Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2383
Peer-review started: July 30, 2015
First decision: August 28, 2015
Revised: September 11, 2015
Accepted: December 12, 2015
Article in press: December 12, 2015
Published online: February 21, 2016
Processing time: 192 Days and 19.7 Hours
A pancreatic tumor was suspected on the abdominal ultrasound of a 72-year-old man. Abdominal computed tomography showed pancreatic enlargement as well as a diffuse, poorly enhanced area in the pancreas; endoscopic ultrasound-guided fine needle aspiration biopsy and endoscopic retrograde cholangiopancreatography failed to provide a definitive diagnosis. Based on the trend of improvement of the pancreatic enlargement, the treatment plan involved follow-up examinations. Later, he was hospitalized with an alveolar hemorrhage and rapidly progressive glomerulonephritis; he tested positive for myeloperoxidase-anti-neutrophil cytoplasmic antibody (ANCA) and was diagnosed with ANCA-related vasculitis, specifically microscopic polyangiitis. It appears that factors such as thrombus formation caused by the vasculitis in the early stages of ANCA-related vasculitis cause abnormal distribution of the pancreatic blood flow, resulting in non-uniform pancreatitis. Pancreatic lesions in ANCA-related vasculitis are very rare. Only a few cases have been reported previously. Therefore, we report our case and a review of the literature.
Core tip: Pancreatic lesions in anti-neutrophil cytoplasmic antibody (ANCA)-related vasculitis are very rare. Only few cases of pancreatic lesions in ANCA-related vasculitis have been reported previously. We encountered a case presenting with pancreatic enlargement and a diffuse, poorly enhanced area in the pancreas during the early stages of ANCA-related vasculitis. In light of the clinical course, it appears that factors such as thrombus formation caused by the vasculitis during the early stages of ANCA-related vasculitis cause abnormal distribution of pancreatic blood flow, resulting in non-uniform pancreatitis manifested in the imaging findings.
- Citation: Iida T, Adachi T, Tabeya T, Nakagaki S, Yabana T, Goto A, Kondo Y, Kasai K. Rare type of pancreatitis as the first presentation of anti-neutrophil cytoplasmic antibody-related vasculitis. World J Gastroenterol 2016; 22(7): 2383-2390
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2383
Pancreatic lesions in anti-neutrophil cytoplasmic antibody (ANCA)-related vasculitis are very rare. We encountered a patient presenting with pancreatic enlargement and a diffuse, poorly enhanced area in the pancreas during the early stages of ANCA-related vasculitis. Although it is difficult to histopathologically prove findings of vasculitis in the pancreas with endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNA) or endoscopic retrograde pancreatography (ERP), in light of the clinical course, it appears that factors such as thrombus formation caused by the vasculitis in the early stages of ANCA-related vasculitis cause to be abnormal distribution of pancreatic blood flow, resulting in non-uniform pancreatitis manifested in the imaging findings described herein. Our report also includes a discussion of the related literature, since there are few previous reports on patients with pancreatic lesions in ANCA-related vasculitis presenting with pancreatitis or nodular shadows in the pancreas.
A 72-year-old man had been regularly visiting a clinic for diabetes exhibited weight loss and exacerbation of his diabetes; a pancreatic tumor was suspected on a subsequent abdominal ultrasound, and he was referred to our department in April 2014.
Upon admission, his height and weight were 175 cm and 63.7 kg, respectively; the following were also measured: blood pressure, 130/80 mmHg; pulse, 80 beats/min (regular); and body temperature, 37.0 °C. He was in a lucid state of consciousness with no neurological abnormalities. There was no anemia in the palpebral conjunctiva or yellowing of the bulbar conjunctiva. Superficial lymph nodes were not palpated. Lung and heart sounds were free of abnormal findings. The abdomen was flat, soft, and without tenderness. There was no lower leg edema.
The laboratory findings upon admission indicated that amylase and lipase were normal (59 IU/L and 53 IU/L, respectively), while trypsin level was slightly elevated (723 ng/mL; normal, 100-500 ng/mL). Findings also indicated mild inflammation: white blood cell count, 10100/μL and C-reactive protein level, 0.97 mg/dL. IgG4 level was normal, and the level of antinuclear antibodies increased 80-fold; carcinoembryonic antigen and cancer antigen 19-9 levels were normal at 2.6 ng/mL and 3.6 U/mL, respectively. Hemoglobin A1c was somewhat elevated at 10.8%.
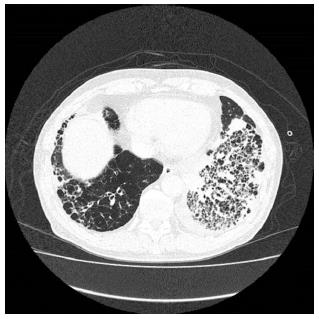
On chest radiography, a reticular shadow from both hilar areas to the lower lung field was observed.
On abdominal ultrasonography, there was mild swelling from the pancreatic body to the tail, with irregular hypoechoic masses observed in both the margins and interior of the same sites. The splenic artery had traveled through the inside of the tumor; however, the boundary was clear, and there was no obvious invasion into the surrounding adipose tissue. In the pancreatic body, the pancreatic duct was disrupted, but there was no dilation of the cephalic main pancreatic duct.
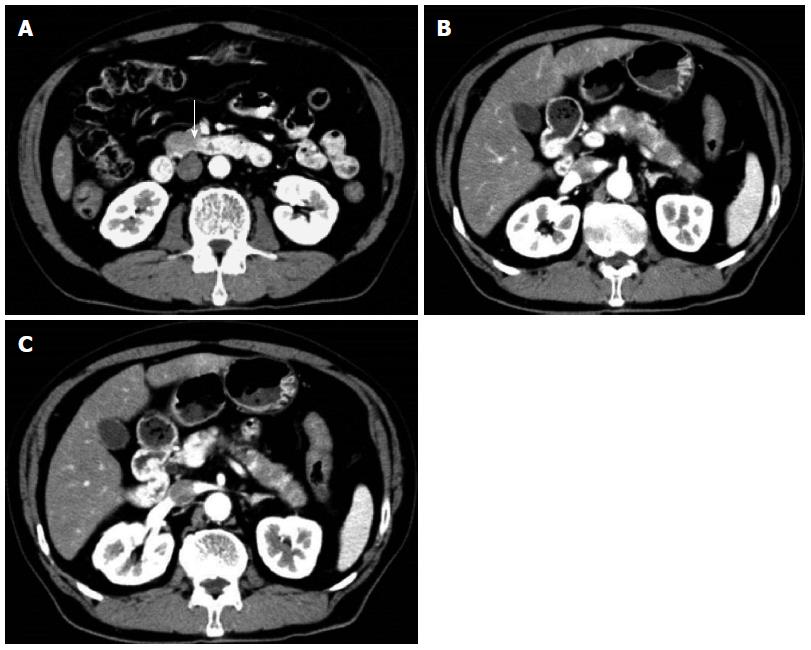
Contrast-enhanced abdominal/pelvic computed tomography (CT) (Figure 1A-C) showed pancreatic enlargement with a diffuse, poorly enhanced area in the uncinate process and pancreatic body tail.
On magnetic resonance cholangiopancreatography, the path of the main pancreatic duct in the body tail could not be identified, but dilation of the cephalic main pancreatic duct was not observed. Diffusion-weighted images showed diffuse signal changes in the pancreatic body tail.
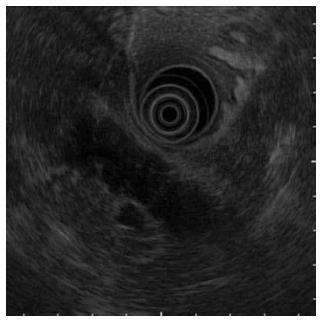
On EUS (Figure 2), a hypoechoic mass was noted in a form in which the pancreatic lobe structure was maintained in the uncinate process and from the pancreatic body to the tail.
On EUS-FNA, the hypoechoic masses of the uncinate process and pancreatic tail were each punctured three times with a 22G puncture needle (ExpectTM, Boston Scientific, Tokyo, Japan); malignant cells were not detected, and the diagnosis was nonspecific pancreatitis.
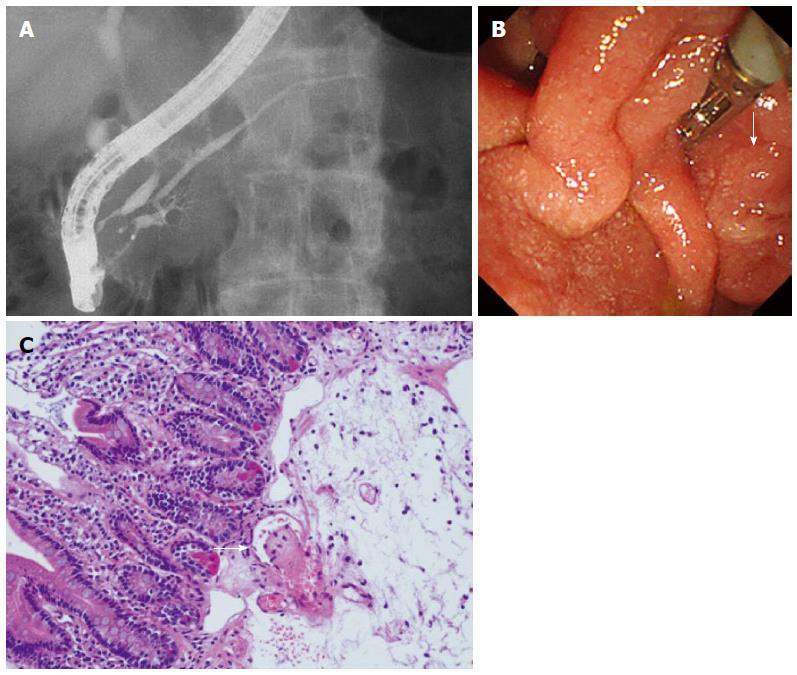
On ERP (Figure 3A-C), a slight disparity of the opening diameter was noted in the main pancreatic duct, but localized stenosis or diffuse narrowing were not observed. No abnormality was observed in the papilla of Vater, but the biopsy revealed thrombus formation.
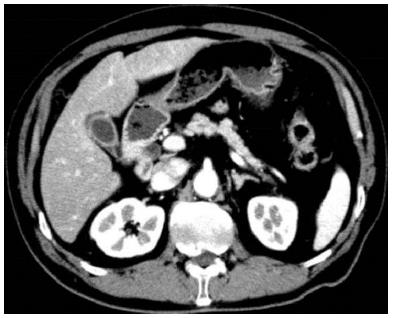
The patient was suspected of having pancreatic cancer presenting with a special distribution, malignant lymphoma, tumor-forming pancreatitis, or autoimmune pancreatitis (AIP); however, despite the various laboratory tests, a definitive diagnosis was not reached. A contrast-enhanced CT performed in May 2014 showed improvement in the pancreatic enlargement (Figure 4); therefore, it was believed to be nonspecific pancreatitis, and follow-up observation was the chosen strategy. Blood collection and CT scans were subsequently performed every three months, but these did not demonstrate any major changes. However, in January 2015, he was admitted to our hospital’s respiratory medicine department for an alveolar hemorrhage and pneumonia (Figure 5); in addition to rapidly progressive glomerulonephritis, he tested positive for myeloperoxidase-ANCA, and he was diagnosed with ANCA-related vasculitis, specifically microscopic polyangiitis (MPA). Contrast-enhanced CT performed during the same period showed that the pancreatic enlargement and poorly enhanced area had disappeared. Despite immunosuppressive therapy with steroids, his respiratory condition worsened, and he passed away in April 2015.
The Chapel Hill Consensus Conference (CHCC) published in 1994 classifies the ten diseases of primary vasculitis into three categories by affected vessel size (large vessels, medium vessels, and small vessels)[1]. Although this classification has been widely used around the world, nearly 20 years have passed, and as research on the etiology and pathological condition of vasculitis has advanced, issues with the classification have arisen. A new classification and definitions have been developed and published in January 2013 as “CHCC2012”[2], in which four categories of 16 diseases, including secondary vasculitis and others, have been added to the original three categories of large-vessel vasculitis, medium-vessel vasculitis, and small-vessel vasculitis; the current understanding of vasculitis now encompasses diverse concepts.
In the CHCC2012, MPA belongs to small-vessel vasculitis, with the definition that “MPA is necrotizing vasculitis, with few or no immune deposits, predominantly affecting small vessels (i.e., capillaries, venules, or arterioles). Necrotizing arteritis involving small and medium arteries might be present. Necrotizing glomerulonephritis is very common. Pulmonary capillaritis often occurs. Granulomatous inflammation is absent[2]. The onset of MPA often occurs at ≥ 50 years of age, and it occurs more frequently in men (male-to-female ratio, 1.5:1)[3]. Sixty percent of cases are positive for myeloperoxidase-ANCA[4], and 51%-94% of cases are reportedly positive for all ANCAs, including PR3-ANCA[4,5]. There are two basic types of findings: those based on bleeding, infarction, and other vascular disorders caused by the rupture of blood vessels and those based on inflammation such as fever and elevated C-reactive protein levels. The main target organs are the kidneys or lungs, with lesions also observed in the skin, muscles, and brain; 30%-50% of lesions are also reportedly observed in the digestive tract[3,6], but it is rare for lesions to be found in the pancreas.
In the present case, it was difficult to histopathologically prove findings of vasculitis in the pancreas with EUS-FNA or endoscopic retrograde cholangiopancreatography; however, lung lesions progressed later, and it is characteristic that imaging showed abnormalities in the pancreas before the diagnosis of ANCA-related vasculitis could be determined. Initially, ANCA was not measured, and the differential diagnoses included pancreatic cancer, malignant lymphoma, autoimmune pancreatitis, and tumor-forming pancreatitis. Irregular, hypoechoic masses were observed in the pancreas, and carcinoembryonic antigen and cancer antigen 19-9 levels were elevated; however, any findings of pancreatic cancer were negated by the fact that no abnormality was observed in the pancreatic ducts and the lesions were scattered (regarding a diagnosis of cancer of the entire pancreas). Malignant lymphoma was considered as a differential disease in part because the artery penetrated the lesion, but enlarged lymph nodes were not observed, and a pathologically definitive diagnosis could not be reached. The diagnosis of AIP was rejected because, although pancreatic enlargement was observed, the diffuse poorly enhanced area was nonspecific, and hyper-IgG4 disease, irregular narrowing of the main pancreatic duct in ERP, or extrapancreatic lesions were not observed. Regarding tumor-forming pancreatitis, tumor signs were not localized, and the pancreatic duct images also showed no abnormalities; therefore, it could not be said to be typical. Although the abdominal pain was not clear, his mild inflammatory reaction, elevated trypsin levels, and pancreatic enlargement suggested pancreatitis. In light of his clinical course, it appears that factors such as thrombus formation caused by the vasculitis in the early stages of ANCA-related vasculitis caused pancreatic blood flow to be abnormally distributed, resulting in non-uniform pancreatitis and manifesting in the presentation of the imaging findings. In AIP, IgG4-positive cells are reportedly detected from the papilla of Vater in a high proportion of biopsies[7]; in the present case, the papilla of Vater was biopsied to exclude AIP even though no abnormalities were observed endoscopically. IgG4-positive cells were not observed, but thrombus formation was present (Figure 3B); conceivably, this also occurred in the peripancreatic vessels, causing an abnormal distribution of the pancreatic blood flow and producing irregular pancreatitis.
When PubMed was searched for previous reports published between 1990 and 2015 of ANCA-related vasculitis presenting with pancreatic lesions, 12 cases were found[8-19] (Table 1). The mean age was 55.2 years (20-84 years), with a male-to-female ratio of 6:7; 11 cases were ANCA-positive, and granulomatosis with polyangiitis was the most frequent diagnosis (9 cases), while there were 4 cases of MPA and no cases of eosinophilic granulomatosis with polyangiitis. In 6 cases, symptoms were accompanied by findings of pancreatitis on imaging; 5 cases presented with nodular shadows that were difficult to differentiate from tumors in the pancreas, and no antemortem pancreatic lesions were indicated, but postmortem autopsy indicated pancreatic lesions in 2 cases. Regarding pathologically definitive diagnoses of pancreatic lesions, 3 cases were diagnosed following a surgical procedure because of the difficulty differentiating the lesions from a tumor, 3 cases were diagnosed by postmortem autopsy, and 7 cases had a diagnosis indirectly proven by biopsies from other organs because a definitive diagnosis could not be reached for the pancreas. None of the cases had a definitive diagnosis of ANCA-related vasculitis based on pancreatic lesions before treatment. Thus, similar to the present case, pancreatic lesions in ANCA-related vasculitis are extremely difficult to diagnose by endoscopic biopsy or similar methods before treatment, and it is important to indirectly diagnose the condition from the involvement of other organs.
| Authors | Year | Age (yr), sex | Symptoms | Pancreatic enzyme | ANCA | Vasculitis | Pancreatic lesionImaging | Diagnostic method | Other organ disorder | TreatmentPancreasOther organ | OutcomePancreasOther organ |
| O'Neil et al[8] | 1992 | 44, M | Jaundice | ND | + | GPA | Ph tumor susp | Renal biopsy | Kidney Nose | Treated | Alive |
| US: hypoechoic | PSL, CYC | Improved | |||||||||
| CT: 3 cm mass | PSL, CYC | Improved | |||||||||
| Stuckey et al[9] | 1992 | 45, M | Epigastric pain | AMY: 55 | - | GPA | Pancreatitis susp | Parotid gland biopsy | Lung | Treated | Alive |
| Nausea | CT:enlargement, sporadic | Conservative | Improved | ||||||||
| low density lesions | PSL, CYC | Improved | |||||||||
| Berney et al[10] | 1997 | 32, M | Epigastric pain | Raised of AMY, Lipase | PR3 | MPA | Pancreatitis susp | Renal biopsy | Kidney | Treated | Alive |
| CT: edematous | Conservative | Improved spontaneously | |||||||||
| PSL, CYC | Improved | ||||||||||
| Matsubayashi et al[11] | 2001 | 65, M | Left abdominal pain | Trypsin: 550 | PR3 | GPA | Pbt tumor susp | Autopsy | Kidney Lung | None | Died |
| Elastase-I: 440 | CT: enlargement, sporadic | Spleen | None | Necrotizing pancreatitis | |||||||
| low density lesions | None | Hemorrhagic pneumonia | |||||||||
| Christl et al[12] | 2004 | 55, F | Abdominal pain | ND | PR3 | GPA | Pt tumor susp CT: | Postoperative pathology Renal biopsy | Kidney | Treated | Alive |
| Weight loss | enlargement, sporadic | Ope | Improved | ||||||||
| low density lesions | PSL, CYC | Improved | |||||||||
| Iwasa et al[13] | 2005 | 84, F | Hematuria | N.D. | MPO | MPA | Normal | Autopsy | Kidney Lung | Treated | Died |
| Fever | PSL | Necrotizing pancreatitis | |||||||||
| PSL | Lung hemorrhage | ||||||||||
| Haraguchi et al[14] | 2005 | 84, F | Fever | AMY: 130 | MPO | MPA | Normal | Autopsy | Kidney | Treated | Died |
| Lung | PSL | Necrotizing pancreatitis | |||||||||
| PSL | Lung hemorrhage | ||||||||||
| Tinazzi et al[15] | 2007 | 48, F | Epigastric pain | N.D. | - | GPA | Ph tumor susp | Postoperative pathology | - | Treated | Alive |
| US: 2cm, mass, hypoechoic | Ope, PSL, CYC, MTX | Improved | |||||||||
| MRCP: obstruction of MPD | - | - | |||||||||
| Joshipura et al[16] | 2007 | 47, M | Epigastric pain Fever | AMY: 874 Lipase: 1294 | PR3 | GPA | Pancreatitis susp | Turbinate biopsy | Nose | Treated | Alive |
| CT: Pbt edematous | PSL, CYC | Improved | |||||||||
| PSL, CYC | Improved | ||||||||||
| Abu-Hilal et al[17] | 2008 | 20, F | Epigastric pain | Normal | PR3 | GPA | Pancreatitis susp CT: Pt | Renal biopsy | Kidney | Treated | Died |
| Lung Skin | Conservative | Improved spontaneously | |||||||||
| Nausea | |||||||||||
| edematous | Intestine | PSL, CYC | Lung | ||||||||
| hemorrhage | |||||||||||
| Chawla et al[18] | 2011 | 60, F | Epigastric pain | Lipase: 1316 | PR3 | GPA | Pancreatitis susp | Renal biopsy | Kidney | Treated | Alive |
| Nausea | CT: diffusely edematous, | Lung | Conservative | Improved spontaneously | |||||||
| Ph hypo attenuated lesion | Heart | PSL, CYC, AZA | Improved | ||||||||
| Valerieva et al[19] | 2013 | 62, F | Epigastric pain | Normal | PR3 | GPA | Pt tumor susp | Postoperative | - | Treated | Alive |
| CT: Pt, 3cm, mass | pathology | Ope, PSL, AZA | Improved | ||||||||
| - | |||||||||||
| Iida et al (this case) | 2015 | 72, M | Weight loss | Trypsin: 723 | MPO | MPA | Pancreatitis susp | Labo data | Kidney | Treated | Died |
| CT: enlargement, multiple | Imaging | Lung | Conservative | Improved spontaneously | |||||||
| hypo attenuated lesions | Vater biopsy | PSL, CYC | Lung hemorrhage |
Regarding the treatment of pancreatic lesions, of the 6 cases that presented with findings of pancreatitis on imaging, 5 were successfully treated with conservative treatment. Therefore, it is possible that pancreatitis caused by ANCA-related vasculitis could follow a transient course; however, there are few reports in which ANCA-related vasculitis resolved spontaneously[20], and this remains largely speculative. Despite the possibility that the pancreatitis is transient, remission of pancreatitis has been followed by organ failure in other cases owing to the manifestation of ANCA-related vasculitis in other organs such as the kidneys or lungs, and the patient died, as in the present case. Therefore, we believe that ANCA-related vasculitis should be differentially included as a cause of pancreatitis of unknown etiology, and an early and appropriate diagnosis is required, followed by the start of treatment.
In addition to cases with ANCA-related vasculitis, some cases with Kawasaki disease, polyarteritis nodosa, or other types of vasculitis have presented with pancreatitis or nodules in the pancreas[21,22]. As already described, although the CHCC2012 classifies vasculitis by vascular diameter, there is considerable overlap in the vessels invaded in each group, and vessels of any size can be affected; therefore, we feel that care should be taken regarding the relationship with pancreatic lesions in all forms of vasculitis, including ANCA-related vasculitis. However, because there are so few previous reports regarding vasculitis and pancreatic lesions, the details of the relationship remain unclear, and additional cases will need to be described.
Pancreatic lesions in anti-neutrophil cytoplasmic antibody (ANCA)-related vasculitis are very rare. This is a case of rare type of pancreatitis as the first presentation of anti-neutrophil cytoplasmic antibody-related vasculitis.
Factors such as thrombus formation caused by the vasculitis in the early stages of ANCA-related vasculitis cause abnormal distribution of the pancreatic blood flow, resulting in non-uniform pancreatitis.
The patient was suspected of having pancreatic cancer presenting with a special distribution, malignant lymphoma, tumor-forming pancreatitis, or autoimmune pancreatitis.
On second admission, myeloperoxidase-ANCA was positive.
First, it was believed to be nonspecific pancreatitis because of improvement in the pancreatic enlargement.
On endoscopic ultrasound-guided fine needle aspiration biopsy, malignant cells were not detected, and the diagnosis was nonspecific pancreatitis. On endoscopic retrograde pancreatography, no abnormality was observed in the papilla of Vater, but the biopsy revealed thrombus formation.
First, it was believed to be nonspecific pancreatitis, and computed tomography showed improvement in the pancreatic enlargement, follow-up observation was the chosen strategy. For an alveolar hemorrhage and pneumonia, immunosuppressive therapy with steroids was performed.
ANCA-related vasculitis presenting with pancreatic lesions published between 1990 and 2015 were found 12 cases in PubMed.
ANCA-related vasculitis should be differentially included as a cause of pancreatitis of unknown etiology, and an early and appropriate diagnosis is required, followed by the start of treatment.
The case report by Iida T and co-workers describes a rare case of ANCA-related vasculitis with pancreatic involvement. It is a well-constructed case report and a short but good review of the corresponding literature.
P- Reviewer: Goetze TO, Kleeff J, Ramia JM S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM
| 1. | Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2763] [Cited by in RCA: 2408] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 2. | Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4416] [Cited by in RCA: 4256] [Article Influence: 354.7] [Reference Citation Analysis (0)] |
| 3. | Villiger PM, Guillevin L. Microscopic polyangiitis: Clinical presentation. Autoimmun Rev. 2010;9:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of Disease: pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol. 2006;2:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Vamvakopoulos J, Savage CO, Harper L. ANCA-associated vasculitides-lessons from the adult literature. Pediatr Nephrol. 2010;25:1397-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Fukushima M, Inoue S, Ono Y, Tamaki Y, Yoshimura H, Imai Y, Inokuma T. Microscopic polyangiitis complicated with ileal involvement detected by double-balloon endoscopy: a case report. BMC Gastroenterol. 2013;13:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A. A new diagnostic endoscopic tool for autoimmune pancreatitis. Gastrointest Endosc. 2008;68:358-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | O‘Neil KM, Jones DM, Lawson JM. Wegener‘s granulomatosis masquerading as pancreatic carcinoma. Dig Dis Sci. 1992;37:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Stuckey SL, Smart PJ. Wegener’s granulomatosis: parotid involvement and associated pancreatitis with C.T. findings. Australas Radiol. 1992;36:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Berney T, Persoz C, Leski M, Morel P. Antineutrophil cytoplasmic antibodies and acute pancreatitis. Pancreas. 1997;15:106-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Matsubayashi H, Seki T, Niki S, Mizumura Y, Taguchi Y, Moriyasu F, Go K. Wegener’s granulomatosis with onset of acute pancreatitis and rapid progress. A case report. Pancreatology. 2001;1:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Christl SU, Borchard F, Keller R, Engemann R, Fischbach W. [Pancreatic tail tumor as an unusual first manifestation of Wegener’s disease]. Z Gastroenterol. 2004;42:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Iwasa S, Katoh R. Autopsy case of microscopic polyangiitis with crescentic glomerulonephritis and necrotizing pancreatitis. Pathol Int. 2005;55:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Haraguchi K, Gunji K, Ito Y, Yokomori N, Kawaguchi A, Ohomori M, Inoue H, Shimura H, Saito T, Kobayashi T. Extensive pancreatic necrosis in microscopic polyangiitis. Clin Exp Nephrol. 2005;9:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Tinazzi I, Caramaschi P, Parisi A, Faccioli N, Capelli P, Biasi D. Pancreatic granulomatous necrotizing vasculitis: a case report and review of the literature. Rheumatol Int. 2007;27:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Joshipura VP, Haribhakti SP, Pandya SC, Soni HN, Patel NR. Wegener’s granulomatosis--an etiology of acute pancreatitis. Indian J Gastroenterol. 2007;26:89-90. [PubMed] |
| 17. | Abu-Hilal M, Abu-Hilal M, McPhail MJ, Zeidan B, Bryant T, Bateman A, Johnson CD. Acute pancreatitis as the first presentation of Wegener’s granulomatosis. JOP. 2008;9:300-304. [PubMed] |
| 18. | Chawla S, Atten MJ, Attar BM. Acute pancreatitis as a rare initial manifestation of Wegener’s granulomatosis. A case based review of literature. JOP. 2011;12:167-169. [PubMed] |
| 19. | Valerieva Y, Golemanov B, Tzolova N, Mitova R. Pancreatic mass as an initial presentation of severe Wegener’s granulomatosis. Ann Gastroenterol. 2013;26:267-269. [PubMed] |
| 20. | Yanagawa T, Andoh T, Ikushima S, Akiyama O, Oritsu M, Takemura T. [A case of Wegener’s granulomatosis which showed early spontaneous remission]. Nihon Kokyuki Gakkai Zasshi. 1998;36:256-261. [PubMed] |
| 21. | Reynaud-Mendel B, Lemann M, David F, Menasché S, Bachelez H, Dubertret L. Adult Kawasaki disease complicated by pancreatitis. Am J Gastroenterol. 1997;92:1239-1240. [PubMed] |
| 22. | Yokoi Y, Nakamura I, Kaneko T, Sawayanagi T, Watahiki Y, Kuroda M. Pancreatic mass as an initial manifestation of polyarteritis nodosa: a case report and review of the literature. World J Gastroenterol. 2015;21:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |