Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10364
Peer-review started: July 8, 2016
First decision: August 8, 2016
Revised: August 25, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: December 21, 2016
Processing time: 167 Days and 5.3 Hours
To explore expression of angiopoietin-like protein 2 (ANGPTL2) and its effect on biological behavior such as proliferation and invasiveness in gastric cancer.
Western blotting was used to detect expression of ANGPTL2 in 60 human normal gastric tissues, 60 human gastric cancer tissues and gastric cell lines including GES-1, N87, SGC7901, BGC823 and PAMC82. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Transwell assay were used to detect the proliferation and invasive ability of gastric cancer cells.
Compared to normal tissues, ANGPTL2 protein levels were significantly upregulated in gastric tissues, and this level was closely correlated with gastric tumor grade, clinical stage and lymph node metastasis. Compared to GES-1 cells, ANGPTL2 mRNA and protein levels were significantly increased in gastric cancer cells including N87, SGC7901, BGC823 and PAMC82. The expression of ANGPTL2 in highly malignant gastric cancer cell lines BGC823 and PAMC82 was significantly higher than in low malignancy gastric cancer cell lines N87 and SGC7901. MTT and Transwell experiments indicated that the proliferation rate and invasive ability of stable overexpressed gastric cancer cells was faster than in cells transfected with Lv-NC and blank control cells, and the invasive ability of stable overexpressed gastric cancer cells was higher than that of cells transfected with Lv-NC and blank control cells.
ANGPTL2 contributed to proliferation and invasion of gastric cancer cells. In clinical treatment, ANGPTL2 may become a new target for treatment of gastric cancer.
Core tip: Expression of angiopoietin-like protein 2 (ANGPTL2) was significantly elevated in gastric cancer tissues and cells. The higher the level of malignancy of the cancer, the higher the expression of ANGPTL2 became. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and Transwell assays estimated that the proliferative and invasive ability of lentivirus-infected gastric cancer cells were evidently improved compared to the Lv-NC and blank control groups, which indicate that ANGPTL2 was conducive to the proliferation and invasion of gastric cancer cells.
- Citation: Sheng WZ, Chen YS, Tu CT, He J, Zhang B, Gao WD. ANGPTL2 expression in gastric cancer tissues and cells and its biological behavior. World J Gastroenterol 2016; 22(47): 10364-10370
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10364.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10364
Cancer has a high morbidity and mortality[1-4]. In China, with the improvement of the economy and the enormous change in lifestyle and eating habits, the morbidity and mortality of gastric cancer have increased annually. In addition, the progression of this disease has become complex. Latest research has found that angiopoietin-like protein 2 (ANGPTL2) is highly expressed in human tissues of the intestine, fat, retina and heart. Furthermore, more research related to the significant high expression of ANGPTL2 in pulmonary cancer tissues, as well as distant metastasis in lung tissues and lymph nodes, has emerged, indicating that ANGPTL2 promotes pulmonary cancer cell proliferation and invasion[5-12]. The study of Teicher[13] also estimated its high expression in soft tissue sarcomas, implicating that ANGPTL2 may accelerate the progression of soft tissue sarcomas. In addition, some studies have reported high expression of ANGPTL2 in various malignant tumors, illustrating that ANGPTL2 is likely to enhance the proliferative and invasive ability of these tumor cells[14-18]. Despite the intimate connection between ANGPTL2 and multiple malignant tumors, there are few studies of its expression level and proliferative and invasive ability in gastric cancer, suggesting a need for further in-depth studies.
Our principle aims in the present study were to investigate the expression level of ANGPTL2 in gastric cancer and normal stomach, and determine its effect in gastric cancer cell proliferation and invasion through western blotting and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Transwell assays. This could help to provide a novel target for better gastric cancer therapy.
Sixty patients diagnosed and confirmed with gastric cancer in Zhongshan Hospital in Shanghai from December 2012 to January 2015 were enrolled in the present study. Patients’ age ranged between 25 and 80 years, with a median age of 51 years. Tumor tissues and normal adjacent cancer tissues were gathered and designated as the experimental and control groups, respectively. Informed consent from patients’ family members and approval from the Ethics Committee of the institution were obtained before the tissues were collected as experimental specimens. In addition, GES-1, N87, SGC7901, BGC823 and PAMC82 cells were all provided by the China Typical Culture Preservation Center.
Total protein was extracted from cell lysates (with protease inhibitors) and protein concentration was measured by the Bradford method. Polyacrylamide gel was prepared by placing in a sufficient running buffer. Then, protein samples were loaded based on the concentration measured before. Electrophoresis was started at 50 V, and adjusted to 100 V when the samples were entered into the separation gel. After electrophoresis was over, all samples were transferred onto a PVDF membrane. The membrane was incubated with blocking buffer for 2 h, followed by primary antibody incubation overnight at 4 °C on a rotator. The next day, the primary antibody was discarded and the membrane was washed three times. The membrane was incubated with a secondary antibody for 1 h at room temperature, and was washed three times after that. Finally, exposure was performed and data was analyzed in detail.
PAMC82 gastric cancer cells were collected and seeded onto six-well plates with a standard 105/mL cell suspension density at 2 mL/well, followed by lentivirus infection after 2 h. Cells were divided into the ANGPTL2 overexpression group (Lv-ANGPTL2), ANGPTL2 interference group (Lv-ANGPTL2-shRNA) and control group (Lv-NC). Ultimately, uninfected cells were removed through screening.
The PAMC82 gastric cancer cell suspension was seeded into 96-well plates, keeping it within 5000 cells and 100 μL of cell suspension per well, and cultured in an incubator. This included three replicates for each group, and four 96-well plates were used in total. A 20-μL MTT solution was added into the plates on days 1, 2, 3 and 4. Then, cells were incubated for 4 h, the culture solution was discarded, and DMSO solution was added. Absorbance value was measured after 10 min.
PAMC82 gastric cancer cell strains were divided into three groups: Lv-ANGPTL2, Lv-ANGPTL2-shRNA and Lv-NC groups. Afterwards, 300 μL of cells at 106/mL density were plated into the invasive chamber, cultured in an incubator for 1 d, and OD value was detected.
SPSS version 17.0 software was used to analyze all data. Gray and OD values of ANGPTL2 and β-actin protein were expressed as mean ± SD. Comparisons of ANGPTL2 protein gray values between high-medium and poor tumor differentiation, T1 + T2 and T3 + T4, and lymph node metastasis and non-lymph node metastasis groups were implemented by t test. t test was also performed on gray and OD values of Lv-ANGPTL2, which were compared with Lv-NC and the blank control group; while Lv-ANGPTL2-shRNA was compared with Lv-NC-shRNA and the blank control group.
Through western blot analysis, gray values of ANGPTL2 in tumor adjacent tissues and gastric cancer tissues were 0.680 ± 0.110 and 0.058 ± 0.009, respectively. Compared to tumor adjacent tissues, the expression of ANGPTL2 in gastric cancer tissues was markedly elevated, and the difference was significant (t = 43.654, P = 0.000). Furthermore, the expression level of ANGPTL2 was closely related to the differentiation of gastric cancer, lymph node metastasis and T stage. The higher the malignant level, the greater the expression of ANGPTL2 (Table 1).
| Group | n | Gray value of ANGPTL2 | T value | P value | |
| Tumor differentiation | High-medium differentiation | 17 | 0.500 ± 0.221 | -4.770 | 0 |
| Low differentiation | 43 | 0.821 ± 0.240 | |||
| T stage | T1 + T2 | 22 | 0.402 ± 0.198 | -5.907 | 0 |
| T3 + T4 | 38 | 0.803 ± 0.280 | |||
| Lymph node metastasis | Metastatic | 35 | 0.680 ± 0.210 | 5.591 | 0 |
| Non-metastatic | 25 | 0.404 ± 0.153 | |||
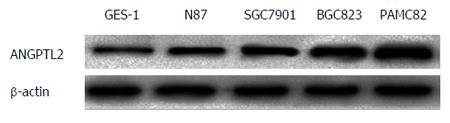
The difference in protein expression was analyzed in normal gastric cell strain GES-1 and the four gastric cancer cells (N87, SGC7901, BGC823 and PAMC82) by western blotting. The protein expression level of ANGPTL2 in the four cancer cells (N87, SGC7901, BGC823 and PAMC82) was 1.1-, 1.3-, 1.8- and 2.1-fold, respectively, compared to the normal human gastric cell strain. ANGPTL2 protein expression levels in the four gastric cancer cell strains were significantly higher than in the normal gastric cell strain GES-1 (P < 0.05). In addition, β-actin protein expression level in the five cell strains was consistent, showing no significant difference (Figure 1).
To investigate the function of ANGPTL2 in gastric cancer cell proliferation, we overexpressed ANGPTL2 in the PAMC82 gastric cancer cell strain through lentivirus infection. Lv-ANGPTL2, Lv-NC, Lv-ANGPTL2-shRNA and Lv-NC-shRNA (four vectors) were constructed to infect the PAMC82 gastric cancer cell strain. ANGPTL2 levels in the above-mentioned cells and two blank control cells were collected and analyzed using western blotting. ANGPTL2 protein level in the Lv-ANGPTL2-infected PAMC82 gastric cancer cell strain was significantly higher than in Lv-NC infected and blank control cells. Protein levels in the infected Lv-ANGPTL2-shRNA PAMC82 gastric cancer cell strain were significantly lower than in the Lv-NC-shRNA and control cell strains. The inner reference protein β-actin had no obvious change (Tables 2 and 3).
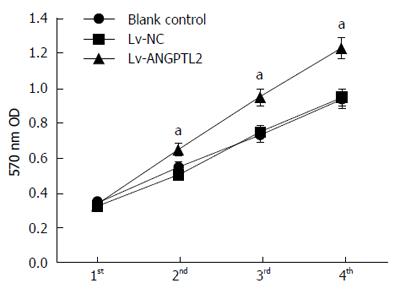
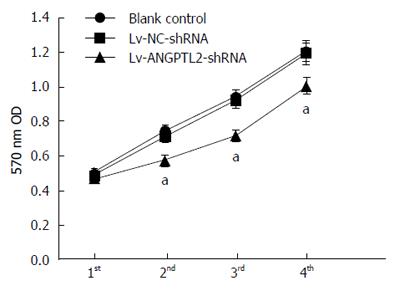
MTT assay was performed to detect the effect of the proliferative ability of PAMC82 gastric cancer cell strains with overexpression and knockdown of ANGPTL2 levels. There was a similar rising tendency without apparent difference at 4 d in the Lv-NC and Lv-NC-shRNA groups, compared to the blank control group. Nevertheless, on days 2-4, cell proliferative ability in the Lv-ANGPTL2 group was evidently higher than in the Lv-NC and blank control groups, and this was significantly lower in the Lv-ANGPTL2-shRNA group (Figures 2 and 3).
Transwell assay was performed to detect the role of ANGPTL2 in PAMC82 gastric cancer cell strain invasion. Results indicate that cell invasive ability in the Lv-ANGPTL2 group was significantly higher than in the Lv-NC and blank control groups. In contrast, invasive ability in the Lv-ANGPTL2-shRNA group was significantly lower compared to the Lv-NC-shRNA and blank control groups (Tables 4 and 5).
The morbidity of gastric cancer has increased with economic development and changes in dietary patterns[19-22]. Furthermore, Helicobacter pylori infection, gastric polyps, and gastritis can lead to gastric cancer[23-27]. Recently, Kadomatsu et al[28] found a novel secreted glycoprotein, ANGPTL, which is highly expressed in adult hearts, as well as in fatty tissues. This protein has a similar function as angiopoietin, which probably regulates vascular endothelial cell function and migration, and angiogenesis. Research has shown that the overexpression of ANGPTL2 in fatty tissues in mice might result in inflammation and invasion of macrophages. Furthermore, the expression of interleukin (IL)-1, IL-6 and tumor necrosis factor inflammation factors in fatty tissues was evidently higher than in wild-type mice[29-31]. Even though we are aware of ANGPTL2, its expression level in gastric cancer and its effects on gastric cancer cell proliferative and invasive ability remain unclear. Hence, our purpose in the present study was to explore the difference in expression level of ANGPTL2 between gastric cancer and normal stomach, as well as its impact on gastric cancer cell proliferation and invasion, thereby providing a novel target for better gastric cancer therapy.
The present study indicates that ANGPTL2 protein level in gastric cancer tissues significantly increased compared to normal stomach tissues, which was also closely related to tumor differentiation, T stages and lymph node metastasis. Gastric cancer cells with poor differentiation had higher ANGPTL2 protein levels; protein levels in T3 + T4 gastric cancer revealed a higher degree than in T1 + T2 gastric cancer; and ANGPTL2 protein expression levels in gastric cancer with lymph node metastasis was significantly higher compared to that in non-metastatic cancer; estimating that ANGPTL2 might accelerate the incidence of gastric cancer. Research has shown that a lifetime after pulmonary cancer operation is negatively correlated with ANGPTL2 expression in pulmonary tissues, and ANGPTL2 expression level in pulmonary cancer cells could accelerate the invasion and metastasis of cells through autocrine and paracrine mechanisms[5,6]. The high expression of ANGPTL2 in gastric cancer probably exerted similar effects as the pulmonary tumor, accelerating gastric cancer cell invasion and metastasis, as well as the incidence and progression of gastric cancer.
Next, western blotting was performed to analyze the protein expression of ANGPTL2 at the translational level. ANGPTL2 protein expression was higher in gastric cancer cell strains BGC823 and PAMC82, and was 1.8- and 2.1-fold compared to that in normal gastric cell strains. As for the two other gastric cancer cell strains, N87 and SGC7901 cells had elevated ANGPTL2 protein expression levels that were slightly lower compared to normal levels. The probable explanation is that the N87 and SGC7901 cell lines had a lower metastatic capacity and the BGC823 and PAMC82 cell lines had a higher degree of malignancy, which exhibited strong migratory ability[32-34]. Recent studies have reported that the carcinogenic capacity of PAMC82 cells is higher than that of N87 cells[35,36]. In addition, compared to the N87 and SGC7901 cell lines, ANGPTL2 protein expression in the BGC823 and PAMC82 cell lines was higher, demonstrating that ANGPTL2 expression was greater in gastric cancer cells with a higher degree of malignancy.
The present study used PAMC82 cells that belonged to poorly differentiated gastric carcinoma cell strains, which strengthened gastric cancer proliferation and invasion studies[37]. Due to the higher efficiency of the lentivirus infection, we used the lentivirus infection to interfere with and overexpress ANGPTL2 protein[38]. The overexpression and knockdown of ANGPTL2 protein was satisfactory, with the confirmation of western blot for the sequential study.
MTT and Transwell assays demonstrated that ANGPTL2 accelerated gastric cancer cell strain PAMC82 propagation and invasion, but interference with ANGPTL2 expression obviously blocked this phenomenon reversibly. The above findings suggested that ANGPTL2 contributed to gastric cancer cell strain proliferation and migration. Gao et al[39] found that ANGPTL2 inhibited apoptosis by disturbing the tumor intracellular signaling pathway, which accelerated cell growth and facilitated invasion. Other studies have indicated that ANGPTL2 can enhance the activity of the Rec-1/NF-кB pathway, promoting angiogenesis and facilitating the migration of THP-1 by conjugation with integrin[40], and playing a critical role in tumor proliferation and invasion[41-43]. As a consequence, we hypothesize that ANGPTL2 is likely to reduce apoptosis through conjugation with integrin, which facilitates the expression of certain proteins, and activates the corresponding pathway to accelerate gastric cancer cell invasion and migration. However, ANGPTL2 probably promotes gastric cancer cell invasion and migration through other approaches as well, which need to be further explored.
Although there were limitations to this study, such as the small number of samples, it was successful in general. In conclusion, ANGPTL2 protein expression levels were higher in gastric cancer tissues compared to tumor adjacent tissues. ANGPTL2 protein expression levels in gastric cancer cell strains were significantly higher than in normal gastric epithelial cell strains. Furthermore, the higher the degree of malignancy of gastric cancer tissues and cell strains, the higher the expression level of ANGPTL2 became. Moreover, ANGPTL2 contributed to gastric cancer cell strain propagation and migration, which may be used as a novel target for the treatment of human gastric cancer. To understand further the function of ANGPTL2 in gastric cancer occurrence and progression, we need to conduct further in-depth studies on the role of ANGPTL2 upstream regulating factors, to provide a new target for gastric cancer therapy.
In China, with the improvement of the economy and the enormous change in lifestyle and eating habits, the morbidity and mortality of gastric cancer has increased annually. In addition, the progression of this disease is complex.
This latest research found that angiopoietin-like protein 2 (ANGPTL2) was highly expressed in human tissues in the intestine, fat, retina and heart. Furthermore, more research related to the high expression of ANGPTL2 in pulmonary cancer tissues, as well as in distant metastasis to lung tissues and lymph nodes, has emerged, indicating that ANGPTL2 promotes pulmonary cancer cell proliferation and invasion. Teicher et al also estimated its high expression in soft tissue sarcoma, implicating that ANGPTL2 might accelerate the progression of soft tissue sarcoma. In addition, some studies have reported high expression of ANGPTL2 in various malignant tumors, illustrating that ANGPTL2 is likely to increase the proliferative and invasive ability of these tumor cells. Despite the intimate connection between ANGPTL2 and multiple malignant tumors, there were few studies on its expression level and proliferative and invasive ability in gastric cancers, suggesting the need for further in-depth studies.
ANGPTL2 protein expression level in gastric cancer tissues and cell strains was significantly higher. The higher the degree of malignancy of the tumor, the higher the expression level of ANGPTL2 protein. ANGPTL2 contributed to the proliferation and invasion of gastric cancer cells. In clinical treatment, ANGPTL2 may become a new target for the treatment of gastric cancer.
Compared to tumor adjacent tissues, ANGPTL2 protein expression levels were higher in gastric cancer tissues. ANGPTL2 protein expression levels in gastric cancer cell strains were significantly higher than in normal gastric epithelial cell strains. The higher the degree of malignancy of gastric cancer tissues and cell strains, the higher the expression level of ANGPTL2. In addition, ANGPTL2 contributed to gastric cancer cell strain propagation and migration, which may be used as a novel target for the treatment of human gastric cancer.
This is an interesting manuscript about the ANGPTL2 expression in gastric cancer tissues and cells.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arredondo M, Mohsen MM S- Editor: Gong ZM L- Editor: Kerr C E- Editor: Wang CH
| 1. | Sobolewski C, Sanduja S, Blanco FF, Hu L, Dixon DA. Histone Deacetylase Inhibitors Activate Tristetraprolin Expression through Induction of Early Growth Response Protein 1 (EGR1) in Colorectal Cancer Cells. Biomolecules. 2015;5:2035-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Lokeshkumar B, Sathishkumar V, Nandakumar N, Rengarajan T, Madankumar A, Balasubramanian MP. Anti-Oxidative Effect of Myrtenal in Prevention and Treatment of Colon Cancer Induced by 1, 2-Dimethyl Hydrazine (DMH) in Experimental Animals. Biomol Ther (Seoul). 2015;23:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Zhang P, Zhao Q, Zhang Y, Cao L, Luan Y. Doxorubicin-loaded polypeptide nanorods based on electrostatic interactions for cancer therapy. J Colloid Interface Sci. 2016;464:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Vassiliou V, Papamichael D, Polyviou P, Koukouma A, Andreopoulos D. Intramedullary spinal cord metastasis in a patient with colon cancer: a case report. J Gastrointest Cancer. 2012;43:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, Ogata A, Odagiri H, Yano M, Araki K. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71:7502-7512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Sasaki H, Suzuki A, Shitara M, Hikosaka Y, Okuda K, Moriyama S, Yano M, Fujii Y. Angiopoietin-like protein ANGPTL2 gene expression is correlated with lymph node metastasis in lung cancer. Oncol Lett. 2012;4:1325-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Doi Y, Ninomiya T, Hirakawa Y, Takahashi O, Mukai N, Hata J, Iwase M, Kitazono T, Oike Y, Kiyohara Y. Angiopoietin-like protein 2 and risk of type 2 diabetes in a general Japanese population: the Hisayama study. Diabetes Care. 2013;36:98-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, Horio E, Tsukano H, Kadomatsu T, Nakashima Y. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32:1400-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Ogata A, Endo M, Aoi J, Takahashi O, Kadomatsu T, Miyata K, Tian Z, Jinnin M, Fukushima S, Ihn H. The role of angiopoietin-like protein 2 in pathogenesis of dermatomyositis. Biochem Biophys Res Commun. 2012;418:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Broxmeyer HE, Srour EF, Cooper S, Wallace CT, Hangoc G, Youn BS. Angiopoietin-like-2 and -3 act through their coiled-coil domains to enhance survival and replating capacity of human cord blood hematopoietic progenitors. Blood Cells Mol Dis. 2012;48:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Umikawa M, Umikawa A, Asato T, Takei K, Matsuzaki G, Kariya K, Zhang CC. Angiopoietin-like protein 2 induces proinflammatory responses in peritoneal cells. Biochem Biophys Res Commun. 2015;467:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wang JY, Xiao HB, Sun ZL, Zhang DS. Angiopoietin-like protein 2 may mediate the inflammation in murine mastitis through the activation of interleukin-6 and tumour necrosis factor-α. World J Microbiol Biotechnol. 2015;31:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Teicher BA. Searching for molecular targets in sarcoma. Biochem Pharmacol. 2012;84:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Ide S, Toiyama Y, Shimura T, Kawamura M, Yasuda H, Saigusa S, Ohi M, Tanaka K, Mohri Y, Kusunoki M. Angiopoietin-Like Protein 2 Acts as a Novel Biomarker for Diagnosis and Prognosis in Patients with Esophageal Cancer. Ann Surg Oncol. 2015;22:2585-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Sato R, Yamasaki M, Hirai K, Matsubara T, Nomura T, Sato F, Mimata H. Angiopoietin-like protein 2 induces androgen-independent and malignant behavior in human prostate cancer cells. Oncol Rep. 2015;33:58-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Endo M, Yamamoto Y, Nakano M, Masuda T, Odagiri H, Horiguchi H, Miyata K, Kadomatsu T, Motokawa I, Okada S. Serum ANGPTL2 levels reflect clinical features of breast cancer patients: implications for the pathogenesis of breast cancer metastasis. Int J Biol Markers. 2014;29:e239-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, Miyamoto T, Kobayashi E, Miyata K, Aoi J. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7:ra7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Yang L, Shu T, Liang Y, Gu W, Wang C, Song X, Fan C, Wang W. GDC-0152 attenuates the malignant progression of osteosarcoma promoted by ANGPTL2 via PI3K/AKT but not p38MAPK signaling pathway. Int J Oncol. 2015;46:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Golpour S, Rafie N, Safavi SM, Miraghajani M. Dietary isoflavones and gastric cancer: A brief review of current studies. J Res Med Sci. 2015;20:893-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Yan W, Qian L, Chen J, Chen W, Shen B. Comparison of Prognostic MicroRNA Biomarkers in Blood and Tissues for Gastric Cancer. J Cancer. 2016;7:95-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kim HS, Kim JH, Kim JW, Kim BC. Chemotherapy in Elderly Patients with Gastric Cancer. J Cancer. 2016;7:88-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Liu F, Yan WL, Liu H, Zhang M, Sang H. Cutaneous metastases from gastric adenocarcinoma 15 years after curative gastrectomy. An Bras Dermatol. 2015;90:46-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Ahn HJ, Lee DS. Helicobacter pylori in gastric carcinogenesis. World J Gastrointest Oncol. 2015;7:455-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Kanat O, O’Neil B, Shahda S. Targeted therapy for advanced gastric cancer: A review of current status and future prospects. World J Gastrointest Oncol. 2015;7:401-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Biondi A, Lirosi MC, D’Ugo D, Fico V, Ricci R, Santullo F, Rizzuto A, Cananzi FC, Persiani R. Neo-adjuvant chemo(radio)therapy in gastric cancer: Current status and future perspectives. World J Gastrointest Oncol. 2015;7:389-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ito S, Ito Y, Misawa K, Shimizu Y, Kinoshita T. Neoadjuvant chemotherapy followed by surgery in gastric cancer patients with extensive lymph node metastasis. World J Clin Oncol. 2015;6:291-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Weigt J, Malfertheiner P. Metastatic Disease in the Stomach. Gastrointest Tumors. 2015;2:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab. 2014;25:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Kitazawa M, Nagano M, Masumoto KH, Shigeyoshi Y, Natsume T, Hashimoto S. Angiopoietin-like 2, a circadian gene, improves type 2 diabetes through potentiation of insulin sensitivity in mice adipocytes. Endocrinology. 2011;152:2558-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Kanda A, Noda K, Oike Y, Ishida S. Angiopoietin-like protein 2 mediates endotoxin-induced acute inflammation in the eye. Lab Invest. 2012;92:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Toyono T, Usui T, Yokoo S, Kimakura M, Nakagawa S, Yamagami S, Miyata K, Oike Y, Amano S. Angiopoietin-like protein 2 is a potent hemangiogenic and lymphangiogenic factor in corneal inflammation. Invest Ophthalmol Vis Sci. 2013;54:4278-4285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Clinical significance of MET in gastric cancer. World J Gastrointest Oncol. 2015;7:317-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Leporini C, Ammendola M, Marech I, Sammarco G, Sacco R, Gadaleta CD, Oakley C, Russo E, De Sarro G, Ranieri G. Targeting mast cells in gastric cancer with special reference to bone metastases. World J Gastroenterol. 2015;21:10493-10501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reißfelder C, Weitz J, Rahbari NN. Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget. 2015;6:35564-35578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Yan Y, Wang LF, Wang RF. Role of cancer-associated fibroblasts in invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:9717-9726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Chen DH, Yu JW, Jiang BJ. Contactin 1: A potential therapeutic target and biomarker in gastric cancer. World J Gastroenterol. 2015;21:9707-9716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 37. | Niccolai E, Taddei A, Prisco D, Amedei A. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol. 2015;21:5778-5793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Horiguchi H, Endo M, Miyamoto Y, Sakamoto Y, Odagiri H, Masuda T, Kadomatsu T, Tanoue H, Motokawa I, Terada K. Angiopoietin-like protein 2 renders colorectal cancer cells resistant to chemotherapy by activating spleen tyrosine kinase-phosphoinositide 3-kinase-dependent anti-apoptotic signaling. Cancer Sci. 2014;105:1550-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Gao L, Ge C, Fang T, Zhao F, Chen T, Yao M, Li J, Li H. ANGPTL2 promotes tumor metastasis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Yoshinaga T, Shigemitsu T, Nishimata H, Takei T, Yoshida M. Angiopoietin-like protein 2 is a potential biomarker for gastric cancer. Mol Med Rep. 2015;11:2653-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 548] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 42. | Zhou M, Ni QW, Yang SY, Qu CY, Zhao PC, Zhang JC, Xu LM. Effects of integrin-targeted photodynamic therapy on pancreatic carcinoma cell. World J Gastroenterol. 2013;19:6559-6567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Kaemmerer E, Kuhn P, Schneider U, Clahsen T, Jeon MK, Klaus C, Andruszkow J, Härer M, Ernst S, Schippers A. Beta-7 integrin controls enterocyte migration in the small intestine. World J Gastroenterol. 2015;21:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |