Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2081
Peer-review started: June 21, 2015
First decision: July 10, 2015
Revised: July 21, 2015
Accepted: October 23, 2015
Article in press: October 26, 2015
Published online: February 14, 2016
Processing time: 217 Days and 17 Hours
AIM: To investigate the therapeutic effects and mechanisms of interleukin (IL)-22 in liver regeneration in mice with concanavalin A (ConA)-induced liver injury following 70% hepatectomy.
METHODS: Mice were injected intravenously with ConA at 10 μg/g body weight 4 d before 70% hepatectomy to create a hepatitis model, and recombinant IL-22 was injected at 0.125 μg/g body weight 30 min prior to 70% hepatectomy to create a therapy model. Control animals received an intravenous injection of an identical volume of normal saline.
RESULTS: IL-22 treatment prior to 70% hepatectomy performed under general anesthesia resulted in reductions in the biochemical and histological evidence of liver injury, earlier proliferating cell nuclear antigen expression and accelerated recovery of liver mass. IL-22 pretreatment also significantly induced signal transducer and activator of transcription factor 3 (STAT3) activation and increased the expression of a variety of mitogenic proteins, such as Cyclin D1. Furthermore, alpha fetal protein mRNA expression was significantly elevated after IL-22 treatment.
CONCLUSION: In this study, we demonstrated that IL-22 is a survival factor for hepatocytes and prevents and repairs liver injury by enhancing pro-growth pathways via STAT3 activation. Treatment with IL-22 protein may represent a novel therapeutic strategy for preventing liver injury in patients with liver disease who have undergone hepatectomy.
Core tip: Interleukin (IL)-22 appears to play a protective role in inflammation and has also been demonstrated to have proliferative effects in a hepatocyte cell line, however, it has rarely been reported that the protective and proliferative effects exist simultaneously. In this article, we investigated the therapeutic effects and mechanisms of IL-22 in liver regeneration in mice with concanavalin A-induced liver injury following 70% hepatectomy. IL-22 played protective and survival roles against liver injury in this model.
- Citation: Zhang YM, Liu ZR, Cui ZL, Yang C, Yang L, Li Y, Shen ZY. Interleukin-22 contributes to liver regeneration in mice with concanavalin A-induced hepatitis after hepatectomy. World J Gastroenterol 2016; 22(6): 2081-2091
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2081.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2081
Interleukin-22 (IL-22) is an inducible cytokine of the IL-10 superfamily that was identified by the Belgian Renauld team as early as 2000 and was previously known as the IL-10-related factor from T cells[1]. IL-22 is produced by activated T cells and natural killer (NK) cells and acts via a heterodimeric receptor complex consisting of IL-22 receptor α (IL-22Rα) and IL-10 receptor β (IL-10Rβ).
IL-22 has been demonstrated to exhibit a variety of effects. IL-22 appears to play an important role in inflammation and has also been noted to exert proliferative effects in a hepatocyte cell line in vitro[2-5]. In 2004, the Bin Gao team demonstrated that IL-22 expression is significantly induced in T cell-mediated hepatitis and that IL-22 blockade markedly enhances liver injury in this model, while administration of recombinant IL-22 prevents concanavalin A (ConA)-induced liver injury[5]. These findings suggest that IL-22 acts as a protective cytokine that attenuates liver injury in T cell-mediated hepatitis. Furthermore, in vitro studies also revealed that IL-22 has no obvious toxicity in liver cell lines or primary liver cells and has proliferative and survival effects on these cells. Additionally, a recent study supported a potential therapeutic role for IL-22 as a protective factor in hepatic resection. The authors of this study observed significant increases in hepatic IL-22 receptor expression and serum IL-22 levels after 70% hepatectomy and a significant decrease in liver regeneration after IL-22 blockade[6]. However, the precise mechanism of IL-22-mediated liver protection remains unclear.
Currently, all of the evidence supporting the role of IL-22 in liver protection is from liver injury models, and the evidence for the liver proliferative effects is almost entirely from the simple 2/3 liver resection model without other injury. However, clinical patients with hepatectomy nearly always have liver disease and thus significantly decreased liver regeneration abilities. In this article, we sought to investigate the therapeutic effects and mechanisms of IL-22-mediated liver regeneration in mice with ConA-induced liver injury following 70% hepatectomy.
Recombinant IL-22 protein was purchased from Pepro Tech Inc (New Jersey, United States). Anti-STAT3, Cyclin D1 and proliferating cell nuclear antigen (PCNA) antibodies were obtained from Cell Signaling Technology Inc (CST, United States). Female C57/BL6 mice were purchased from HFK Bioscience Co., Ltd. (Beijing, China).
Female C57/BL6 mice (6-8 wk of age, 20-25 g) were maintained under specific pathogen-free conditions with free access to water and food before each experiment. The animals were anesthetized with chloral hydrate injections. After a midline incision was created under microscopic guidance, and the middle and left hepatic lobes of the liver were fully freed, 7-0 vascular sutures were used to ligate the branches of the hepatic artery and portal vein of the median and left lateral lobes of the liver. Next, the bile duct was ligated with 7-0 vascular sutures, and the gallbladder was removed. Finally, the median and left lateral lobes of the liver were resected after a 4-0 silk suture ligation was secured around the base of each lobe.
ConA was injected intravenously at 10 μg/g body weight 4 d before the operation, and the 70% hepatectomy model animals received intravenous injections of identical volumes of normal saline.
ConA was injected intravenously at 10 μg/g body weight, and 70% hepatectomies were performed 4 d later.
Four days after the intravenous injections of ConA at 10 μg/g body weight, recombinant murine IL-22 was injected intravenously at 0.125 μg/g body weight 30 min prior to 70% hepatectomy. Control animals received intravenous injections of identical volumes of normal saline.
At 32 h, 40 h, 48 h, 1 wk, and 2 wk, the mice were humanely killed under general anesthesia once moribund. The liver and body weights of all mice in each group were measured, and the liver weight/body weight ratio was then calculated to observe the liver regeneration conditions.
To assess the damage to the hepatic parenchyma, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using a serum analyzer (Cobas-Mira Plus, Roche, Manheim, Germany). The liver specimens were fixed in 10% buffered formalin and embedded in paraffin, and the paraffin embedded liver tissue sections were then stained with hematoxylin and eosin (HE) for histological examinations.
Tissue specimens were fixed in neutral buffered formalin and then embedded in paraffin. The 4-μm paraffin sections were deparaffinized in xylene and rehydrated in a graded series of alcohol. Endogenous peroxidase was inhibited with 0.3% H2O2 in methanol. The sections were heated in a microwave oven (in 10 mmol/L citrate buffer, pH 6.0) for 20 min for epitope retrieval, followed by incubation with the primary antibody against PCNA (1:4000; Abcam). The slides were then incubated with a biotinylated bridging antibody (dilution: 1/200, DAKO) for 60 min. The sections were counterstained with Mayer’s hematoxylin. PCNA antigen expression levels were evaluated by counting the positively stained cells in the portal triads of five high-power fields (HPFs) per slide, and the results are expressed as the average number of positive cells/HPF.
RNA was extracted from snap-frozen liver tissue samples using the TRIzol reagent. Five micrograms of RNA was reverse-transcribed into cDNA using oligo-dT primers with a Superscript III First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR was performed with iCycler IQ system (Bio-Rad, Hercules, CA). The primer sequences for alpha fetal protein (AFP) gene were 5’-CAA AGC ATT GCA CGA AAA TGA G-3’ (forward) and 5’-AAC AAA CTG GGT AAA GGT GAT GGT-3’ (reverse). β-actin was measured as a housekeeping gene. The cycling conditions comprised a 5 min polymerase activation at 95 °C, 40 cycles of 95 °C for 5 s and 60 °C for 30 s and a single fluorescence measurement. Melting curve analysis based on increasing the temperature from 60 to 95 °C at a rate of 0.5 °C/s with continuous fluorescence measurement revealed a single, narrow peak for the suspected fusion temperature.
The mice were euthanized at baseline and at 32 h, 40 h, 48 h, 1 wk and 2 wk after hepatectomy, and liver samples were obtained for Western blot analyses. The proteins were extracted from the liver tissues and quantified using a protein assay (Bio-Rad Laboratories, CA). The protein samples (30 μg) were fractionated by SDS-PAGE and transferred to a nitrocellulose membrane. Immunoblotting was conducted using antibodies against STAT3 and Cyclin D1 (Cell Signaling Technology Inc., United States). The results were visualized via an enhanced chemiluminescent detection system (Pierce ECL Substrate Western blot detection system, Thermo Scientific, IL) and exposure to autoradiography film (Kodak XAR film).
All parametric data are presented as the mean ± SD. The data were analyzed for significance using Student’s t-tests. One-way analyses of variance with Fisher’s protected least significant difference (PLSD) tests were used to compare the means. The log-rank test was applied to compare survival curves. Differences were considered statistically significant at P < 0.05.
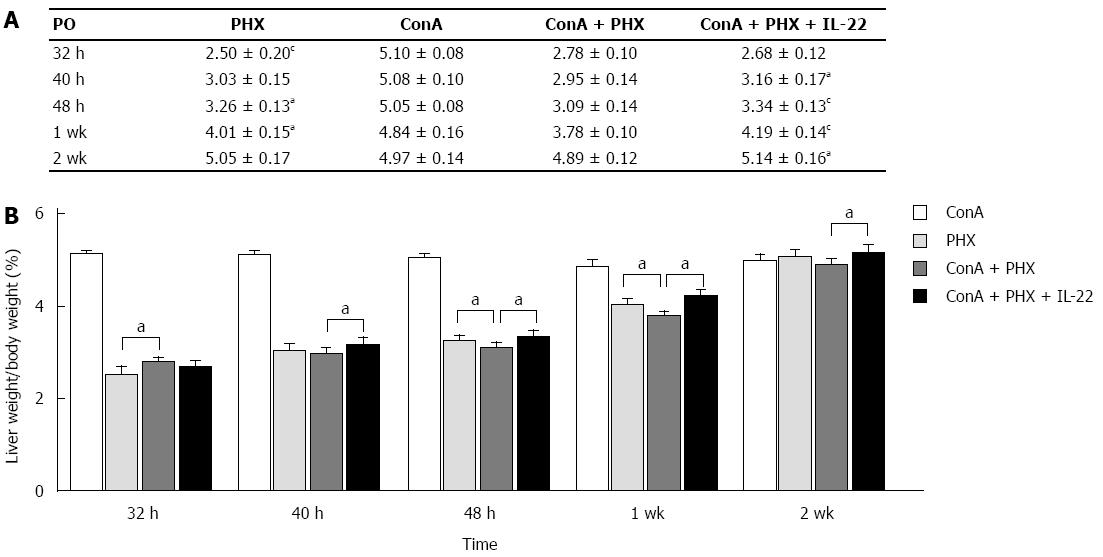
At 32 h, 40 h, 48 h, 1 wk, or 2 wk, the mice were humanely killed under general anesthesia once moribund. The liver and body weights were measured, and the liver weight/body weight ratios were then calculated. As illustrated in Figure 1, increases in the liver weight/body weight ratios were observed in the PHX, ConA + PHX and ConA + PHX + IL-22 groups, and all groups returned to normal liver weights by 2 w. Compared with the ConA + PHX group, the ratio of the ConA + PHX + IL-22 group increased more rapidly, and significant differences between these two groups were observed at 40 h, 48 h, 1 wk and 2 wk. Similarly, compared to the ConA + PHX group, the ratios of the PHX group increased more rapidly, and the differences reached significance at 48 h or 1 wk. However, the increase in the PHX group was less than that in the ConA + PHX group at 32 h. These data correlated with the cellular swelling in the liver at 32 h in the ConA + PHX group.
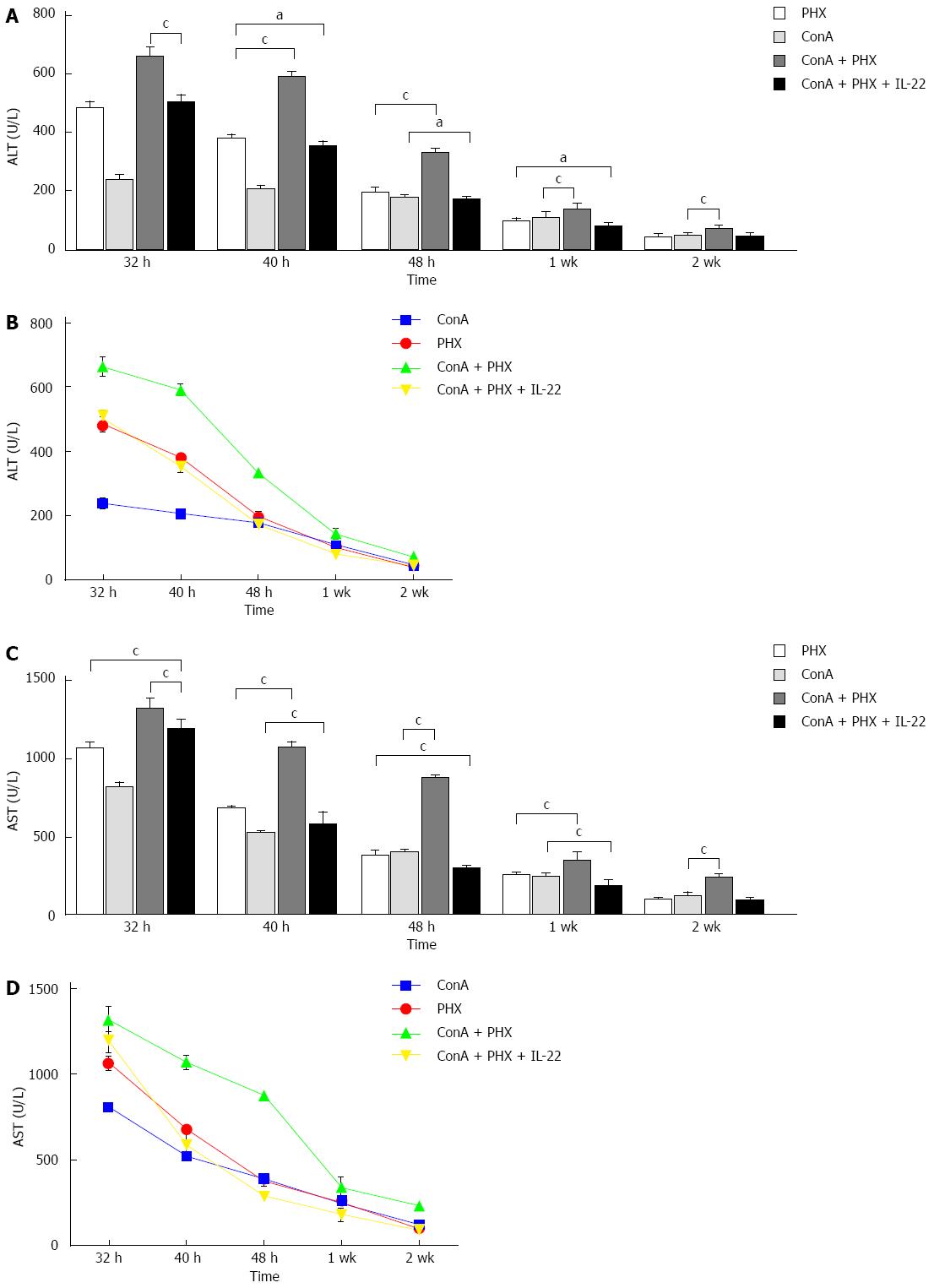
At 32 h, 40 h, 48 h, 1 wk and 2 wk after 70% hepatectomy, serum samples were collected, and the ALT and AST levels were measured via biochemical analyses. As illustrated in Figure 2, compared with the ConA + PHX group, the ALT and AST serum levels in the ConA + PHX + IL-22 group were reduced, and these differences were significant at all of the time points. Furthermore, with the recombinant IL-22 pretreatment, the decreases in the ALT and AST levels of the ConA + PHX + IL-22 group were significantly greater than those of the ConA and PHX groups at 40 h, 48 h and 1 wk.
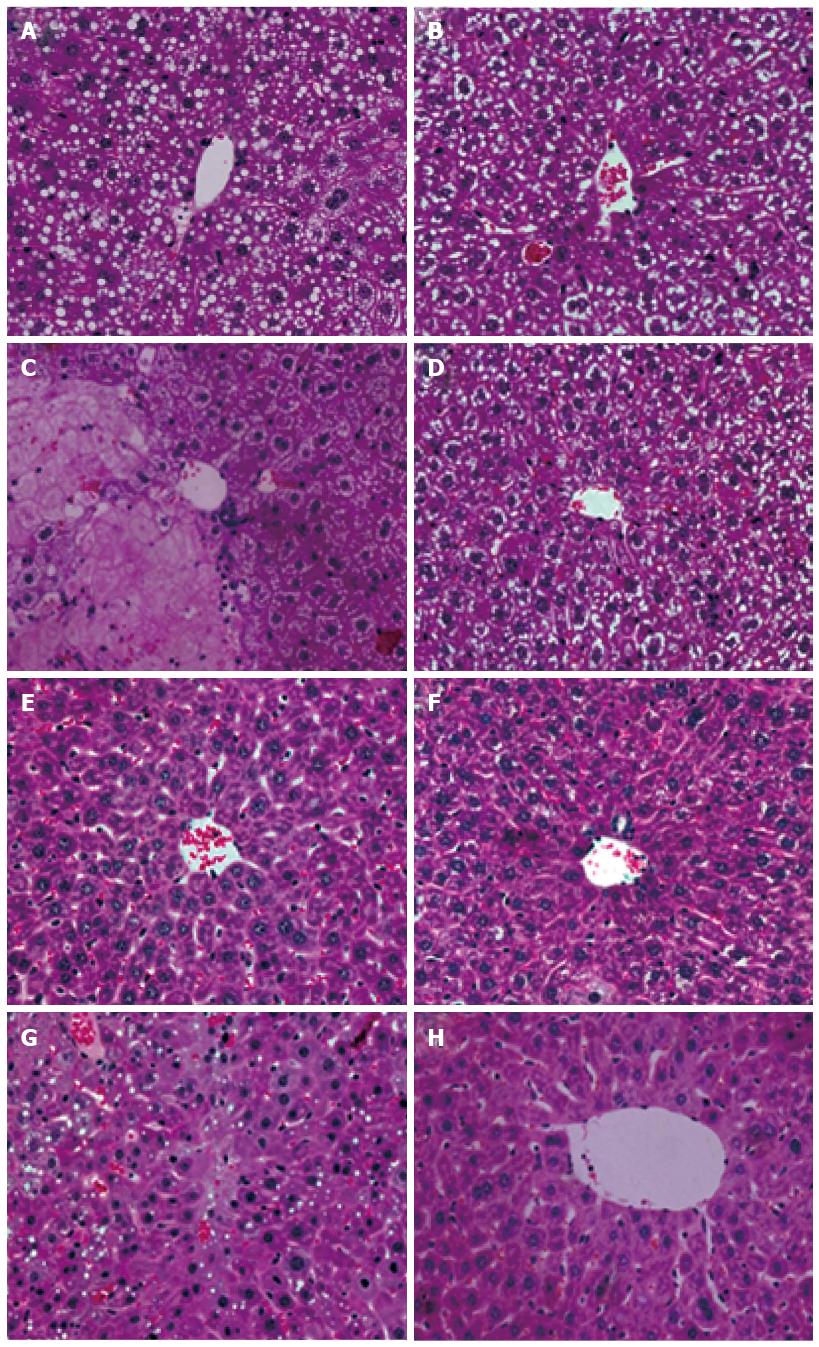
We also investigated the histologic features of the liver by HE staining. As illustrated in Figure 3, the HE staining of the ConA + PHX group demonstrated severe sinusoidal narrowing that was noted as early as 32 h after hepatectomy. By 48 h, swelling, nuclear condensation and laminar necrosis of the hepatocytes were observed in addition to the near-total loss of the hepatic sinusoids. By 2 wk, the hepatic sinusoids and the complete structure of the hepatic lobule were not observed in the hepatocytes. In contrast, the HE staining of the ConA + PHX + IL-22 group revealed much less evidence of injury at 48 h; the cytoplasm was preserved, and some less severe swelling was present. At 2 wk, normal nuclear morphologies, hepatic sinusoids, complete structures of the hepatic lobules and significant regeneration in the hepatocytes were observed.
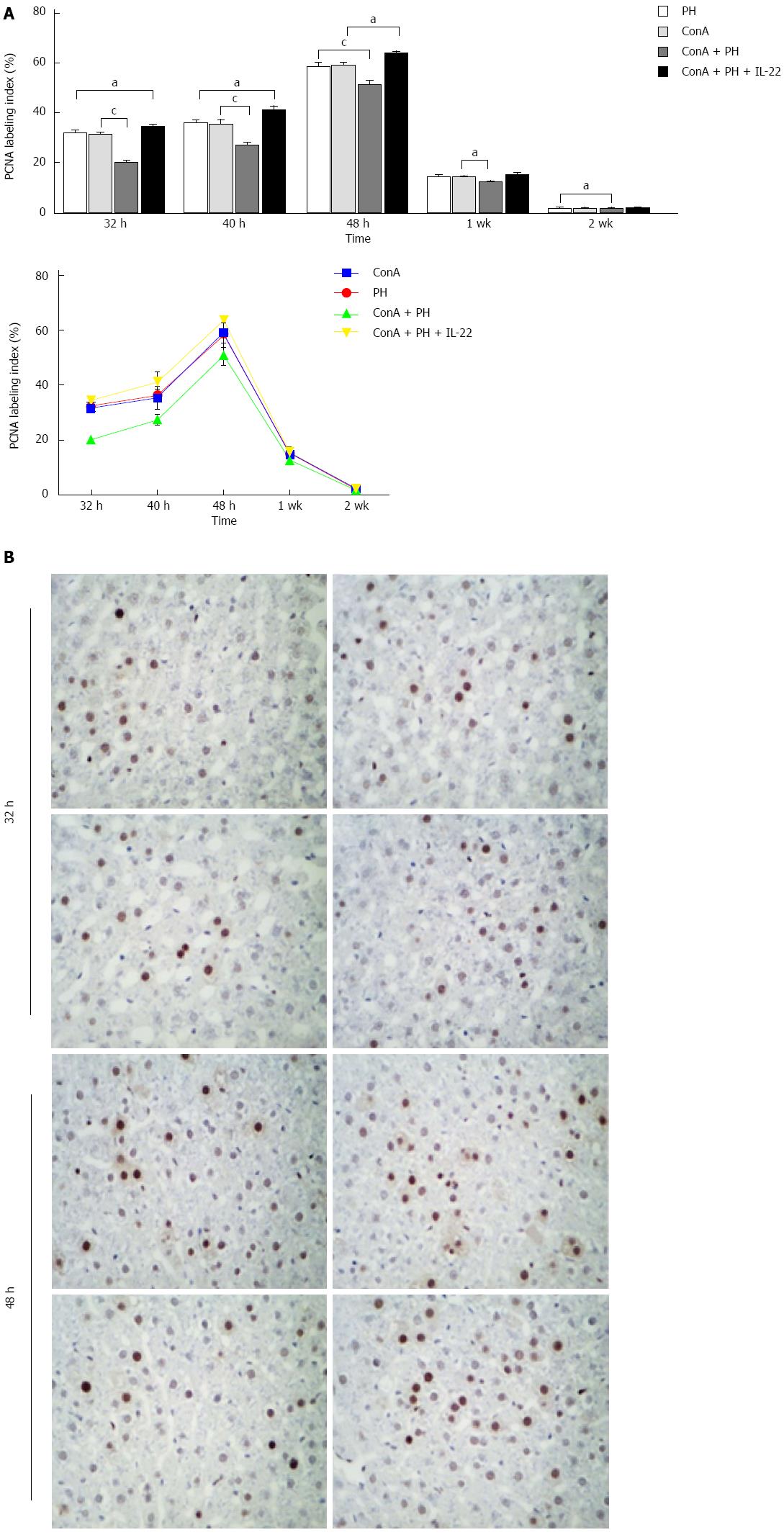
Hepatocyte proliferation was determined by the expression of PCNA, which is a nuclear antigen that is associated with hepatocyte proliferation. As illustrated in Figure 4, the PCNA labeling indices in the four groups began to increase postoperatively and peaked at 48 h. Compared with the ConA + PHX group, the PCNA labeling indices were significantly increased in the ConA, PHX and ConA + PHX + IL-22 groups at all of the time points, particularly at 32, 40, and 48 h. Furthermore, the PCNA levels in the ConA + PHX + IL-22 group increased to greater extents than those of the ConA and PHX groups at 32 h, 40 h and 48 h.
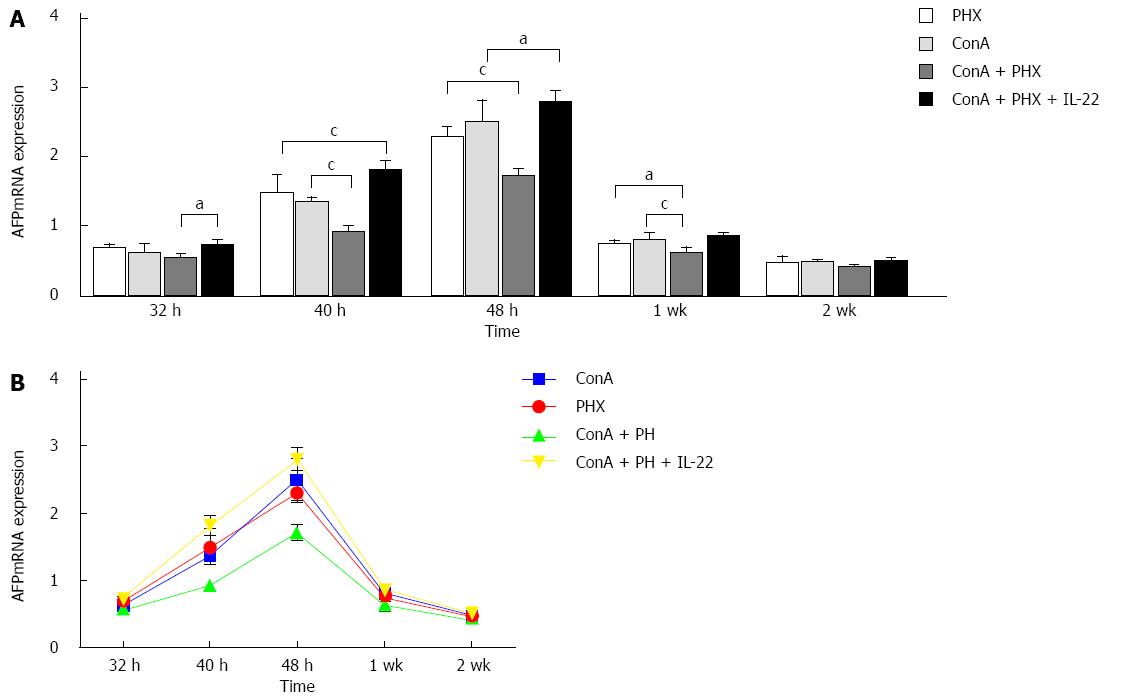
The mice underwent 70% hepatectomy or sham laparotomy, and quantitative analysis of the liver AFP mRNA expression was performed by real-time RT-PCR. At 32 h, 40 h, 48 h, 1 wk, and 2 wk, the hepatic AFP mRNA expression levels in the ConA + PHX group were 0.55 ± 0.06, 0.93 ± 0.08, 1.72 ± 0.11, 0.66 ± 0.05 and 0.43 ± 0.04, respectively. In the IL-22 pretreatment group, the corresponding values were 0.74 ± 0.08, 1.81 ± 0.14, 2.80 ± 0.26, 0.86 ± 0.05 and 0.51 ± 0.03. Figure 5 illustrates that the AFP mRNA expression began to increase at 32 h and had significantly increased by 48 h after hepatectomy in all four groups. Additionally, the AFP mRNA levels in the ConA + PHX + IL-22 group were significantly increased at 32 h, 40 h, 48 h and 1 wk after partial hepatectomy compared with the ConA + PHX group.
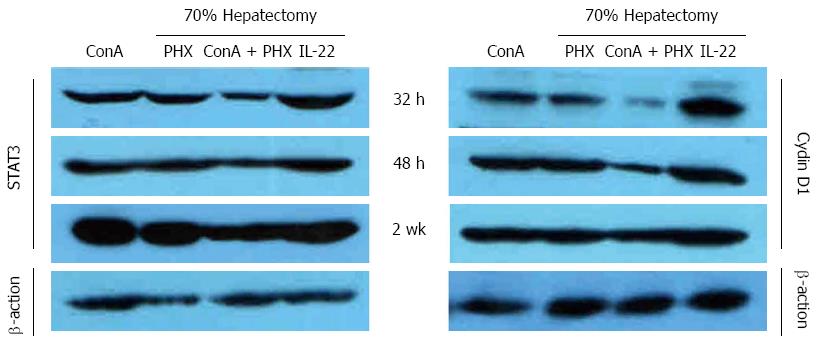
The activation of the STAT3 and Cyclin D1 was measured using Western blot analysis to assess the effects of IL-22 after partial hepatectomy. As illustrated in Figure 6, no significant activation of STAT3 or Cyclin D1 was observed at 32 h after partial hepatectomy in the ConA + PHX group, and the activation increased gradually at 48 h and 2 wk. Although STAT3 and Cyclin D1 activation was apparent in the remnant livers of the other three groups, STAT3 and Cyclin D1 were activated much earlier in the ConA + PHX + IL-22 group than the PHX and ConA groups. Furthermore, among the four groups, the STAT3 and Cyclin D1 levels exhibited the greatest increases in the ConA + PHX + IL-22 group and the smallest increases in the ConA + PHX group across all of the time points.
IL-22 has previously been shown to have a variety of effects. IL-22 appears to play a protective role in inflammation[7-10] and has also been demonstrated to have proliferative effects in a hepatocyte cell line[11]. Hepatectomy in T cell-mediated hepatitis induced by ConA models clinical hepatectomy with liver disease well. We demonstrated that IL-22 acts as a protective cytokine that attenuates liver injury in this model. The present study revealed that pre-treatment with IL-22 prior to hepatectomy significantly decreases the serum ALT and AST levels and increases the serum ALB level following 70% hepatectomy. Our findings suggest that IL-22 plays a protective role against liver injury in ConA-induced hepatitis following 70% hepatectomy and that IL-22 is a survival factor for hepatocytes. With the administration of exogenous IL-22, the liver weight/body weight ratio increased significantly and returned to the normal level by 2 wk. Additionally, the nuclear morphologies, hepatic sinusoids, complete structures of the hepatic lobules returned to normal, and significant regeneration was observed in the hepatocytes. In contrast, the ConA + PHX group that was not administered exogenous IL-22 exhibited swelling, nuclear condensation and laminar necrosis of the hepatocytes and a near-total loss of the hepatic sinusoids.
In the liver, IL-22 plays an important role in the acute-phase response and possibly also plays a role in the promotion of liver regeneration[2,6,12,13]. IL-22 acts via a heterodimeric receptor complex that consists of IL-22Rα and IL-10Rβ[2,5,14,15]. Examinations of the downstream signaling events following IL-22 administration in the context of partial hepatectomy demonstrated an increase in STAT3 activation. A substantial volume of published evidence supports the notion of STAT3-mediated cell survival and proliferation[16-21]. Our present investigation revealed that the injection of IL-22 rapidly induced STAT3 activation in the liver and that STAT3 induced the expression of genes that are important for cell cycle progression (e.g., Cyclin D1) and concurrently significantly increased PCNA staining to eventually promote cell survival and proliferation. These findings suggest that IL-22 was also partially responsible for hepatic STAT3 activation in this model. Thus, the activation of STAT3 by IL-22 was likely responsible for the protective role of IL-22 in the hepatocytes.
AFP is a specific marker of liver cancer tumors and is closely related to individual development, tissue regeneration, apoptosis and tumorigenesis[22-25]. The main role of AFP in liver regeneration is the regulation of hepatocyte growth. It has also been demonstrated that in the context of the synergy of various growth factors, AFP mediates cell growth regulation via an interaction with a special cell membrane receptor that results in the uptake of arachidonic acid and AFP into the cell. The process provides the necessary substrate and signal transduction for the M phase of mitosis[26,27]. Our present study found that following pretreatment with recombinant IL-22, AFP mRNA begin to be expressed from 32 h, and this expression increased significantly by 48 h after hepatectomy. This increase in expression was probably due to the loss of the negative regulation of the transcription inhibitory factor. This expression trend reflects the promotion of cell proliferation in the liver by AFP mRNA. These findings suggest that IL-22 can decrease the expression of the transcription inhibitory factor to induce the expression of AFP mRNA and provide the necessary material basis for mitosis and thus eventually promote cell survival and proliferation.
In summary, the model of hepatectomy in T cell-mediated hepatitis induced by ConA simulates clinical hepatectomy with liver disease accurately, and our findings suggest that IL-22 played protective and survival roles against liver injury in this model. Thus, IL-22 treatment should be considered to be a novel therapeutic option for liver injury and regeneration.
Interleukin (IL)-22 appears to play a protective role in inflammation and has also been demonstrated to exert proliferative effects in a hepatocyte cell line; however, it has rarely been reported that the protective and proliferative effects exist simultaneously. In this article, the authors sought to investigate the therapeutic effects and mechanisms of IL-22 in liver regeneration in mice with concanavalin A (ConA)-mediated liver injury following 70% hepatectomy.
IL-22 has been demonstrated to play a protective role in inflammation and proliferative effects in a hepatocyte cell line, however, it has rarely been reported that the protective and proliferative effects exist simultaneously.
In this article, the authors investigated the therapeutic effects and mechanisms of IL-22 in liver regeneration in mice with ConA-mediated liver injury following 70% hepatectomy. IL-22 was demonstrated to play a protective role and have proliferative effects together.
IL-22 treatment should be considered to be a novel therapeutic option for liver injury and regeneration.
In this study, the authors demonstrated that IL-22 is a valuable factor for hepatocyte proliferation and can protect the liver from injury induced by ConA. The work is well-done and provides the interesting results.
P- Reviewer: Mensah-Brown EPK, Wang GY S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 5. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 502] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 6. | Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74-G80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702. [PubMed] [DOI] [Full Text] |
| 8. | Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827-G838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, Shieh CC, Cheng KC, Lee MF, Chiang SR. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol. 2004;173:6712-6718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MH, Weiss TS, Prüfer T, Olszak T, Steib CJ, Storr M. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019-G1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003;38:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1117] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 15. | Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 16. | Ransohoff DF. Colon cancer in ulcerative colitis. Gastroenterology. 1988;94:1089-1091. [PubMed] |
| 17. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1767] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 18. | Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 19. | Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 2011;133:397-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1573] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 21. | Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407-2416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Liu L, Zhang CZ, Cai M, Fu J, Chen GG, Yun J. Downregulation of polo-like kinase 4 in hepatocellular carcinoma associates with poor prognosis. PLoS One. 2012;7:e41293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Spear BT. Alpha-fetoprotein gene regulation: lessons from transgenic mice. Semin Cancer Biol. 1999;9:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Boss JH, Rosenmann E, Zajicek G. Alpha-fetoprotein and liver cell proliferation in rats fed choline-deficient diet. Z Ernahrungswiss. 1976;15:211-216. [PubMed] |
| 25. | Yuan Q, Loya K, Rani B, Möbus S, Balakrishnan A, Lamle J, Cathomen T, Vogel A, Manns MP, Ott M. MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration. Hepatology. 2013;57:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Karvountzis GG, Redeker AG. Relation of alpha-fetoprotein in acute hepatitis to severity and prognosis. Ann Intern Med. 1974;80:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Shen H, Luan F, Liu H, Gao L, Liang X, Zhang L, Sun W, Ma C. ZHX2 is a repressor of alpha-fetoprotein expression in human hepatoma cell lines. J Cell Mol Med. 2008;12:2772-2780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |