras(G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res. 2007;67:7068-7071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Salvatore T, Marfella R, Rizzo MR, Sasso FC. Pancreatic cancer and diabetes: A two-way relationship in the perspective of diabetologist. Int J Surg. 2015;21 Suppl 1:S72-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Meier JJ, Giese A. Diabetes associated with pancreatic diseases. Curr Opin Gastroenterol. 2015;31:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 106. | Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, Petersen GM. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 107. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 108. | Bobrowski A, Spitzner M, Bethge S, Mueller-Graf F, Vollmar B, Zechner D. Risk factors for pancreatic ductal adenocarcinoma specifically stimulate pancreatic duct glands in mice. Am J Pathol. 2013;182:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Trajkovic-Arsic M, Kalideris E, Siveke JT. The role of insulin and IGF system in pancreatic cancer. J Mol Endocrinol. 2013;50:R67-R74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 110. | Cohen R, Neuzillet C, Tijeras-Raballand A, Faivre S, de Gramont A, Raymond E. Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget. 2015;6:16832-16847. [PubMed] |
| 111. | Sousa CM, Kimmelman AC. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 2014;35:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 112. | Mamedova LK, Yuan K, Laudick AN, Fleming SD, Mashek DG, Bradford BJ. Toll-like receptor 4 signaling is required for induction of gluconeogenic gene expression by palmitate in human hepatic carcinoma cells. J Nutr Biochem. 2013;24:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Bao B, Wang Z, Li Y, Kong D, Ali S, Banerjee S, Ahmad A, Sarkar FH. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta. 2011;1815:135-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci USA. 2010;107:10791-10798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 115. | Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2003;48:1972-1978. [PubMed] |
| 116. | Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, Solomides C, Peiper SC, Witkiewicz AK. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3484-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 117. | Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47:R1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 118. | Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 119. | Ooi GT, Tseng LY, Tran MQ, Rechler MM. Insulin rapidly decreases insulin-like growth factor-binding protein-1 gene transcription in streptozotocin-diabetic rats. Mol Endocrinol. 1992;6:2219-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Zechner D, Radecke T, Amme J, Bürtin F, Albert AC, Partecke LI, Vollmar B. Impact of diabetes type II and chronic inflammation on pancreatic cancer. BMC Cancer. 2015;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila). 2012;5:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 122. | Povoski SP, Fenoglio-Preiser CM, Sayers HJ, McCullough PJ, Zhou W, Bell RH. Effect of streptozotocin diabetes on development of nitrosamine-induced pancreatic carcinoma when diabetes induction occurs after nitrosamine exposure. Carcinogenesis. 1993;14:961-967. [PubMed] |
| 123. | Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 124. | Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 125. | Summer R, Walsh K, Medoff BD. Obesity and pulmonary arterial hypertension: Is adiponectin the molecular link between these conditions? Pulm Circ. 2011;1:440-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 126. | Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 467] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 127. | Tishinsky JM, Dyck DJ, Robinson LE. Lifestyle factors increasing adiponectin synthesis and secretion. Vitam Horm. 2012;90:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Wang GR, Li L, Pan YH, Tian GD, Lin WL, Li Z, Chen ZY, Gong YL, Kikano GE, Stange KC. Prevalence of metabolic syndrome among urban community residents in China. BMC Public Health. 2013;13:599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84:289-298. [PubMed] |
| 130. | Marroquí L, Gonzalez A, Ñeco P, Caballero-Garrido E, Vieira E, Ripoll C, Nadal A, Quesada I. Role of leptin in the pancreatic β-cell: effects and signaling pathways. J Mol Endocrinol. 2012;49:R9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 131. | Kuehnen P, Laubner K, Raile K, Schöfl C, Jakob F, Pilz I, Päth G, Seufert J. Protein phosphatase 1 (PP-1)-dependent inhibition of insulin secretion by leptin in INS-1 pancreatic β-cells and human pancreatic islets. Endocrinology. 2011;152:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 132. | Krechler T, Zeman M, Vecka M, Macasek J, Jachymova M, Zima T, Zak A. Leptin and adiponectin in pancreatic cancer: connection with diabetes mellitus. Neoplasma. 2011;58:58-64. [PubMed] |
| 133. | Kellerer M, Lammers R, Fritsche A, Strack V, Machicao F, Borboni P, Ullrich A, Häring HU. Insulin inhibits leptin receptor signalling in HEK293 cells at the level of janus kinase-2: a potential mechanism for hyperinsulinaemia-associated leptin resistance. Diabetologia. 2001;44:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 134. | Mendonsa AM, Chalfant MC, Gorden LD, VanSaun MN. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS One. 2015;10:e0126686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 135. | Fan Y, Gan Y, Shen Y, Cai X, Song Y, Zhao F, Yao M, Gu J, Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120-16134. [PubMed] |
| 136. | Ren H, Jia L, Zhao T, Zhang H, Chen J, Yang S, Liu J, Yu M, Hao J. Hypoxia inducible factor (HIF)-1α directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014;354:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 137. | Brown DR, Berkowitz DE, Breslow MJ. Weight loss is not associated with hyperleptinemia in humans with pancreatic cancer. J Clin Endocrinol Metab. 2001;86:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 138. | Fujiwara Y, Kobayashi T, Chayahara N, Imamura Y, Toyoda M, Kiyota N, Mukohara T, Nishiumi S, Azuma T, Yoshida M. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS One. 2014;9:e113259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 139. | Gąsiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Małecka-Panas E. Role of adipocytokines and its correlation with endocrine pancreatic function in patients with pancreatic cancer. Pancreatology. 2013;13:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Okitsu K, Kanda T, Imazeki F, Yonemitsu Y, Ray RB, Chang C, Yokosuka O. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer. 2010;1:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359-6374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 142. | Catalano S, Leggio A, Barone I, De Marco R, Gelsomino L, Campana A, Malivindi R, Panza S, Giordano C, Liguori A. A novel leptin antagonist peptide inhibits breast cancer growth in vitro and in vivo. J Cell Mol Med. 2015;19:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 143. | Dong Z, Fu S, Xu X, Yang Y, Du L, Li W, Kan S, Li Z, Zhang X, Wang L. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer. 2014;110:1801-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 144. | Feng H, Liu Q, Zhang N, Zheng L, Sang M, Feng J, Zhang J, Wu X, Shan B. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol Res. 2013;21:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 145. | Kato S, Abarzua-Catalan L, Trigo C, Delpiano A, Sanhueza C, García K, Ibañez C, Hormazábal K, Diaz D, Brañes J. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: an explanation for poor outcomes in obese women. Oncotarget. 2015;6:21100-21119. [PubMed] |
| 146. | Noda T, Kikugawa T, Tanji N, Miura N, Asai S, Higashiyama S, Yokoyama M. Long-term exposure to leptin enhances the growth of prostate cancer cells. Int J Oncol. 2015;46:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 147. | Yuan HJ, Sun KW, Yu K. Leptin promotes the proliferation and migration of human breast cancer through the extracellular-signal regulated kinase pathway. Mol Med Rep. 2014;9:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 148. | Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. [PubMed] |
| 149. | Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697-10703. [PubMed] |
| 150. | Jortay J, Senou M, Abou-Samra M, Noel L, Robert A, Many MC, Brichard SM. Adiponectin and skeletal muscle: pathophysiological implications in metabolic stress. Am J Pathol. 2012;181:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 151. | Crawford LJ, Peake R, Price S, Morris TC, Irvine AE. Adiponectin is produced by lymphocytes and is a negative regulator of granulopoiesis. J Leukoc Biol. 2010;88:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 152. | Simpson F, Whitehead JP. Adiponectin--it’s all about the modifications. Int J Biochem Cell Biol. 2010;42:785-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 153. | Tan PH, Tyrrell HE, Gao L, Xu D, Quan J, Gill D, Rai L, Ding Y, Plant G, Chen Y. Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res. 2014;74:5711-5722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 154. | Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, Mizutani A, Yokoyama H, Irie R, Sumimoto H. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 155. | Grote VA, Rohrmann S, Dossus L, Nieters A, Halkjaer J, Tjønneland A, Overvad K, Stegger J, Chabbert-Buffet N, Boutron-Ruault MC. The association of circulating adiponectin levels with pancreatic cancer risk: a study within the prospective EPIC cohort. Int J Cancer. 2012;130:2428-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19:1926-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 157. | Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, Karmaniolas K, Pelecanos N, Tseleni-Balafouta S, Dionyssiou-Asteriou A. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer Causes Control. 2009;20:625-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 158. | Chang MC, Chang YT, Su TC, Yang WS, Chen CL, Tien YW, Liang PC, Wei SC, Wong JM. Adiponectin as a potential differential marker to distinguish pancreatic cancer and chronic pancreatitis. Pancreas. 2007;35:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 159. | Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 160. | Stolzenberg-Solomon RZ, Weinstein S, Pollak M, Tao Y, Taylor PR, Virtamo J, Albanes D. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am J Epidemiol. 2008;168:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 161. | Yang JP, Li X, Wang F, Gao M, Li SL, Chen KS. Association analysis of genetic variants of adiponectin gene and risk of pancreatic cancer. Int J Clin Exp Med. 2015;8:8094-8100. [PubMed] |
| 162. | Kuruma S, Egawa N, Kurata M, Honda G, Kamisawa T, Ueda J, Ishii H, Ueno M, Nakao H, Mori M. Case-control study of diabetes-related genetic variants and pancreatic cancer risk in Japan. World J Gastroenterol. 2014;20:17456-17462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 163. | Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623-33631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 164. | Herreros-Villanueva M, Hijona E, Cosme A, Bujanda L. Mouse models of pancreatic cancer. World J Gastroenterol. 2012;18:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 165. | Lanza-Jacoby S, Yan G, Radice G, LePhong C, Baliff J, Hess R. Calorie restriction delays the progression of lesions to pancreatic cancer in the LSL-KrasG12D; Pdx-1/Cre mouse model of pancreatic cancer. Exp Biol Med (Maywood). 2013;238:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 166. | Kato M, Watabe K, Tsujii M, Funahashi T, Shimomura I, Takehara T. Adiponectin inhibits murine pancreatic cancer growth. Dig Dis Sci. 2014;59:1192-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 167. | Dicitore A, Caraglia M, Colao A, Zappavigna S, Mari D, Hofland LJ, Persani L, Vitale G. Combined treatment with PPAR-γ agonists in pancreatic cancer: a glimmer of hope for cancer therapy? Curr Cancer Drug Targets. 2013;13:460-471. [PubMed] |
| 168. | Dicitore A, Caraglia M, Gaudenzi G, Manfredi G, Amato B, Mari D, Persani L, Arra C, Vitale G. Type I interferon-mediated pathway interacts with peroxisome proliferator activated receptor-γ (PPAR-γ): at the cross-road of pancreatic cancer cell proliferation. Biochim Biophys Acta. 2014;1845:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 169. | Nakajima A, Tomimoto A, Fujita K, Sugiyama M, Takahashi H, Ikeda I, Hosono K, Endo H, Yoneda K, Iida H. Inhibition of peroxisome proliferator-activated receptor gamma activity suppresses pancreatic cancer cell motility. Cancer Sci. 2008;99:1892-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 170. | Sawai H, Liu J, Reber HA, Hines OJ, Eibl G. Activation of peroxisome proliferator-activated receptor-gamma decreases pancreatic cancer cell invasion through modulation of the plasminogen activator system. Mol Cancer Res. 2006;4:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 171. | Duffy MJ. The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des. 2004;10:39-49. [PubMed] |
| 172. | Koga H, Selvendiran K, Sivakumar R, Yoshida T, Torimura T, Ueno T, Sata M. PPARγ potentiates anticancer effects of gemcitabine on human pancreatic cancer cells. Int J Oncol. 2012;40:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 173. | Maniati E, Bossard M, Cook N, Candido JB, Emami-Shahri N, Nedospasov SA, Balkwill FR, Tuveson DA, Hagemann T. Crosstalk between the canonical NF-κB and Notch signaling pathways inhibits Pparγ expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121:4685-4699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 174. | Ninomiya I, Yamazaki K, Oyama K, Hayashi H, Tajima H, Kitagawa H, Fushida S, Fujimura T, Ohta T. Pioglitazone inhibits the proliferation and metastasis of human pancreatic cancer cells. Oncol Lett. 2014;8:2709-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 175. | Eibl G, Takata Y, Boros LG, Liu J, Okada Y, Reber HA, Hines OJ. Growth stimulation of COX-2-negative pancreatic cancer by a selective COX-2 inhibitor. Cancer Res. 2005;65:982-990. [PubMed] |
| 176. | Sun WH, Chen GS, Ou XL, Yang Y, Luo C, Zhang Y, Shao Y, Xu HC, Xiao B, Xue YP. Inhibition of COX-2 and activation of peroxisome proliferator-activated receptor gamma synergistically inhibits proliferation and induces apoptosis of human pancreatic carcinoma cells. Cancer Lett. 2009;275:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 177. | Kadera BE, Toste PA, Wu N, Li L, Nguyen AH, Dawson DW, Donahue TR. Low expression of the E3 ubiquitin ligase CBL confers chemoresistance in human pancreatic cancer and is targeted by epidermal growth factor receptor inhibition. Clin Cancer Res. 2015;21:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 178. | Giaginis C, Katsamangou E, Tsourouflis G, Zizi-Serbetzoglou D, Kouraklis G, Theocharis S. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor-alpha expression in pancreatic ductal adenocarcinoma: association with clinicopathological parameters, tumor proliferative capacity, and patients’ survival. Med Sci Monit. 2009;15:BR148-BR156. [PubMed] |
| 179. | Kristiansen G, Jacob J, Buckendahl AC, Grützmann R, Alldinger I, Sipos B, Klöppel G, Bahra M, Langrehr JM, Neuhaus P. Peroxisome proliferator-activated receptor gamma is highly expressed in pancreatic cancer and is associated with shorter overall survival times. Clin Cancer Res. 2006;12:6444-6451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 180. | Zhang Y, Luo HY, Liu GL, Wang DS, Wang ZQ, Zeng ZL, Xu RH. Prognostic significance and therapeutic implications of peroxisome proliferator-activated receptor γ overexpression in human pancreatic carcinoma. Int J Oncol. 2015;46:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 181. | Gupta D, Jetton TL, Mortensen RM, Duan SZ, Peshavaria M, Leahy JL. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. J Biol Chem. 2008;283:32462-32470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 182. | Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55 Suppl 2:S16-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 183. | Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. 1998;101:22-32. [PubMed] |
| 184. | Im SS, Kim JW, Kim TH, Song XL, Kim SY, Kim HI, Ahn YH. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp Mol Med. 2005;37:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 185. | Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1694] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 186. | Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne TF. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci USA. 2009;106:5819-5824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 187. | O’Sullivan KE, Reynolds JV, O’Hanlon C, O’Sullivan JN, Lysaght J. Could signal transducer and activator of transcription 3 be a therapeutic target in obesity-related gastrointestinal malignancy? J Gastrointest Cancer. 2014;45:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 188. | Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B. Thiazolidinediones (PPARgamma agonists) but not PPARalpha agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. FASEB J. 2001;15:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 189. | Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci USA. 1998;95:14751-14756. [PubMed] |
| 190. | Kajita K, Mori I, Kitada Y, Taguchi K, Kajita T, Hanamoto T, Ikeda T, Fujioka K, Yamauchi M, Okada H. Small proliferative adipocytes: identification of proliferative cells expressing adipocyte markers. Endocr J. 2013;60:931-939. [PubMed] |
| 191. | Rebours V, Gaujoux S, d’Assignies G, Sauvanet A, Ruszniewski P, Lévy P, Paradis V, Bedossa P, Couvelard A. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin Cancer Res. 2015;21:3522-3528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 192. | Hollenberg AN, Susulic VS, Madura JP, Zhang B, Moller DE, Tontonoz P, Sarraf P, Spiegelman BM, Lowell BB. Functional antagonism between CCAAT/Enhancer binding protein-alpha and peroxisome proliferator-activated receptor-gamma on the leptin promoter. J Biol Chem. 1997;272:5283-5290. [PubMed] |
| 193. | Tomaru T, Steger DJ, Lefterova MI, Schupp M, Lazar MA. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding proteins. J Biol Chem. 2009;284:6116-6125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 194. | Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472-476. [PubMed] |
| 195. | Rios FJ, Koga MM, Pecenin M, Ferracini M, Gidlund M, Jancar S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm. 2013;2013:198193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 196. | Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1074] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 197. | Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, Kim TS. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem. 2000;275:32681-32687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 281] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 198. | Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844-4849. [PubMed] |
| 199. | Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, Shapiro CL, Chen CS. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74:4783-4795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 200. | Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab. 2015;26:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |