INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. It is responsible for an estimated 1 million deaths annually and, in most cases, complicates the clinical course of liver cirrhosis, resulting in a poor prognosis due to its rapidly infiltrating growth[1,2]. A careful multidisciplinary assessment of tumor characteristics, liver function, and physical status is required for proper therapeutic management. However, consensus about a common treatment strategy for patients with HCC has not been reached worldwide, even if several proposals have been published. The most recent one is the Barcelona-Clinic Liver Cancer (BCLC) staging classification and treatment schedule that is currently used for clinical management of patients with HCC.
According to the BCLC staging system, surgical approaches, including surgical liver resection (SR) and liver transplantation (LT), as well as image-guided tumor ablation, such as radiofrequency ablation (RFA), are regarded as potentially curative treatments for HCC but are only recommended in patients with early stage tumor. Patients diagnosed with intermediate stage HCC are candidates for trans-arterial chemoembolization (TACE), which has proven to control symptoms and prolong survival[1]. However, it is important to emphasize that this option is considered as a palliative and not a curative treatment, characterized in most instances by an unsatisfactory long-term outcome due to the inability to achieve complete tumor necrosis. Furthermore, repeated TACE is often required to completely eradicate the residual tumors, but its efficiency is limited and the rate of tumor recurrence or relapse after initial remission or stable disease is very high[3-6].
However, the early stage also includes patients with a single large HCC exceeding 3 cm in diameter, and the intermediate stage includes many patients with very different presentations of HCC. Indeed, patients with 4 small HCC nodules, with multinodular unilobar or bilobar disease and well-compensated liver function, are all classified as intermediate stage. The principal purpose of research in this field should be to increase the rate of patients who are suitable for non-surgical curative treatment and, consequently, to reduce indications for palliation alone[7].
In this scenario, the purpose should be to expand the indication for RFA, that is a curative treatment for nodules smaller than 3 cm, by increasing its effectiveness in the treatment of single larger HCC nodules and for use during the intermediate stage. To this end, a combination of intervention therapies has been widely developed and performed in recent years. One such combined strategy is based on the association of the percutaneous approach, such as RFA, and the intra-arterial locoregional approach, such as TACE[8]. Several types of evidence have indicated the feasibility and benefit of combined therapy, despite some studies reporting conflicting results and outcomes[9].
Hence, the aim of this review was to explain the technical aspects of different combined treatments and to analyze and comprehensively compare the clinical efficacy and safety of this combined treatment option and monotherapy, either TACE or RFA alone, in order to provide clinicians with an unbiased opinion and valuable information.
RATIONALE
It is well known that RFA is only indicated for early stage HCC patients with fewer than 3 tumors, due to the local range of the treatment action, and with tumors less than 3-cm in size, due to a complete response rate lower than 50% in larger lesions, which is clearly poor for a treatment intended to cure the tumor. Furthermore, a high rate of local recurrence in lesions exceeding the 3 cm threshold has been demonstrated even after an initial complete post-treatment response[10].
To expand indication for RFA, we should look at the limitations of RFA. The first consideration is the number of lesions; due to its local range of action, patients with multiple monolobar nodules should be considered less ideal for RFA than TACE, which acts by combining ischemia, anoxia and chemotoxicity at the tumor level with a range of action that could be regional or global. On the other hand, when considering the lesion size, the RFA induced volume of coagulation necrosis should be increased, with the potential result of also completely treating lesions exceeding the 3 cm threshold. We know that the extent of coagulation necrosis is a function of the energy delivered into the tumor, of the local tissue interaction and of the negative factor of heat loss due to perfusion-mediated tissue cooling. Indeed, lesions bordering a large vessel (> 3 mm) may not achieve complete necrosis due to the thermal protection provided by the adjacent blood flow, a phenomenon termed “heat sink”. Blood flow promotes heat loss, and reducing or eliminating blood flow during the RFA procedure is known to increase the volume of the ablative zone as confirmed more than 10 years ago by Rossi et al[11]. More specifically, the authors demonstrated the strict relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. This ex vivo study brought the same group to test this assumption in vivo, performing RFA after the occlusion of tumor blood supply, resulting in a significant increase in RFA coagulation necrosis[12]. This study was essential in demonstrating that, in order to increase the volume of coagulation necrosis (ablation zone size), arterial occlusion of the tumor feeding vessels obtained using balloon occlusion, embolization, and chemoembolization can be combined with RFA.
On the basis of these results, we should alter our final goal of looking for a new bio-kill formula. We can achieve tumor death not only from using the RFA induced thermal damage or the TACE derived ischemic-cytotoxic injury. These treatments can be combined in order to overcome the drawbacks when they are applied alone.
COMBINATION OPTIONS STRATEGY
Several studies have evaluated a multimodal approach to increasing the effectiveness of single treatments for single large or intermediate stage HCC. The available data suggest that combined therapy with RFA and TACE is superior to RFA or TACE alone in preventing the incomplete necrosis of HCC and in improving patient survival, but it is not clear which is the best combination of these two procedures[13-15].
The first and more common option is represented by TACE followed by RFA. TACE can reduce the cooling effect of hepatic blood flow by decreasing hepatic arterial flow and increasing the necrotizing effect of RFA therapy at the tumor level. Furthermore, the edematous change in the tumor tissue induced by ischemia and inflammation after TACE is expected to enlarge the area of tumor necrosis during RFA treatment, thereby increasing the ablation safety margin and reducing local recurrence.
The second option is to perform RFA followed by TACE. Instead of using only lethal heating, which is obtained with RFA, you can actually try to obtain a sustained anticancer effect from the sublethal heating created in the large area surrounding the heating zone. In this area we have a number of phenomena, including increased blood flow, increased vascular permeability and effects on multiple cell targets. TACE performed after RFA could increase its therapeutic effect acting on the large zones of sublethal heating obtained during RFA application in tissues surrounding the electrode. In detail, the chemotherapy drug should be concentrated on a relatively small volume of residual viable neoplastic tissue, characterized by reduced cell resistance to the drug due to previous exposure to sublethal heating. Furthermore, the delivery of a chemotherapy drug could be enhanced by the reactive hyperemia induced by RFA application. The rationale of performing TACE after RFA was based on the hypothesis that there could be a loss in efficacy of the drugs used during TACE when they are exposed to high temperatures[16]. Furthermore, TACE performed before RFA could have a negative impact on the quality of the ultrasonography (US) images; as a matter of fact, after TACE, the lesions become hyperechoic, thereby hampering the identification of viable tissue in the tumor area.
The last option, described in our previously published paper, could be to perform a single-step combination therapy, applying RFA to the lesion during balloon-occlusion of the hepatic artery supplying the tumor, thereby enhancing the thermal damage, followed by selective TACE to enhance the cytotoxic injury[17]. In detail, balloon occlusion of the tumor arterial supply increases the area of coagulation necrosis (ablation zone size) obtained with RFA, reducing arterial blood flow and minimizing heat loss, as already shown by other authors[12,18].
A demonstration of the superiority of one approach over the other is not possible due to the lack of randomized comparative studies.
TIME-INTERVAL OPTIONS STRATEGY
To date, there has been no clear consensus about the time interval between TACE and RFA for balancing local therapeutic efficacy and safety. In a study by Choe et al[19], it was reported that the time-interval between TACE and RFA treatments should be chosen carefully to achieve a balance between successful tumor eradication and adequate preservation of liver function. A longer time interval between the two treatments might preserve liver function because sufficient time is allowed for hepatic functional recovery[19]. However, this extended time prolongs the hospital stay required or may increase the number of patient admissions to the hospital. Conversely, a short interval can lead to better local efficacy because of the more synergistic effect of the combination of TACE and RFA. However, a short interval might increase the potential risk of liver function injury, mainly in cirrhotic patients with mild to moderate liver dysfunction.
In our opinion, only using a single-step “combined” approach makes it possible to obtain and amplify the synergistic effects of RFA and TACE. This approach entails further relevant advantages, such as the reduction of hospitalization days, decrease in patient discomforts, and cost saving due to the performance of both procedures in the same session. However, in many published papers, the treatments were performed during different sessions, separated by a time-interval of 1-30 d[10,20,21]. In fact, the administration of treatments in sequential order is common practice in clinical medicine, particularly when a treatment fails; it could also be performed per protocol independently from the partial effectiveness or failure associated with a specific therapy. In other words, instead of administering different therapies together, there is planned sequential administration based on some specific effects induced by each therapy, which provides additional benefits over time. Based on this definition, it could be more appropriate and correct to define this approach as “sequential treatment” and not “combined treatment”. In our opinion, when dealing with the association of different locoregional HCC treatments, it should be mandatory to distinguish between sequential and combined treatments in order to provide more comparable results to the scientific community.
TREATMENT PROCEDURES
All of the combined treatments should be performed in a single-step approach, using antibiotic prophylaxis, patient monitoring and anesthesiological assistance, in an angiographic suite that has the structural characteristics of an operating room.
TACE
Hepatic artery angiography is usually performed through a right common femoral approach to map liver vascular anatomy, check for arteriovenous shunts and identify the arterial tumor supply. A superselective catheterization and chemoembolization is performed using a coaxial technique and by placing a 2.7-Fr microcatheter (Progreat; Terumo, Tokyo, Japan) into the distal segmental hepatic artery feeding the HCC. In the case of balloon-occluded RFA, a 0.014-inch guide wire is advanced into the segmental hepatic artery feeding the lesion, enabling optimal guidance of a low-profile 4-5 mm monorail percutaneous transluminal angioplasty (PTA)-balloon. Conventional chemoembolization is performed by infusing an emulsion of chemotherapeutic agent (Epirubicin-Doxorubicin/Cisplatin/Mytomicin-C) and iodized oil (Lipiodol Ultra Fluid; Mitsui, Tokyo, Japan), followed by embolization performed with gelatin sponge particles (Gelfoam; Pfizer, Tokyo, Japan). Drug-eluting bead TACE is performed with a slow injection of a 100-300 μm DC-Bead (Terumo, Tokyo, Japan) loaded with Epirubicin (Farmorubicin® 50 mg Powder) until the complete intended dose is administered and slow flow is observed.
RFA
Before or after TACE, RFA is usually performed using US-guidance with the patient under sedation with Fentanyl citrate (0.1-0.2 mg, Phentanest; Daiichi Sankyo, Tokyo, Japan) and local anesthesia. In detail, US-guidance has been most widely implemented as the standard guiding modality for RFA (Figure 1). The advantages of US are several, including easy availability, lower cost and real-time multiplanar imaging capability. However, ultrasound also has serious drawbacks. For multiple overlapping ablations, the characteristic hyperechoic area of microbubbles generated by previous ablation cycles often obscures the index tumor and may hinder accurate placement of the electrode for subsequent ablation cycles. Furthermore, ultrasound-guided targeting is difficult for tumors in sonographic blind spots, such as the liver dome. In addition, for combined treatment, prior chemoembolization may alter the sonographic conspicuity of the index tumor owing to variable uptake of iodized oil and the chemotherapeutic agent in the tumor and the adjacent hepatic parenchyma. For this reason, computed tomography (CT) fluoroscopy has also been used to guide RFA alone or RFA combined with TACE (sequential approach)[22-24]. It provides several contiguous axial images through near real-time image reconstruction during the interventional procedure. When the CT plane includes both the electrode path and the index tumor, the needle advancement into the tumor can be monitored in real-time. One major drawback of this guiding modality is the high radiation dose to both patient and operator, which is on the order of centigrays per second of exposure, whereas conventional fluoroscopy is on the order of centigrays per minute of exposure[25]. This concern is obviously magnified for cases requiring multiple overlapping ablations. Furthermore, targeting the index tumor tends to be more time-consuming than other guidance modalities, such as ultrasound and conventional fluoroscopy. For a tumor in the dome of the liver, oblique advancement of the electrode is preferred to avoid violation of the thorax or the pleura. However, such an oblique approach might have been technically cumbersome with CT fluoroscopy guidance owing to a limited range of CT gantry tilting. Although the transthoracic approach for dome lesions has been generally accepted as safe, pneumothorax can complicate 38%-70% of cases, of which 18%-40% will require chest tube placement[13,26,27]. Alternatively, the oblique approach for a dome lesion under CT guidance with coronal/sagittal reformatted imaging is also useful to avoid this complication.
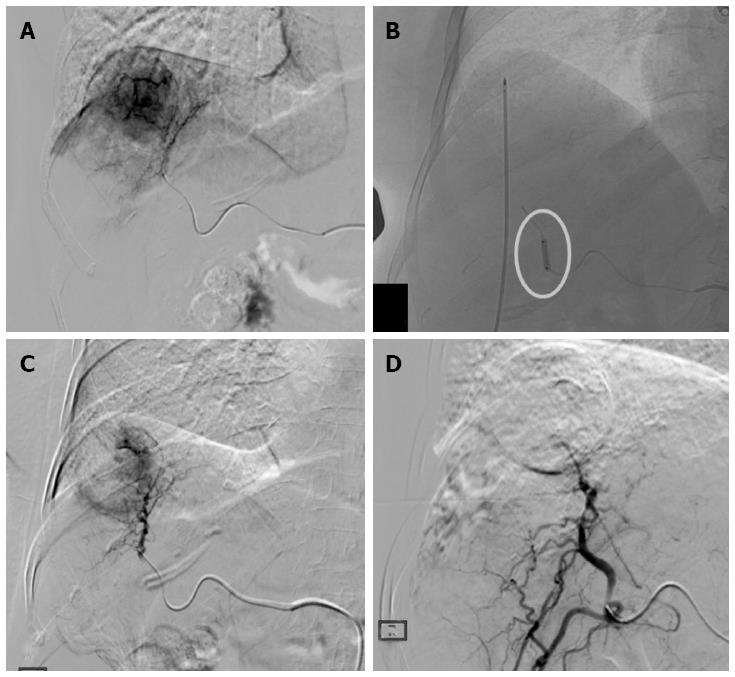
Figure 1 Treatment procedure.
A: Hepatocellular carcinoma in SVIII (4.5 cm in size) confirmed on digital subtraction angiography; B: Radiofrequency ablation (RFA) electrode placed into the tumor under ultrasonography-guidance with ablation performed during balloon-occlusion (circle); C: Post-RFA digital subtraction angiography showing the central devascularized area with peripheral reactive hyperemia; D: Complete devascularization obtained with superselective Drug-eluting bead trans-arterial chemoembolization.
Another potential guiding-technique is represented by biplane fluoroscopy[28]. For combined treatment, this technique has several potential strengths. First, the visible fluoroscopically index tumor can be easily targeted regardless of location and successfully ablated with a single session procedure. Furthermore, registration and fusion of intraprocedural ultrasound with pre-procedural CT, magnetic resonance imaging (MRI) or positron emission tomography images have been reported as feasible for thermal ablation of liver tumors[29,30]. With these fusion techniques, inconspicuous dome HCCs on ultrasounds could be targeted for percutaneous RFA. Second, proper needle placement into the tumor can be more confidently made because biplane fluoroscopy provides real-time orthogonal projectional imaging for the simultaneous delineation of the electrode and the index tumor. Enhanced targeting confidence may shorten the overall procedure time, especially for cases requiring multiple overlapping ablations. In addition, fluoroscopy provides much higher resolution and artifact-free images than CT or CT fluoroscopy, allowing for more precise overlapping ablations. Unlike ultrasounds, microbubbles generated by previous cycles of ablation do not obscure the radio-opaque index tumor in fluoroscopy. Third, this method allows for a greater degree of freedom for electrode insertion than ultrasounds or CT guidance. This is pertinent not only for liver dome tumors as described above but also for subcapsular tumors that may be better accessed through an oblique approach with biplane fluoroscopy than with ultrasound or CT to avoid direct puncture and to minimize the risk of bleeding or seeding[31]. However, a major drawback of this guiding method is obviously that fluoroscopy cannot provide cross-sectional imaging with the soft-tissue contrast available when using ultrasound or CT. Therefore, ultrasound as an accessory guidance modality is required to avoid a traversal of critical intrahepatic or extrahepatic structures during targeting and to estimate the ablation zone. Future perspectives could be based on the use of MRI to guide RFA alone or in combination with TACE in a sequential approach[21].
CLINICAL INDICATIONS
Combined vs curative treatments
A recent meta-analysis showed that RFA plus TACE significantly improved the overall survival (OS) rates at 1 and 3 years compared with RFA alone in patients with a single HCC, without significant differences in major complications. Subgroup analyses by tumor size showed that RFA plus TACE significantly improved the OS at 1, 3 and 5 years compared with RFA alone for patients with HCC larger than 3 cm. However, there was no advantage for patients with HCC smaller than 3 cm; the reason for this may be that RFA alone can already achieve complete necrosis in treating small (< 3 cm) HCC nodules, suggesting that adding TACE to RFA could be redundant[32].
There are conflicting results in the literature when analyzing the comparison between combined treatment and surgical resection (SR). Some retrospective studies have suggested that TACE-RFA may yield OS rates comparable to those from SR[8,12,33]. Yamakado et al[34] reported that patients with early-stage HCC who underwent TACE-RFA had OS and disease-free survival (DFS) rates similar to those found in patients who underwent SR. In contrast, Kagawa et al[4] reported that compared with SR, TACE-RFA in patients with early-stage HCC yielded a similar rate of OS but a lower rate of DFS. In a study by Takuma et al[35], the OS and DFS rates after TACE-RFA in patients with early stage HCCs within the Milan criteria were significantly lower than those observed after SR. These findings may be explained by differences in baseline patient characteristics; in fact, after adjusting for propensity score matching, patients who underwent TACE-RFA had a similar OS rate but had poorer DFS compared to patients who underwent SR. The difference in DFS rates may be mainly due to local tumor progressions, which are higher after TACE-RFA. Based on published papers, it seems that TACE-RFA is safe and may confer an OS rate comparable with that of SR after adjusting for potential confounders. However, SR improves the DFS rate compared with the rate attained with TACE-RFA. We can conclude that combined treatments may be considered an effective treatment modality in patients with early/intermediate HCC when SR is not feasible.
Combined vs palliative treatments (TACE)
The combined use of TACE and RFA may have an advantage over TACE alone in the treatment of HCC because they are mutually complementary, thereby significantly improving the efficacy, quality of life and long-term survival of HCC patients[33,36,37]. In detail, TACE plus RFA increases the chance of peritumoral satellitosis clearance, reduces the possibility of tumor recurrence, thereby enhancing the possibility of complete tumor necrosis and conceivably improves the OS rate. In a recent study, we demonstrated that balloon-occluded RFA immediately followed by DEB-TACE was effective at achieving prolonged local disease control in single large HCCs (> 3 cm), with a sustained complete response in 62.5% of the treated lesions, a 2-year cumulative HCC recurrence rate of 48.1%, and an overall survival rate of 91.1%, which is significantly better than that achieved in a comparable control group of patients treated with DEB-TACE alone[38]. It is worth noting that these promising results were obtained in a single-step procedure without significantly increasing the procedural time, and the benefit of combined therapy was not offset by any important side effects or worsening of liver function, as none of our patients experienced an increased Child-Pugh score at 1-mo post treatment. Furthermore, in patients with lesions > 5 cm, a large necrotic area was obtained in most cases, and a sustained CR was achieved in about half of the cases with a mean number of only 1.3 procedures per patient. This implies the potential reduction of the therapeutic procedures number, and consequently of the liver function failure risk. Finally, in some cases, the combined therapy appeared to be promising as an effective bridge treatment to liver transplantation.
Complex lesions/complex patients
Combined treatments could expand the indication for RFA to previously contraindicated cases[39]. In detail, it could be possible to effectively also treat complex lesions with RFA, i.e., hepatic tumors adjacent to the diaphragm with a consequent high risk of thermal injury, or tumors located next to the intra-abdominal free surface, or proximal to the hepatic portal region. As a matter of fact, the aim to ensure a safety margin is tempered by the high risk of damaging big arterial or portal vein vessels, bile ducts, or intestinal loops with subsequently serious complications, such as hepatic infarction, biloma, abscesses or intestinal perforation. On the other hand, less aggressive treatments, using lower RFA power or shortened exposure time to the RFA needle, may result in local recurrence. Taking this into consideration, in these cases the RFA should be limited to the tumor portions located far from the diaphragm or intra-abdominal free surface as well as adjacent to vascular/biliary structures; these tumor portions can then be treated with post-RFA chemoembolization in order to obtain an effective and secure safety margin (Figure 2). Finally, it’s noteworthy that using this single-step approach could make it possible to also safely treat “complex patients” with high risk for bleeding complications without requiring blood transfusion or other prophylactic treatment. As a matter of fact, transarterial chemoembolization performed after RFA could effectively and immediately treat any eventual RFA-induced hepatic bleeding.
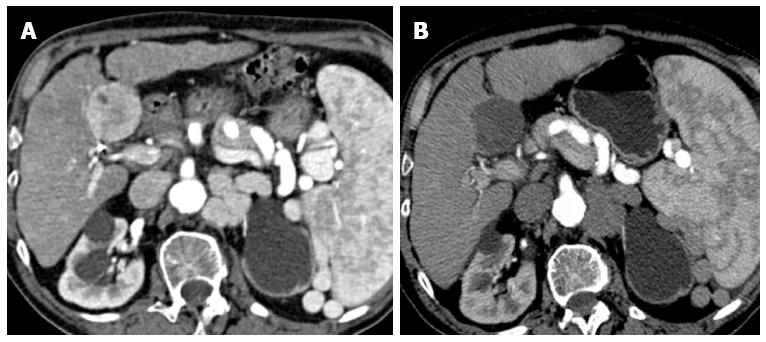
Figure 2 Complex lesion.
A: Hepatocellular carcinoma in SV (5 cm in size), located on the intra-abdominal free-surface, adjacent to a gastrointestinal structure; B: Combined treatment allows obtaining a central safe necrosis with radiofrequency ablation (RFA); subsequent post-RFA trans-arterial chemoembolization was used to treat a peripheral portion of the tumor, obtaining a safe complete response.
CONCLUSION
The combined use of TACE and RFA (combined treatment) is a safe and effective option in the treatment of patients with HCC. In detail, combined treatments may be considered an alternative treatment modality in patients with single large or multinodular HCC when surgical resection is not feasible. In particular, this approach seems to provide better results than RFA and DEB-TACE alone for the treatment of large HCC exceeding 3 cm in size, significantly improving the efficacy, quality of life and long-term survival of patients. Finally, it could also expand the indication for RFA to previously contraindicated “complex cases”, in which the application of RFA alone entails an increased risk of complications, or to “complex patients” with high risk of RFA-related bleeding complications.
P- Reviewer: Joh JW S- Editor: Gong ZM L- Editor: A E- Editor: Ma S