Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2118
Peer-review started: August 5, 2015
First decision: September 9, 2015
Revised: November 26, 2015
Accepted: December 12, 2015
Article in press: December 14, 2015
Published online: February 14, 2016
Processing time: 171 Days and 11.4 Hours
AIM: To examine healthcare resource utilization patterns and costs accrued by carcinoid syndrome (CS) patients with and without diarrhea.
METHODS: We conducted a retrospective cohort study using MarketScan® data from 1/1/2002-12/31/2012. Newly diagnosed CS patients had 1 medical claim for CS (ICD-9-CM code 259.2) plus either ≥ 1 additional claim for CS or for carcinoid tumors (ICD-9-CM 209.x), and had no evidence of CS for 1 year prior to index CS diagnosis, in commercially-insured patients < 65 years old. Patients were required to have continuous enrollment one year prior and after index date (first claim with CS diagnosis in the ID period). We identified patients with evidence of non-infectious diarrhea (ICD-9-CM codes 564.5 and 787.91) within one year from the index date. Overall and CS-related healthcare resource utilization and costs were compared between patients with and without non-infectious diarrhea during the one year period after the index date.
RESULTS: There were 2822 newly diagnosed CS patients; 534 (18.9%) had evidence of non-infectious diarrhea. Compared to patients without non-infectious diarrhea, non-infectious diarrhea patients more commonly had at ≥ 1 CS-related hospitalization (13.7% vs 7.2%), ≥ 1 CS-related ED visit (11.0% vs 4.4%), and CS-related office visits in one year (6.9 vs 4.1; all P < 0.001). After adjusting for demographics, region, number of chronic conditions and the Charlson Comorbidity Index, the proportions of patients with any and with CS-related hospitalizations were 9.7% and 6.8% higher, respectively, among non-infectious diarrhea patients compared to those with without non-infectious diarrhea (P < 0.001). Unadjusted costs were significantly higher among non-infectious diarrhea patients vs those without non-infectious diarrhea. The non-infectious diarrhea group was also more costly, with adjusted mean annual costs of $81610, compared to $51719 in the group without non-infectious diarrhea (P < 0.001).
CONCLUSION: Diarrhea is burdensome and costly in CS patients. Reduction of CS-related healthcare expenditures may be achievable through preventive treatment and appropriate management of diarrhea in CS.
Core tip: Healthcare resource utilization patterns and costs accrued by carcinoid syndrome (CS) patients with and without diarrhea have not been well described. We examined newly diagnosed CS patients using MarketScan® commercial claims data from 2003-2012 and found that non-infectious diarrhea (NID) is particularly burdensome and costly in CS patients. The adjusted proportions of patients with any and with CS-related hospitalizations were 9.7% and 6.8% higher in patients with NID than in those with no NID, respectively (P < 0.001). The NID group was also significantly more costly, with adjusted mean annual healthcare costs of $81610, compared to $51719 in the no NID group (P < 0.001).
- Citation: Broder MS, Chang E, Romanus D, Cherepanov D, Neary MP. Healthcare and economic impact of diarrhea in patients with carcinoid syndrome. World J Gastroenterol 2016; 22(6): 2118-2125
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2118
Neuroendocrine tumors (NETs), historically known as carcinoids, are rare lesions that originate in clusters of secretory cells in the gastrointestinal, respiratory, and urogenital tracts and are typically indolent in nature[1]. These neoplasms produce peptides and neuroamines that induce characteristic hormonal syndromes, including carcinoid syndrome (CS)[2,3]. NETs comprised 0.66% of malignancies in the United States from 1973 to 2004[1]. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database found that the incidence of NETs has increased from 1.09 per 100000 individuals in 1973 to 5.25 per 100000 in 2004, with a prevalence of NETs in the United States may exceed 100000[4].
The classic description of CS by Oberndofer[5,6] in 1907 included the triad of diarrhea, flushing, and bronchospasm. In a prospective single-institution database of over 900 patients with NETs, Ter-Minassian et al[7] found that diarrhea, abdominal pain, and flushing were the most common presenting symptoms. Of the myriad of symptoms that afflict patients with CS, diarrhea appears to have a particularly profound impact on patients’ sense of well-being. Based on surveys using the RAND version of the Short Form-36 (SF-36) and the Patient-Reported Outcomes Measurement Information System (PROMIS-29), patients with CS experience worse quality of life (QOL) compared to the general population and to other cancer patients and survivors. Those with uncontrolled diarrhea have reported even poorer QOL[8].
The healthcare and economic burden of diarrhea in CS patients has not been previously quantified. The goal of the current study was to examine healthcare resource utilization (HRU) patterns and healthcare costs accrued by CS patients with and without diarrhea in an insured United States population.
We conducted a cross-sectional, retrospective cohort study of newly diagnosed patients with CS using the Truven Health Analytics MarketScan® Database, using data between January 1, 2002 and December 31, 2012. In 2007, this claims database contained data for approximately 23 million employer-insured beneficiaries in the United States. The database includes patient-level medical and pharmacy claims submitted by large employers, managed care organizations, hospitals, and Medicare and Medicaid programs. It contains longitudinal records of reimbursable services by insurance, including medical claims (hospital stays, outpatient visits, emergency department visits, home care services, laboratory and imaging services) and pharmacy claims (outpatient prescription drug claims). Claims include information on each physician visit, medical procedure, hospitalization, drugs dispensed, dates of service, number of days of medication supplied, test performed, and complete payment information. Each medical claim has a principal diagnosis and secondary diagnoses codes associated with it. Available patient demographic information includes age, gender, and geographic region. The database also includes enrollment information such as date of enrollment and disenrollment. The database is fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) and meets the criteria for a limited-use dataset. Since the patient and provider data included in this analysis were fully de-identified, this study was considered exempt from approval by the Institutional Review Board.
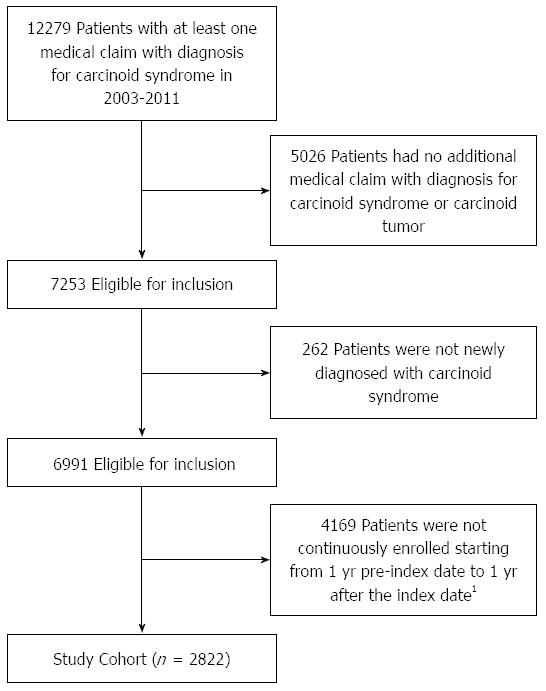
The study cohort comprised newly diagnosed patients with CS. Patients with a diagnosis of CS were identified if they had a medical claim for CS [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 259.2] between January 1, 2003 and December 31, 2011 [the identification (ID) period][9]. A single diagnosis code in claims may represent services provided to rule out a diagnosis. To increase the specificity of our cohort selection algorithm, we further required that eligible patients have either at least one additional claim for (1) CS; or (2) carcinoid tumors (ICD-9-CM 209.x), in any diagnostic field during the ID period. The date of the first claim with a diagnosis code for CS in the ID period was defined as the index date. To ensure the study population only included newly diagnosed cases of CS, patients were required to have no claims with a diagnosis code for CS during the year prior to the index date (disease-free period). All patients were followed for one year after the index date (study period). Patients were excluded if they were not continuously enrolled both during the disease-free period and for one year following the index date.
Among all identified newly diagnosed CS patients, we identified patients with at least one claim for non-infectious diarrhea (ICD-9-CM codes 564.5 and 787.91) in any diagnosis field. We grouped CS patients according to whether or not they had experienced non-infectious diarrhea.
Patient demographic characteristics (age, gender, United States census region) were derived from the enrollment files. Baseline measures of disease burden were also included in the analysis. First, we calculated the Charlson Comorbidity Index by examining the ICD-9-CM diagnostic codes appearing in medical claims within one-year prior to the index date[10]. The Charlson Comorbidity Index is calculated using weights for comorbidities derived from medical claims through a validated prediction formula for 1-year mortality. Second, the number of chronic conditions experienced by each patient within one-year prior to the index date was calculated using the Healthcare Cost and Utilization Project Chronic Condition Indicator (CCI)[9,11]. The CCI is a validated index which captures chronic conditions that last at least one year, and place limitations on self-care, independent living, and social interactions, or require ongoing medical care or special equipment.
HRU, including hospitalizations, emergency department (ED) visits, and physician office visits, was identified in medical claims during the study period. We examined the overall annual HRU related to any diagnosis occurring in the same period. In addition, we identified HRU related to CS based on claims with a primary diagnosis associated with carcinoid syndrome, CS-related symptoms, or carcinoid tumor progression. CS-related symptoms were identified with claims for non-infectious diarrhea (564.5, 787.91), nausea/vomiting (787.0x), flushing (782.62), asthma (493.x), dyspnea/wheezing (786.0x), cardiac palpitations (785.1), hypotension (458.0x), asthenia/fatigue (728.87, 780.71, 780.79), and dizziness (780.4). Carcinoid tumor progression was identified with claims for intestinal obstruction (560.0, 560.2, 560.9).
Cumulative annual healthcare costs were summed up for each patient from the index date. Costs were reported as total costs. In addition, we disaggregated the costs into medical costs (defined as costs related to medical claims), pharmacy costs (defined as related to pharmacy claims), inpatient hospitalization costs, ED visit costs, and outpatient (non-ED) costs.
Descriptive statistics were conducted for all study measures. We reported means and SDs for continuous variables, and patient counts and percentages for categorical variables. To compare differences between groups with and without non-infectious diarrhea, χ2 tests or t-tests were used for categorical and continuous variables, respectively. Our three key outcomes of interest were overall and CS-related hospitalizations, and total healthcare costs. We conducted multivariate analyses to compare the risk of overall and CS-related hospitalizations, and total healthcare costs between patients with and without non-infectious diarrhea. To model number of inpatient hospitalizations and number of ED visits, we used negative binomial models. All models were adjusted for age, gender, CCI, Charlson comorbidity index, and census region.
Costs were adjusted to 2012 United States dollars (the latest year of data in the study database) using the medical care components of the Consumer Price Index. All reported P values are two-sided with a significance level of 0.05. To compare costs between patients with and without non-infectious diarrhea, we used multivariate analyses to adjust for baseline characteristics, including age, gender, region, number of chronic conditions and the Charlson Comorbidity Index. We used linear regression models to estimate overall healthcare costs, pharmacy costs, non-pharmacy costs, outpatient non-ED costs, and number of office visits, and logistic regression models for risk of inpatient hospitalization and ED visits. As the majority patients had no hospitalization or ED visit, we used a 2-step approach to estimate adjusted inpatient and ED costs. We first conducted logistic regression models to estimate the risk of an event then used linear regression to estimate the adjusted costs among patients who had such event. Data transformations and statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC).
The overall study cohort included 2822 patients newly diagnosed with CS (Figure 1). Mean age among patients was 51.5 years, 56.9% were women and 43.0% lived in the southern United States. Patients had a mean Charlson Comorbidity Index of 3.6 and were diagnosed with a mean 3.5 chronic conditions. Of the total study cohort, 534 patients (18.9%) had at least one claim associated with non-infectious diarrhea. There were no significant differences between those with non-infectious diarrhea and those without when considering age, geographic region and Charlson Comorbidity Index. A significantly higher number of chronic conditions was observed among those with diarrhea compared to those without (4.0 vs 3.4, P < 0.001). Additionally, a higher percentage of patients with diarrhea were female compared to those without (62.4% vs 55.6%, P = 0.005) (Table 1).
| With non-infectious diarrhea | Without non-infectious diarrhea | All newly diagnosed CS patients | P value | |
| n = 534 | n = 2288 | n = 2822 | ||
| Age, yr | 51.3 ± 9.9 | 51.6 ± 10.1 | 51.5 ± 10.1 | 0.639 |
| Charlson comorbidity index | 3.7 ± 3.9 | 3.6 ± 3.8 | 3.6 ± 3.8 | 0.643 |
| No. of chronic conditions | 4.0 ± 2.4 | 3.4 ± 2.0 | 3.5 ± 2.1 | < 0.001 |
| Age group, yr | 0.120 | |||
| ≤ 17 | 1 (0.2) | 22 (1.0) | 23 (0.8) | |
| 18-24 | 8 (1.5) | 30 (1.3) | 38 (1.3) | |
| 25-34 | 33 (6.2) | 114 (5.0) | 147 (5.2) | |
| 35-44 | 80 (15.0) | 279 (12.2) | 359 (12.7) | |
| 45-54 | 157 (29.4) | 752 (32.9) | 909 (32.2) | |
| 55-64 | 255 (47.8) | 1091 (47.7) | 1346 (47.7) | |
| Sex | 0.005 | |||
| Female | 333 (62.4) | 1273 (55.6) | 1606 (56.9) | |
| Region | 0.961 | |||
| North Central | 134 (25.1) | 563 (24.6) | 697 (24.7) | |
| Northeast | 86 (16.1) | 358 (15.6) | 444 (15.7) | |
| South | 224 (41.9) | 989 (43.2) | 1213 (43.0) | |
| West | 90 (16.9) | 378 (16.5) | 468 (16.6) |
CS patients with diarrhea had significantly higher rates of unadjusted healthcare resource utilization compared to patients without diarrhea (Table 2). In comparison to those without diarrhea, those with diarrhea more commonly had at least one hospitalization (49.6% vs 39.6%, P < 0.001), at least one ED visit for any cause (37.6% vs 20.9%, P < 0.001), as well as more all-cause office visits in one year (25.5 vs 18.7, P < 0.001). Moreover, the mean duration of all-cause hospitalization among patients with diarrhea was longer than in those without diarrhea (11.6 d vs 8.0 d, P < 0.001).
| With non-infectious diarrhea | Without non-infectious diarrhea | P value | |
| n = 534 | n = 2288 | ||
| Overall healthcare utilization | |||
| Patients with hospitalizations | 265 (49.6) | 907 (39.6) | < 0.001 |
| Average LOS among hospitalized patients (d) | 11.6 ± 13.4 | 8.0 ± 9.2 | < 0.001 |
| Patients with ED visits | 201 (37.6) | 479 (20.9) | < 0.001 |
| Outpatient physician visits | 25.5 ± 18.4 | 18.7 ± 15.8 | < 0.001 |
| CS-related utilization1 | |||
| Patients with hospitalizations | 73 (13.7) | 165 (7.2) | < 0.001 |
| Average LOS among hospitalized patients (d) | 7.4 ± 7.1 | 5.5 ± 3.6 | 0.029 |
| Patients with ED visits | 59 (11.0) | 101 (4.4) | < 0.001 |
| Outpatient physician visits | 6.9 ± 7.8 | 4.1 ± 6.1 | < 0.001 |
| Healthcare costs (USD) | |||
| Total costs | 82032 ± 90181.7 | 51621 ± 63890.8 | < 0.001 |
| Total medical costs | 74654 ± 86742.3 | 47083 ± 61214.0 | < 0.001 |
| Total pharmacy costs | 7378 ± 13949.8 | 4538 ± 10314.2 | < 0.001 |
Similar trends were observed in unadjusted CS-related healthcare resource utilization. Compared to patients without diarrhea, patients with diarrhea more commonly had at least one CS-related hospitalization (13.7% vs 7.2%, P < 0.001), at least one CS-related ED visit (11.0% vs 4.4%, P < 0.001), as well as more CS-related office visits in one year (6.9 vs 4.1, P < 0.001). The mean duration of CS-related hospitalization among patients with diarrhea was also longer than in those without diarrhea (7.4 d vs 5.5 d, P = 0.029).
Unadjusted healthcare costs - both in total, and divided into medical and pharmacy costs-were also significantly higher among patients with diarrhea compared to those without (Table 2). CS patients with diarrhea incurred $82032 in annual total costs, 58.9% higher than the $51621 among those without diarrhea (P < 0.001). In the one-year post index date, those with diarrhea also had higher medical ($74654 vs $47083, P < 0.001) and pharmacy costs ($7378 vs $4538, P < 0.001) compared to those without diarrhea. Components of medical cost also differed significantly between groups. Inpatient costs were $27018 in patients with diarrhea compared to $16609 in those without (P < 0.001). Outpatient medical costs were $46917 vs $30140 (P < 0.001), and ED costs were $719 vs $334 (P < 0.001) in CS patients with vs without diarrhea.
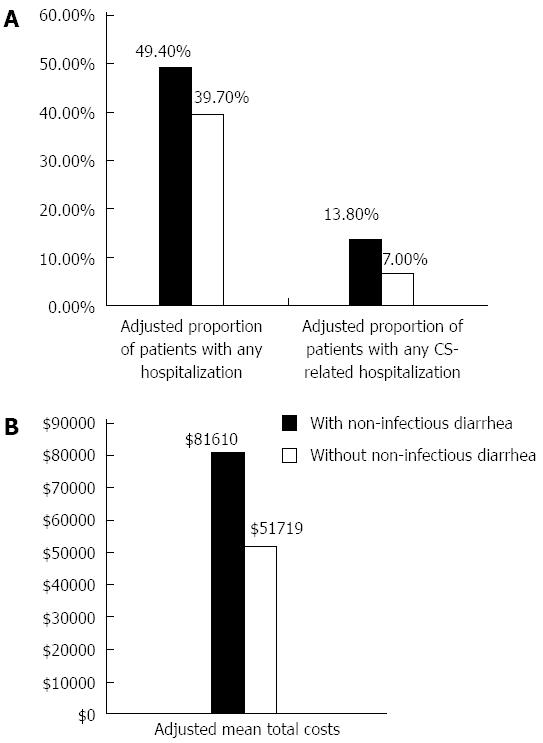
After adjusting for age, gender, geographic region, number of chronic conditions and the Charlson Comorbidity Index, the differences in our primary outcomes of interest were still significant in the one-year post index date. The odds of any hospitalization were 1.48 (95%CI: 1.22-1.80, P < 0.001) (Table 3) times greater among those with diarrhea compared to those without. The adjusted proportion of patients with any hospitalization was 9.7% higher among those with diarrhea [49.4% (45.1%-53.6%) vs 39.7% (95%CI: 37.7%-41.7%)] (Table 4, Figure 2). Patients with diarrhea had 24.2 (95%CI: 22.9-25.5) office visits in the study period compared to 19.0 (95%CI: 18.4-19.7) in those without (P < 0.001).
| Any hospitalization | Any CS-related hospitalization1 | Total costs | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | mean (SE) | P value | |
| Age group, yr | ||||||
| ≤ 17 vs 55-64 | 0.84 (0.35-2.02) | 0.702 | 2.38 (0.78-7.22) | 0.127 | $-8004 (13971.7) | 0.567 |
| 18-24 vs 55-64 | 1.33 (0.69-2.57) | 0.389 | 0.81 (0.24-2.70) | 0.726 | $-8328 (10946.6) | 0.447 |
| 25-34 vs 55-64 | 0.93 (0.65-1.33) | 0.687 | 0.52 (0.25-1.10) | 0.087 | $-10875 (5867.4) | 0.064 |
| 35-44 vs 55-64 | 0.97 (0.76-1.23) | 0.785 | 0.80 (0.52-1.24) | 0.314 | $-5474 (3993.0) | 0.171 |
| 45-54 vs 55-64 | 0.93 (0.78-1.11) | 0.412 | 0.85 (0.63-1.16) | 0.307 | $-2433 (2855.8) | 0.394 |
| Sex | ||||||
| Female vs Male | 0.88 (0.75-1.02) | 0.093 | 0.89 (0.68-1.17) | 0.401 | $-7165 (2539.7) | 0.005 |
| Region | ||||||
| North Central vs West | 1.01 (0.79-1.28) | 0.951 | 0.88 (0.58-1.35) | 0.562 | $-6623 (3954.7) | 0.094 |
| Northeast vs West | 0.91 (0.69-1.19) | 0.477 | 1.18 (0.76-1.85) | 0.458 | $-7458 (4393.6) | 0.090 |
| South vs West | 1.02 (0.82-1.27) | 0.842 | 0.88 (0.60-1.29) | 0.521 | $-11343 (3601.1) | 0.002 |
| No. of chronic conditions | 1.03 (0.99-1.08) | 0.107 | 0.98 (0.91-1.05) | 0.521 | $1115 (665.6) | 0.094 |
| Charlson comorbidity index | 1.02 (1.00-1.04) | 0.099 | 0.99 (0.95-1.03) | 0.658 | $5231 (354.5) | < 0.001 |
| Non-infectious diarrhea | ||||||
| Yes vs No | 1.48 (1.22-1.80) | < 0.001 | 2.12 (1.57-2.86) | < 0.001 | $29890 (3204.5) | < 0.001 |
| Non-infectious diarrhea | Adjusted proportion of patients with any hospitalization1 | Adjusted proportion of patients with any CS-related hospitalization12 | Adjusted total cost1 |
| % (95%CI) | % (95%CI) | mean (95%CI) | |
| Yes | 49.4 (45.1-53.6) | 13.8 (11.1-17.1) | $81610 ($75962-$87258) |
| No | 39.7 (37.7-41.7) | 7.0 (6.0-8.2) | $51719 ($49007-$54432) |
The difference in odds was also significantly greater for CS-related hospitalizations. Those with diarrhea were at 2.12 (95%CI: 1.57-2.86, P < 0.001) (Table 3) times greater odds of CS-related hospitalization compared to those without diarrhea. The adjusted proportion of patients with any CS-related hospitalization was 6.8% higher among those with diarrhea [13.8% (95%CI: 11.1%-17.1%) vs 7.0% (95%CI: 6.0%-8.2%)] (Table 4, Figure 2).
The adjusted total healthcare costs were significantly higher, by $29890 (P < 0.001) for those with diarrhea at $81610 (95%CI: $75962-$87258) compared to those without diarrhea at $51719 (95%CI: $49007-$54432) (Tables 3 and 4, Figure 2). Pharmacy costs were $2557 (P < 0.001) more than in patients without diarrhea. Medical costs were higher by $27334 (P < 0.001). Medical costs comprised outpatient visits (cost of which was $16695 higher) and inpatient hospitalization ($11431 higher) (P = 0.003).
Beyond its deleterious influence on quality of life, our results demonstrate that diarrhea in patients with CS has a significant medical and economic impact. Our findings suggest that diarrhea associated with CS accounts for 1.5-fold higher total healthcare spending and almost a 2-fold higher risk of CS-related hospitalizations compared to when diarrhea is not present. The adjusted mean total healthcare costs in our analysis were $81610 compared $51719 per year among patients with CS who suffered from diarrhea compared to those who did not have diarrhea (P < 0.001). The adjusted risk of CS-related hospitalizations increased from 7.0% among CS patients with no evidence of diarrhea to 13.8% who were diagnosed with diarrhea symptoms (P < 0.001). Effective preventive treatment of diarrhea in patients suffering from CS would be a reasonable approach to reducing healthcare resource utilization and costs in this population.
Our results indicate that diarrhea symptoms are common in patients with CS. In the present analysis, we found 18.9% of cases with at least one claim for the diagnosis of non-infectious diarrhea in the one-year study period. These findings are comparable to an analysis of commercially insured patients with NETs in which 17.6% of patients had symptoms of diarrhea and up to 72% of patients had CS within the same time period[12]. In that analysis the annualized total healthcare costs for patients with NETs were estimated at approximately $106000 (2011 United States $)[12]. While those cost estimates were not disaggregated by CS symptoms, both analyses highlight the substantial economic burden associated with treatment of patients with CS.
Patients with NETs have significantly higher rates of mortality and hepatic and gastrointestinal morbidities compared to patients without NETs or other cancers matched by age, sex, and year of diagnosis[13]. They also have worse health-related QOL than general population controls, with evidence that this is attributable to symptoms such as diarrhea, fatigue, and depression[14,15]. Fröjd et al[14] used the EORTC QLQ-C30 instrument to demonstrate that diarrhea, along with fatigue, had particularly prominent adverse impact on physical, emotional, and social well-being in a cohort of Swedish patients with NETs.
Chronic secretory diarrhea in patients with NETs results from imbalances in intestinal absorptive and secretory processes, leading to dehydration, renal insufficiency, and various serum electrolyte imbalances, and improper digestion of food[16]. Nutritional deficiencies caused by inadequate digestion may further exacerbate weight loss and fatigue caused by fluid and electrolyte loss[16]. NETs are among eight different neoplastic diseases known to cause chronic diarrhea, but because these conditions as a group comprise less than 1% of all chronic diarrhea, they are often ignored in the differential diagnosis, which may lead to delay in care[17]. Preventive treatment and management of non-infectious diarrhea in patients with CS could directly reduce health service use and cost, and it may also help resolve fatigue and other secondary consequences of the condition previously associated with CS. Effective management of diarrhea also could potentially contribute to a reduction in emotional distress, which has been shown repeatedly to be associated with greater resource utilization in other populations[18-21]. The NCCN Clinical Practice Guidelines in Oncology on NETs recommend the use of the long-acting somatostatin analogues, octreotide and lanreotide, should result in improvement of diarrhea and flushing symptoms of carcinoid syndrome[22]. Octreotide LAR dose and frequency may be further increased for symptom control as needed[22].
The results of this study need to be interpreted in the context of several limitations. First, in our analysis we attributed all diagnoses of non-infectious diarrhea to CS. We mitigated the possibility of misdiagnosis by excluding certain ICD-9-CM codes that were not clearly indicative of non-infectious diarrhea, such as gastroenteritis (e.g., 558.9: other and unspecified noninfectious gastroenteritis and colitis). However, claims do not attribute diarrhea to a cause, they merely note the presence of the condition. Some cases of non-infectious diarrhea could have been from causes other than CS, resulting in an overestimate of the presence of CS-related diarrhea. On the other hand, codes for infectious diarrhea may have been applied to CS-related diarrhea simply because clinicians were more familiar with them. Studies of diarrhea in other conditions have been inconsistent in the ICD-9-CM codes used for non-infectious diarrhea[23-25]. Nonetheless, our estimate of an 18.9% annual prevalence of diarrhea was remarkably close to another published estimate of 17.6% in a cohort of NET patients in which the majority had CS (72%)[12]. Second, we considered HRU to be CS-related if codes for a variety of conditions associated with CS were identified in the primary position on a claim. This may have overestimated utilization, although limiting the definition of CS-related to only those claims with CS in the primary position would have almost certainly have underestimated utilization (e.g., a patient admitted for management of intestinal obstruction from a growing tumor would be likely to have obstruction, rather than CS, coded as the primary diagnosis). Third, our patient identification algorithm allowed a relatively long interval to pass between the first and confirmatory diagnosis. This decision may have reduced the specificity of our algorithm, although such reduction should have affected both groups equally. Fourth, we adjusted for a variety of potential confounders in our comparisons, but we did not adjust for pre-diagnosis health care resource use or cost. We felt that although prior utilization can predict future utilization, the patients in this analysis were all newly diagnosed with CS and thus controlling for pre-diagnosis resource use would be of limited value. Finally, this study included only patients with commercial insurance coverage. Our cohort of incident cases with CS was younger (mean age: 52 years) than a population-representative sample of incident NET cases from the Surveillance, Epidemiology, and End Results (SEER) database (mean age: 62 years)[4]. Our results may not be generalizable to the United States population at large, but they are representative of a commercially-insured population.
Diarrhea is a particularly burdensome and costly symptom suffered by patients with CS. Our study demonstrates that health care costs and resource utilization in newly diagnosed CS patients with diarrhea are consistently and significantly higher than in those without diarrhea. Reduction of healthcare expenditures attributable to diarrhea may be achievable through preventive treatment and appropriate management of diarrhea in patients with CS.
The authors thank Gordon H Sun, MD, MS and Elya Papoyan, MPH for their contributions to study design and drafting of the initial manuscript.
Non-infectious diarrhea (NID) is particularly burdensome and costly in carcinoid syndrome (CS) patients. The authors found that the proportions of patients with any and with CS-related hospitalizations were 9.7% and 6.8% higher, respectively, among NID patients than in those with no NID (P < 0.001). The NID group was also significantly more costly, with adjusted mean annual healthcare costs of $81610, compared to $51719 in the no NID group (P < 0.001).
The healthcare and economic burden of diarrhea in CS patients has not been previously quantified.
This study shows diarrhea is burdensome and costly in CS patients.
Reduction of CS-related healthcare expenditures may be achievable through preventive treatment and appropriate management of diarrhea in CS.
Neuroendocrine tumors that produce peptides and neuroamines induce characteristic hormonal syndromes, including CS. Most common presenting symptoms of CS are diarrhea and flushing. Diarrhea appears to have a particularly profound impact on CS patients’ well-being. Given the lack of evidence about the economic burden of diarrhea in CS patients, we examined healthcare resource utilization patterns and healthcare costs accrued by CS patients with and without NID.
This research is interesting, and it is well executed methodologically and clearly described.
P- Reviewer: Helfer B, Teramoto-Matsubara OT S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the diffuse neuroendocrine system. Curr Opin Oncol. 2008;20:1-12. [PubMed] |
| 2. | Pearse AG. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969;17:303-313. [PubMed] |
| 3. | Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502-1522. [PubMed] |
| 4. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3240] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 5. | Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, Wright NA, Kidd M. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust. 2010;193:46-52. [PubMed] |
| 6. | Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol. 2004;35:1440-1451. [PubMed] |
| 7. | Ter-Minassian M, Chan JA, Hooshmand SM, Brais LK, Daskalova A, Heafield R, Buchanan L, Qian ZR, Fuchs CS, Lin X. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocr Relat Cancer. 2013;20:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Chronic Condition Indicator (CCI) for ICD-9-CM. Rockville, MD: Agency for Health Care Policy and Research, 2009. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. |
| 10. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] |
| 11. | Hwang W, Weller W, Ireys H, Anderson G. Out-of-pocket medical spending for care of chronic conditions. Health Aff (Millwood). 2011;20:267-278. [PubMed] |
| 12. | Chuang CC, Bhurke S, Chen SY, Brulais S, Gabriel S. Clinical characteristics, treatment patterns, and economic burden in patients treated for neuroendocrine tumors in the United States: a retrospective cohort study. J Med Econ. 2015;18:126-136. [PubMed] |
| 13. | Hess GP, Chen CC, Liu Z, Yao JC, Phan AT, Hill JW. Clinical burden of illness in patients with neuroendocrine tumors. Pancreas. 2012;41:1058-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Fröjd C, Larsson G, Lampic C, von Essen L. Health related quality of life and psychosocial function among patients with carcinoid tumours. A longitudinal, prospective, and comparative study. Health Qual Life Outcomes. 2007;5:18. [PubMed] |
| 15. | Haugland T, Vatn MH, Veenstra M, Wahl AK, Natvig GK. Health related quality of life in patients with neuroendocrine tumors compared with the general Norwegian population. Qual Life Res. 2009;18:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Harris AG, O’Dorisio TM, Woltering EA, Anthony LB, Burton FR, Geller RB, Grendell JH, Levin B, Redfern JS. Consensus statement: octreotide dose titration in secretory diarrhea. Diarrhea Management Consensus Development Panel. Dig Dis Sci. 1995;40:1464-1473. [PubMed] |
| 17. | Jensen RT. Overview of chronic diarrhea caused by functional neuroendocrine neoplasms. Semin Gastrointest Dis. 1999;10:156-172. [PubMed] |
| 18. | Berghöfer A, Roll S, Bauer M, Willich SN, Pfennig A. Screening for depression and high utilization of health care resources among patients in primary care. Community Ment Health J. 2014;50:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:963-971. [PubMed] |
| 20. | Moraska AR, Chamberlain AM, Shah ND, Vickers KS, Rummans TA, Dunlay SM, Spertus JA, Weston SA, McNallan SM, Redfield MM. Depression, healthcare utilization, and death in heart failure: a community study. Circ Heart Fail. 2013;6:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Shippee ND, Rosen BH, Angstman KB, Fuentes ME, DeJesus RS, Bruce SM, Williams MD. Baseline screening tools as indicators for symptom outcomes and health services utilization in a collaborative care model for depression in primary care: a practice-based observational study. Gen Hosp Psychiatry. 2014;36:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Neuroendocrine Tumors. Version 1. 2015. 1998; Available from: http://www.nccn.org. |
| 23. | Bunnapradist S, Neri L, Wong W, Lentine KL, Burroughs TE, Pinsky BW, Takemoto SK, Schnitzler MA. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis. 2008;51:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Cortes JE, Curns AT, Tate JE, Parashar UD. Trends in healthcare utilization for diarrhea and rotavirus disease in privately insured US children & lt; 5 years of age, 2001-2006. Pediatr Infect Dis J. 2009;28:874-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001;20:14-19. [PubMed] |