Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2153
Peer-review started: September 21, 2015
First decision: October 14, 2015
Revised: November 16, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: February 14, 2016
Processing time: 125 Days and 18.3 Hours
Double pylorus (DP), or duplication of the pylorus, is an uncommon condition that can be either congenital or acquired. Acquired DP (ADP) occurs when a peptic ulcer erodes and creates a fistula between the duodenal bulb and the distal stomach. The clinical features and endoscopic characteristics of four patients with ADP were reviewed and compared with previously reported cases. An accessory channel connects the lesser curvature of the prepyloric antrum with the duodenal bulb, and in all cases, a peptic ulcer was located in or immediately adjacent to the accessory channel. In one of the patients, the bridge between the double-channel pylorus disappeared, resulting in a single large opening and duodenal kissing ulcer after two years and three months. Finally, nonsteroidal anti-inflammatory drugs, Helicobacter pylori and other risk factors associated with ADP are assessed.
Core tip: Double pylorus, which can be congenital or acquired, is a relatively rare condition consisting of two openings connecting the antrum to the duodenal bulb. This disease has a prevalence that ranges from 0.001%-0.4% of upper gastrointestinal endoscopies, and only a few reports have documented long-term endoscopic observations for this disease. In this report, we present the clinical and endoscopic characteristics and four years of follow-up observations of four patients with acquired double pylorus complicated with gastric ulcer.
- Citation: Lei JJ, Zhou L, Liu Q, Xu CF. Acquired double pylorus: Clinical and endoscopic characteristics and four-year follow-up observations. World J Gastroenterol 2016; 22(6): 2153-2158
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2153
Double pylorus (DP), also called double-channel pylorus, is a rare condition involving a double communication between the gastric antrum and the duodenal bulb. DP is observed in 0.001% to 0.4% of upper gastrointestinal endoscopies[1] and is twice as frequent in males than females[2]. In most cases, DP is a complication of a penetrating ulcer, a condition that is called acquired double pylorus (ADP), pyloric duodenal fistula, antral duodenal fistula and peripyloric gastroduodenal fistula[3]. DP occasionally occurs as a congenital abnormality, either isolated or in combination with other congenital abnormalities, such as heterotopic pancreatic tissue[4], pancreatic divisum or gastric duplication[5]. Herein, we describe four cases of gastric ulcer disease complicated by ADP and discuss risk factors for the occurrence of this disease.
The patients included three men and one woman ranging from 41 to 62 years of age. The disease durations ranged from 2 mo to 18 years. Two of the male patients presented to the emergency department with melena and/or coffee-ground vomitus suggestive of upper gastrointestinal hemorrhage; the other male patient was an outpatient who underwent an endoscopic examination due to epigastric pain for 2 mo. The only female patient was admitted to the hospital because of abdominal pain and dyspepsia with concomitant gouty arthritis. Physical examinations of the four patients indicated mild abdominal tenderness; the female patient exhibited gouty tophi in both hands and feet. For the two emergency patients, full blood counts revealed normocytic anemia, and blood biochemistry revealed enterogenous azotemia (i.e., elevated blood urea nitrogen and normal creatinine). The other two (nonemergency) patients showed normal hemoglobin and urea nitrogen levels. All four patients exhibited normal platelet, prothrombin time, creatinine, liver enzyme, and calcium levels. Helicobacter pylori (H. pylori) urease breath test results were positive for all four patients. Three of the patients abused nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of concomitant diseases (Table 1).
| Characteristic | Case number | |||
| 1 | 2 | 3 | 4 | |
| Age (yr) | 41 | 61 | 58 | 62 |
| Sex | Male | Male | Male | Female |
| Disease duration | 2 mo | 1 yr | 8 yr | 2 mo |
| Symptoms at presentation | Epigastric pain | Melena | Coffee-ground vomitus | Abdominal pain |
| Previous history of gastric ulcer | - | - | + | - |
| Abdominal tenderness | + | + | + | + |
| NSAID use | - | Diclofenac | AKaFenSan2 | AKaFenSan2 |
| Helicobacter pylori infection | + | + | + | + |
| Hemoglobin (g/L) | - | 82 | 101 | 115 |
| Blood urea nitrogen (mmol/L) | - | 14.3 | 26.3 | 6.1 |
| Blood creatinine (μmol/L) | - | 80.46 | 98 | 75.11 |
| Blood uric acid (μmol/L) | - | 334.2 | 270.75 | 565.5 |
| Concomitant disease | - | Osteoarticular degenerative disease | Headache | Gout arthritis |
| Duration (yr) | - | 3 | 18 | 20 |
| Localization of the accessory pylorus | Lesser curve | Lesser curve | Lesser curve | Lesser curve |
| Localization of the gastric ulcer | Lesser curve | Within the accessory pylorus | Anterior wall | Within the accessory pylorus |
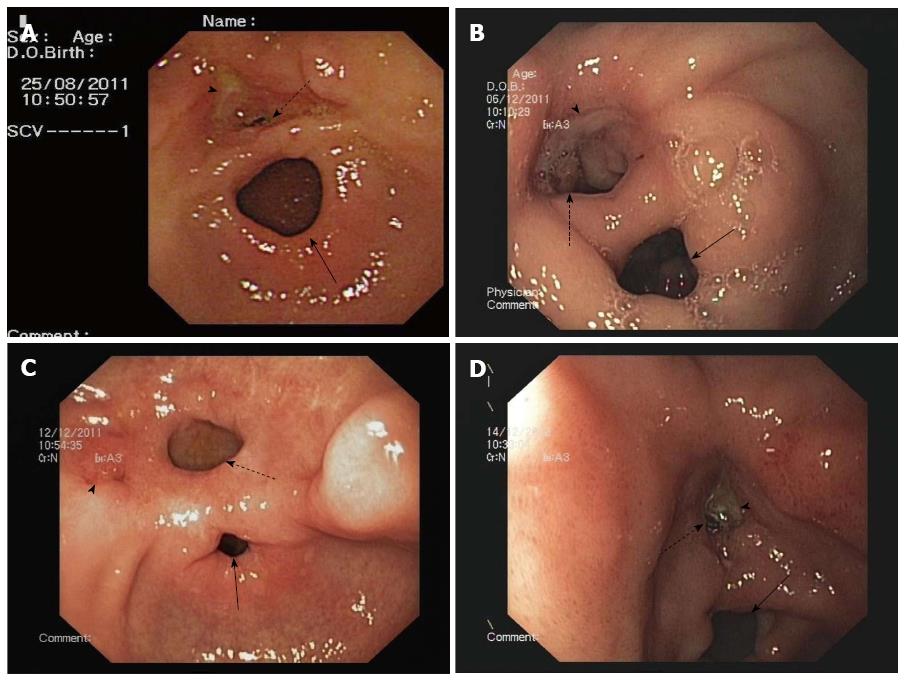
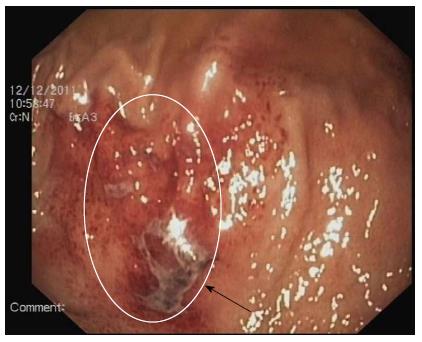
Esophagogastroduodenoscopies of all four patients revealed normal esophagi along the full length, without any pathological changes, and the bulbus and postbulbar duodenums were normal in appearance. However, each of the patients had DP and gastric ulcer disease, and the observed channel contractions suggested that their conditions might be related to the true pyloric rings. In all four patients, the accessory pylorus was located along the lesser curvature of the prepyloric antrum, connecting the lesser curvature with the duodenal bulb (Figure 1). A gastric ulcer was found in two of the patients: in one case on the lesser curve of the antrum and on the anterior wall adjacent to the accessory pylorus in the other case (Figure 1A and C). In contrast, the white bases of the ulcers were located in the accessory channel in the other two patients (Figure 1B and D). The duodenum could easily be entered via both of the pyloric channels. Additionally, the 58-year-old male patient exhibited mucosal erosion of the gastric fundus, active bleeding and irregular shallow ulcers (Figure 2) that were suggestive of acute erosive hemorrhagic gastropathy, which caused upper gastroenteral bleeding in addition to the ADP and the accompanying gastric ulcer itself. This condition was the result of the patient’s consumption of 8 bags of TouTongFen, which is also known as AkaFenSan (an over-the-counter analgesic consisting of acetaminophen, aspirin, and caffeine powder), to treat a long duration headache. No endoscopic interventions were performed in any of the patients. Real-time abdominal ultrasonography and computerized tomography findings were normal.
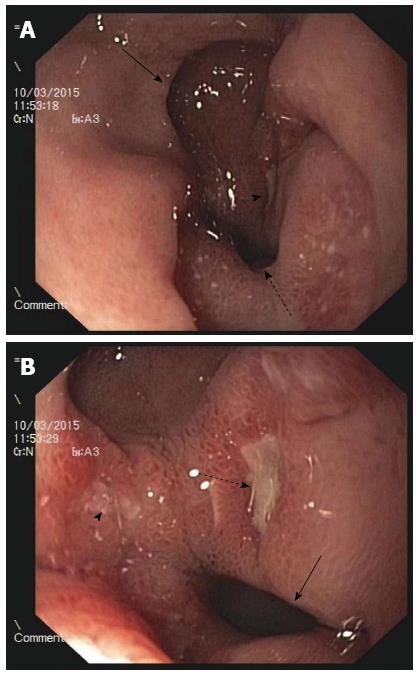
The two patients with gastrointestinal hemorrhages were immediately resuscitated with crystalloids and treated with intravenous pantoprazole (40 mg) twice daily until they left hospital. Bleeding did not recur, and epigastric pain was resolved. These patients were discharged on days 7 and 10. All patients were treated with H. pylori eradication therapy for 10 d. The 58-year-old male patient dropped out of the follow-up treatment regimen after discharge. The other three patients underwent urea breath tests after one month that indicated eradication of H. pylori in the two male patients. In addition, the 41-year-old male patient underwent an endoscopic examination at one month, which revealed that the gastric ulcer had healed, though the DP remained. The other two patients declined follow-up gastroscopies. The female patient’s urea breath test remained positive, but she declined further therapy for the bacterial infection due to adverse reactions to the drugs. Both of these patients were asymptomatic and were instructed to avoid all NSAIDs and to use proton pump inhibitors (PPIs) if necessary to prevent ulcer recurrence and other complications. These two patients remained relatively healthy with the exception of occasional epigastralgia that was improved upon the use of over-the-counter drugs. Two years and three months after the initial endoscopy in March 2015, the female patient exhibited a recurrence of persistent epigastric pain. She consequently underwent a second endoscopic examination revealing that the bridge between the two channels had disappeared, resulting in a single large opening (Figure 3A) and a duodenal kissing ulcer (Figure 3B). A urea breath test indicated persistent H. pylori infection. This patient also admitted to the continued use of TouTongFen to treat gouty arthritis attacks. Because the ulcer remained, this patient was treated with triplex H. pylori eradication therapy and PPI maintenance therapy. The patient’s abdominal pain rapidly improved, and she was healthy at the last follow-up.
DP can be congenital or acquired. The first case of congenital DP (CDP) was reported by Christien et al[6] in 1971, and only a few additional cases have been reported since that time. In CDP, a defect in canalization appears to occur during early embryonic development[4]. Diagnosis is based on normal histology of both channels, the coexistence of another congenital abnormality, a bridge between the two channels with a normal muscle layer, a lack of a peptic ulcer disease history, and a lack of radiologic or endoscopic evidence of an ulcer[7]. Conversely, ADP is a complication of a prepyloric or duodenal ulcer that perforates the gastric and duodenal walls to create a fistula. Although the first reported cases was in 1861, this condition was not considered a real entity until 1969[8]. In our four patients, the presence of a peptic ulcer on endoscopic examination and/or a previous history of gastric ulcer indicated that the lesions were acquired.
ADP has no specific autonomous clinical manifestations and is not associated with upper abdominal pain or dyspepsia. ADP can present with chronic upper abdomen pain and/or discomfort, dyspepsia, vomiting and gastrointestinal bleeding[9-11] due to an associated peptic ulcer or other diseases. For example, the 58-year-old male patient was admitted to the hospital due to upper gastroenteral bleeding. Upon admission, acute gastroscopy confirmed that the cause of the bleeding was acute erosive hemorrhagic gastropathy (Figure 2) caused by the overuse of TouTongFen. In the 61-year-old man with osteoarticular degenerative disease, the cause of bleeding was a peptic ulcer within the accessory pylorus.
On endoscopy, the gastric antrum may appear normal[1], inflamed, or ulcerated[11] (as in the present four cases). The fistula may vary in size from a few millimeters to several centimeters, and in the majority of patients, these fistulae are located in the lesser curvature of the gastric antrum and the superior wall of the duodenal bulb[2], as observed in all four patients described herein. However, the fistula can form an ulcer that penetrates from the posterior part of the antrum to the third or fourth part of the duodenum[12] or even the jejunum[13]. Rarely, ADP can be found in patients with duodenal ulcers or gastric cancer[10,14]. According to a follow-up study by Hu et al[2], the accessory pylorus channel frequently persists for life (60%); nonetheless, in some patients, the accessory pylorus closes (25%) or connects with the true pylorus to form a single channel (5%)[2,12,15]. The latter occurred in the female patient in this report. There are few reports of clinical improvement with fistula formation.
Several etiologies of ADP have been proposed. First, systemic diseases, such as diabetes, cirrhosis and chronic obstructive pulmonary disease, may be associated with ADP[16-18]. We believe that damage to the gastric mucosal microcirculation can cause ADP[16-18]. Second, long histories of treatment with drugs, including NSAIDs and corticosteroids, can affect peptic ulcer healing. Atiq et al[19] reported a 54-year-old African woman with ADP and a clean-based ulcer in the accessory pylorus that resulted from the failure to follow medication instructions as well as the use of an over-the-counter analgesic consisting of acetaminophen, aspirin and caffeine. Peixoto et al[9] described a 73-year-old man with multiple concomitant diseases who was admitted to their emergency department with first-episode melena. These authors believed that formation of the DP in these cases resulted from NSAID abuse. Moreover, H. pylori infection was absent in both of the above-mentioned cases[9,19], suggesting that NSAIDs were responsible for the observed ADP. Yousuf et al[20] reported a case of DP that was diagnosed endoscopically in a male patient with an adrenal adenoma; these authors believed that formation of the DP in this case resulted from a recurrent peptic ulcer that was likely induced by the hypersecretion of endogenous corticosteroids by the adrenal adenoma. Third, H. pylori plays a role in the pathogeneses of duodenal ulcer disease and the majority of gastric peptic diseases. Indeed, H. pylori is potentially responsible for refractory cases and the lack of healing. Akazawa[15] reported a patient with none of the above-mentioned concomitant diseases or drug use, though this patient did have a continuous H. pylori infection for 14 years; recurrent gastric ulcers in this patient finally led to ADP. Fourth, poor compliance with medication regimens might be an important factor in ADP formation.
Among our four patients, all had H. pylori infections, and three abused NSAIDs. When the symptoms of the latter three patients were relieved, they continued using NSAIDs and refused regular recertification and follow-up gastroscopies. In addition, the female patient’s NSAID abuse to treat her gouty arthritis and persistent H. pylori infection may have been related to formation of the fistula and subsequent duodenal kissing ulcer.
ADP is most frequently an incidental finding during investigations of other conditions. The diagnosis is typically made based on endoscopic findings and is occasionally made based on radiologic findings. Endoscopy is generally the preferred method of visualization; however, ADP by endoscopy should be differentiated from gastric diverticulum, one of the rarest and controversial gastrointestinal pathologies. Very few cases of gastric diverticulum have been reported in the literature[4,21]; such cases are typically asymptomatic, singular, saccular in shape, 1 to 4 cm in size and predominantly encountered in the 5th or 6th decade of life. Although DP has a characteristic appearance on upper gastrointestinal series, it may easily be misinterpreted as polyps, tumors, or large mucosal folds[22,23].
Therapy should focus on the removal of the factors that impair mucosal healing. Ulcerogenic medications, such as NSAIDs and corticosteroids, should be avoided, and H. pylori infection should be eradicated. For patients who cannot stop using NSAIDs and those in whom H. pylori eradication fails, PPI maintenance therapy is necessary. ADP in the majority of patients responds well to medical treatments such as PPI, H2-receptor antagonist and antacid therapies and gastric mucosal protective agents. The eradication of H. pylori, anti-acid treatments and the cessation of NSAID use are beneficial in terms of symptom relief, ulcer recurrence prevention, and fistula closure. For patients with symptoms of gastric outlet obstruction, endoscopic division of the tissue bridge with a sphincterotome should be considered first because the reestablishment of a normal pyloric aperture will alleviate symptoms[24]. Indications for surgery include other complications, such as free perforations, obstructions that are refractory to endoscopic treatment, refractory bleeding, and failure to heal under maximum medical therapy with persistent symptoms not due to the fistula per se[25].
Acquired double pylorus (ADP) can present with chronic upper abdomen pain and/or discomfort, dyspepsia, vomiting and gastrointestinal bleeding due to an associated peptic ulcer or other diseases.
The diagnosis of ADP is typically made based on endoscopic findings and is occasionally made based on radiologic findings. Endoscopy is generally the preferred method of visualization.
ADP should be differentiated from gastric diverticulum on endoscopy; double pylorus (DP) has a characteristic appearance but may easily be misinterpreted as polyps, tumors, or large mucosal folds on upper gastrointestinal series.
Full blood counts and blood biochemistry are often normal, but in patients with gastrointestinal bleeding, full blood counts may show anemia, and blood biochemistry may indicate enterogenous azotemia (i.e., elevated blood urea nitrogen and normal creatinine). Helicobacter pylori (H. pylori) urease breath test results are often positive.
The real-time abdominal ultrasonography and computerized tomography findings were normal.
ADP arises from ulceration and fistulization between the gastric antrum and duodenal bulb; not all of the normal histological layers are present in this form.
In the majority of patients, ADP responds well to medical treatments, such as proton pump inhibitor, H2-receptor antagonist, and antacid therapies and gastric mucosal protective agents.
DP, also called double-channel pylorus, is a rare condition involving a double communication between the gastric antrum and the duodenal bulb. DP is observed in 0.001% to 0.4% of upper gastrointestinal endoscopies and occurs at twice the frequency in males compared with females.
TouTongFen, which is also called AKaFenSan, is an over-the-counter analgesic consisting of acetaminophen, aspirin, and caffeine powder.
PPI maintenance therapy is necessary in patients who cannot stop using NSAIDs and in those in whom H. pylori eradication fails.
This is a rare condition and an interesting report, and the manuscript is well written. It is a pity that follow-up endoscopy was performed on only one patient, and that the follow-up had to be based on clinical symptoms.
P- Reviewer: Tovey FI, Pavlovic M S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Wiseman SM, Tan D, Hill HC. Double pylorus: an unusual endoscopic finding. Endoscopy. 2005;37:277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Hu TH, Tsai TL, Hsu CC, Lu SN, Hsiao M, Changchien CS. Clinical characteristics of double pylorus. Gastrointest Endosc. 2001;54:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Safatle-Ribeiro AV, Ribeiro Júnior U, Habr-Gama A, Gama-Rodrigues JJ. Double pylorus: case report and review of the literature. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Wolters VM, Nikkels PG, Van Der Zee DC, Kramer PP, De Schryver JE, Reijnen IG, Houwen RH. A gastric diverticulum containing pancreatic tissue and presenting as congenital double pylorus: case report and review of the literature. J Pediatr Gastroenterol Nutr. 2001;33:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sisman G. Concomitant pancreas divisum and double pylorus: a case report. JOP. 2014;15:632. [PubMed] |
| 6. | Christien G, Branthomme JM, Volny L, Deschamps P, Morice A. [Double pylorus: a congenital malformation]. Sem Hop. 1971;47:1485-1488. [PubMed] |
| 7. | Mylonas A, Papaziogas B, Paraskevas G, Fragos E, Gigis P, Papaziogas T. Congenital double pyloric ostium in the adult. Surg Endosc. 2002;16:1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Smith VM, Tuttle KW. Gastroduodenal (pyloric) band. Endoscopic findings and first reported case. Gastroenterology. 1969;56:331-336. [PubMed] |
| 9. | Peixoto P, Sadio A, Cancela E, Castanheira A, Ministro P, Silva A, Caldas A. Acute upper bleeding due to an unusual complication of peptic ulcer disease--double pylorus. Rev Esp Enferm Dig. 2010;102:451-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Arhan M, Oztas E, Ibis M, Sezgin S, Ozin Y. A rare endoscopic finding: acquired double pylorus. Surg Endosc. 2010;24:244-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Almeida N, Romãozinho JM, Ferreira M, Amaro P, Tomé L, Gouveia H, Correia Leitão M. Double pylorus with bleeding gastric ulcer - a rare event. Rev Esp Enferm Dig. 2008;100:600-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Czajkowski A, Rosołowski M, Lukaszyk A. Double pylorus: strong evidence for the acquired etiology of this rare abnormality. Endoscopy. 2007;39 Suppl 1:E84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Culafić DM, Matejić OD, Dukić VS, Vukcević MD, Kerkez MD. Spontaneous gastrojejunal fistula is a complication of gastric ulcer. World J Gastroenterol. 2007;13:483-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Matsuyama E, Nagashima R, Watanabe S, Takahashi T. Endoscopic hemostasis for hemorrhage from gastric cancer complicated by double-channel pylorus. Gastrointest Endosc. 2001;53:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Akazawa Y, Mizuta Y, Osabe M, Nakamura T, Morikawa S, Isomoto H, Takeshima F, Kohno S, Murata I. A case of double pylorus caused by recurrent gastric ulcers: a long-term endoscopic observation. Dig Dis Sci. 2005;50:2125-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Hu TH, Tai DI, Changchien CS, Chen TY, Chang WC. Double pylorus: report of a longitudinal follow-up in two refractory cases with underlying diseases. Am J Gastroenterol. 1995;90:815-818. [PubMed] |
| 17. | Fattahi MR, Homayoon K, Hamidpour L. Double pylorus in a cirrhotic patient: a case report and review of the literature. Middle East J Dig Dis. 2012;4:130-132. [PubMed] |
| 18. | Costa S, Dias VC, Peixoto P, Machado A, Gonçalves R. Double pylorus. Rev Esp Enferm Dig. 2015;107:377. [PubMed] |
| 19. | Atiq O, Abrams GA. Case study in gastroenterology & hepatology: An Uncommon Complication of Peptic Ulcer Disease. Gastroenterol Hepatol (N Y). 2014;10:333-334. [PubMed] |
| 20. | Yousuf M, Kameya S, Noda A, Watanabe T. A case of double pylorus accompanied by adrenal adenoma. Am J Gastroenterol. 1989;84:173-175. [PubMed] |
| 21. | Bhattacharya K. Gastric diverticulum - ‘Double pylorus appearance’. J Minim Access Surg. 2005;1:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Bennike S, Hegedüs V. The double pylorus. Br J Radiol. 1976;49:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Friehling JS, Rosenthal LE. Gastric carcinoma presenting as double-channel pylorus on upper gastrointestinal series. Dig Dis Sci. 1985;30:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Graham SM, Lin F, Flowers JL. Symptomatic double-channel pylorus. Successful treatment with a biliary sphincterotome. Surg Endosc. 1994;8:792-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Goh BK, Tan HK. Double pylorus. Am J Surg. 2006;191:515-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |