Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10254
Peer-review started: August 1, 2016
First decision: August 19, 2016
Revised: September 1, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: December 14, 2016
Processing time: 134 Days and 3.2 Hours
Gallbladder cancer (GBC), although considered as a relatively rare malignancy, is the most common neoplasm of the biliary tract system. The late diagnosis and abysmal prognosis present challenges to treatment. The overall 5-year survival rate for metastatic GBC patients is extremely low. BRCA1 and BRCA2 are the breast cancer susceptibility genes and their mutation carriers are at a high risk for cancer development, both in men and women. Olaparib, an oral poly ADP-ribose polymerase inhibitor, has been approved by the Food and Drug Administration and the European Commission for the treatment of ovarian cancer with any BRCA1/2 mutations. The first case of a BRCA1-mutated GBC patient who responded to olaparib treatment is reported here.
Core tip: Gallbladder cancer (GBC) is the most common neoplasm of the biliary tract system. BRCA1, the first major breast cancer susceptibility gene, has been widely studied in breast and ovarian cancers. Olaparib, an oral poly ADP-ribose polymerase (PARP) inhibitor, has been approved by the Food and Drug Administration and the European Commission for the treatment of ovarian cancer with any BRCA1/2 mutations. However, there is no report of a germline BRCA1 functional mutation in GBC prior to this case. Even further, the GBC with a BRCA1 mutation responded to the PARP inhibitor olaparib.
- Citation: Xie Y, Jiang Y, Yang XB, Wang AQ, Zheng YC, Wan XS, Sang XT, Wang K, Zhang DD, Xu JJ, Li FG, Zhao HT. Response of BRCA1-mutated gallbladder cancer to olaparib: A case report. World J Gastroenterol 2016; 22(46): 10254-10259
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10254.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10254
Gallbladder cancer (GBC) derives from the mucosal epithelial lining of the gallbladder and the cystic duct. It is a relatively rare malignancy, but is the most frequent malignant neoplasm of the biliary tract system. Epidemiological studies have demonstrated that the incidence of GBC is characterized by remarkable geographic distribution and ethnic disparities. The incidence is extraordinarily high in American Indians, elevated in Southeast Asia and quite low elsewhere in the Americas[1]. Although GBC limits in Southeast Asia, with increasing global migration, the incidence is also increasing in the west, and spreads worldwide. The prognosis of GBC is dismal and the median survival for locally advanced GBC with non-surgical treatment is about 8 mo[2]. Some patients detected incidentally during routine cholecystectomy for cholelithiasis have a long-term survival, but they only account for 2% of all cases with GBC[3]. Clinically, the adjuvant treatment for GBC is gemcitabine or 5-fluorouracil-based chemotherapy, with or without radiotherapy[4]. Even though the response rate remains low, there is no effective treatment. Here we report that a BRCA1-mutated GBC patient responded to the poly ADP-ribose polymerase inhibitor (PARPi) olaparib.
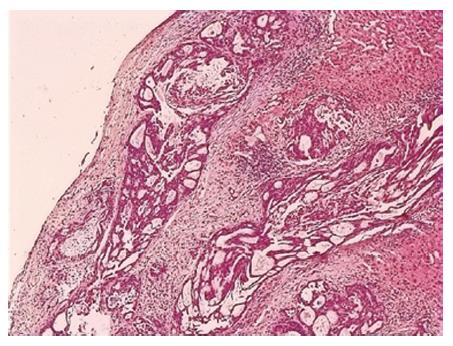
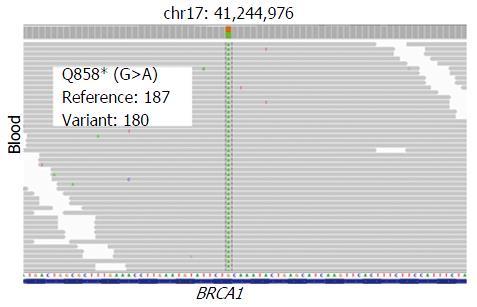
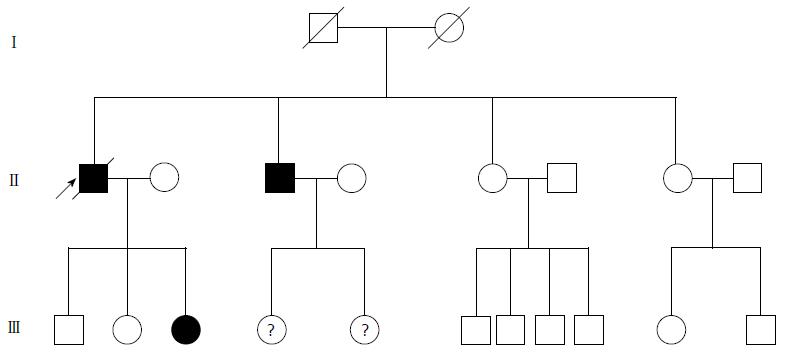
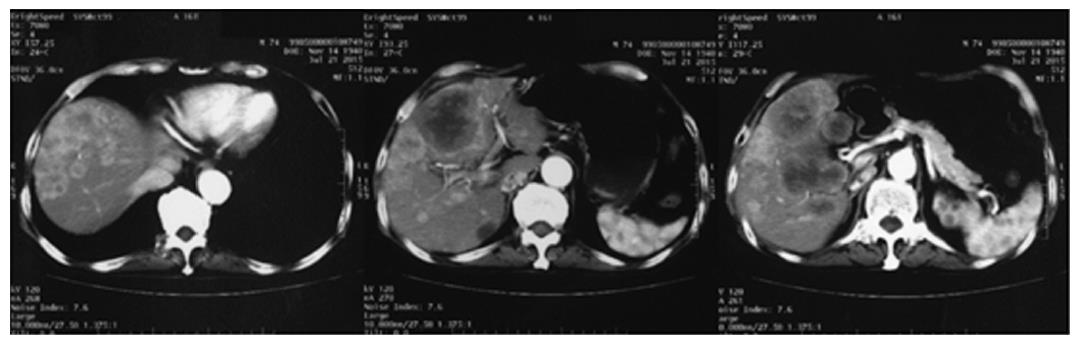
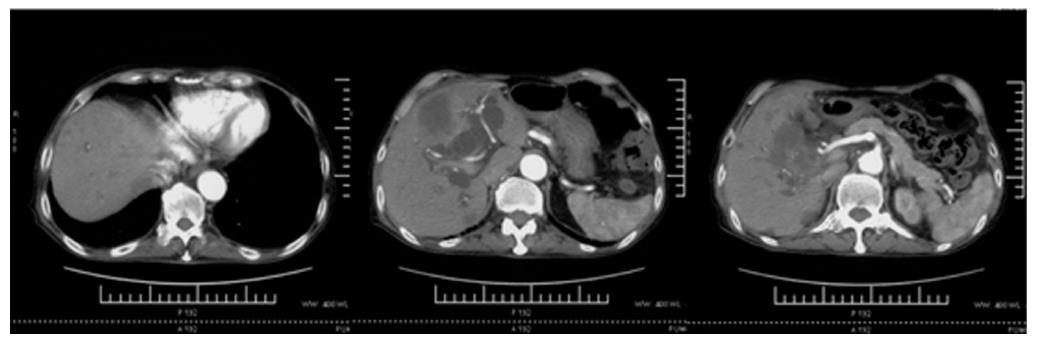
A 74-year-old man, with a past history of primary hypertension, atrial fibrillation, coronary disease and cholelithiasis, presented with epigastric pain. The patient underwent a robot-assisted prostate cancer surgery on November 29, 2013, and his mother had died of esophageal cancer. Computed tomography (CT) of the abdomen revealed multiple low-density intrahepatic lesions as well as the gallbladder lesion on May 7, 2015. PET-CT revealed multiple hypermetabolic intrahepatic lesions apart from the porta hepatis on May 14, 2015. A laparoscopic exploration was performed and an intrahepatic biopsy was conducted on May 26, 2015. Histologic examination indicated GBC (Figure 1). Considering the dismal prognosis and his poor physical condition, systemic chemotherapy was not preferred. After having obtained consent from the patient and his family, we tested the tissue. Two specimens from different liver metastases and a blood sample were sent for next generation sequencing panel. We detected all genomic alteration types on over 390 genes commonly associated with cancers and found a somatic MET P1086A mutation in one of two liver metastases, but there was no literature to confirm this was a functional mutation. Bioinformatics analysis also suspected MET P1086A could have an impact on MET function. However, we also detected a germinal BRCA1 Q858* mutation in both liver metastases and further Sanger sequencing confirmed this result (Figure 2). Furthermore, the patient’s offspring and siblings also had been screened for BRCA mutation from their saliva samples, and some family members were also BRCA1 Q858* mutation carriers (Figure 3). The nonsense mutation may lead to the premature termination of BRCA1 protein translation and nonsense-mediated mRNA decay, and the loss-of-function disenables its involvement in transcriptional regulation of gene expression and repair of DNA damage, particularly double-strand breaks[5]. Several studies have demonstrated that BRCA1 mutations increase the risks of breast, ovarian, prostate and pancreatic cancer[5-7]. Poly ADP-ribose polymerase (PARP) inhibitors have been studied as potential cancer therapeutics by means of inhibiting base excision repair (BER) as well as by trapping PARP[8,9]. A number of clinical trials have shown patients with germline BRCA1/2 mutations, especially in breast and ovarian cancer, to receive PARP inhibitor olaparib with survival benefit[10-12]. Based on the gene alteration testing report and the clinical trial studies, the patient was started on olaparib 400 mg twice daily on July 21, 2015 (Figure 4). The patient could tolerate the dose, and subsequently his pain was relieved significantly. On August 23, 2015, CT of the abdomen revealed the shrinkage of both intra- and extra-hepatic lesions and some extra-hepatic lesions even appeared to be invisible (Figure 5). The patient responded well to olaparib until the occurrence of obstructive jaundice. On October 9, 2015, CT of the abdomen indicated that intrahepatic lesions had dwindled; nevertheless, extrahepatic lesions became large and progressed (Figure 6). Subsequently, percutaneous transhepatic cholangiodrainage was performed to reduce the serum bilirubin level and the olaparib treatment was suspended from that time. We intended to resume olaparib treatment in combination with platinum agents at a later date. Unfortunately, the patient passed away as a result of severe biliary tract infection on November 25, 2015.
Like other cancers, substantial molecular alterations in genes contribute to the pathogenesis of GBC. Hitherto, in GBC, over 1450 single nucleotide variants, 34 deletions have been reported. The most frequent mutations are TP53 (18%-63%), KRAS, ERRB3 and ERBB2 (HER2)[13]. BRCA1, the first major breast cancer susceptibility gene, has been widely studied in breast and ovarian cancers. However, there is no report of germline BRCA1 functional mutation in GBC prior to this case. Even further, the GBC with a BRCA1 mutation responded to the PARP inhibitor olaparib.
Association of BRCA1/2 mutations with susceptibility to breast and ovarian cancer has been investigated for years. It is estimated that about 60% of women with BRCA1/2 mutations have developed breast cancer[14]. A woman who carries a germline BRCA1/2 mutation could be 5 times more likely to develop breast cancer than one who does not carry any BRCA1/2 mutation[15]. Men who have BRCA1/2 mutations are more likely to have prostate or pancreatic cancers. Men are 3.5 times and 8.6 times more likely to develop prostate cancer for BRCA1 and BRCA2 mutation carriers by age 65, respectively[16]. Similar to prostate cancer, BRCA1/2 poses a risk of pancreatic cancer development. Overall, BRCA1 mutation increases the risk by 0- to 4.11-fold, while the BRCA2 mutation increases the risk by 2.13- to 21.7-fold[17].
The BRCA proteins play a pivotal role in repair of double-strand DNA breaks via homologous recombination (HR). Due to deficiency in BRCA proteins, BRCA-mutated cells are not capable of locating the DNA recombinase RAD51 to damaged DNA and hence are unable to perform HR efficiently. Subsequently, an error-prone DNA repair mechanism, such as non-homologous end joining, is compelled to be used by cells, which often leads to cell death. BER, as one of the single-strand DNA break repair mechanisms, is crucial to address damaged single-strand DNA. Olaparib is an oral PARPi, and it was approved by the Food and Drug Administration and European Commission for the treatment of ovarian cancer with any BRCA1/2 mutations in 2014. Olaparib, by means of blocking BER, can convert single-strand DNA breaks to double-strand breaks, which gives rise to selective death of HR-deficient tumor cells. Mounting evidence has indicated that BRCA-mutated cancers are highly sensitive to PARP inhibitors and platinum agents. Compared with wild-type cells, BRCA-mutated cells are 1000-fold and 5-fold more sensitive to PARPi and platinum agents, respectively[18,19].
In this case, we observed that the intrahepatic lesions had a favorable response to olaparib, while the extrahepatic lesions had a progression with the emergence of olaparib resistance. Despite the fact that olaparib holds considerable promise in targeted therapies for BRCA-mutated breast or ovarian cancers, drug resistance has became a potential issue. So far, several resistance mechanisms have been proposed. Olaparib-triggered secondary BRCA mutations are perhaps considered as the most well-validated mechanism in patients; others include up-regulation of PgP transporter, loss of 53BP1 as well as PARP expression[20-22]. The comprehensive genomic alteration testing may provide novel clinical strategies for personalized therapy in advanced GBC. More mechanisms regarding chemoresistance are expected to be explored and understood in the future, which will help develop strategies to re-sensitize tumor cells to PARPi and improve the long-term effectiveness.
A 74-year-old man, with a past history of primary hypertension, atrial fibrillation, coronary disease and cholelithiasis, presented with epigastric pain.
The physical examination revealed tenderness of the epigastrium, without rebound tenderness and muscle tonus.
Hepatocellular carcinoma, intrahepatic cholangiocarcinoma, metastatic lesions of non-hepatic origins, gallbladder cancer (GBC).
The blood test for tumor markers revealed elevation of carbohydrate antigen 19-9 (4815.0 U/mL) and carcinoembryonic antigen (12.5 ng/mL), while alpha-fetoprotein and prostate specific antigen were within normal limits. The blood test for liver function revealed elevation of total bilirubin (23.0 μmol/L) and direct bilirubin (9.2 μmol/L), while alanine aminotransferase was within normal limits and the test for hepatitis virus was negative.
Computed tomography (CT) revealed multiple low-density intrahepatic lesions as well as the gallbladder lesion. Positron emission tomography-CT (PET-CT) revealed multiple hypermetabolic intrahepatic lesions apart from the porta hepatis.
Pathological examination revealed GBC with hepatic infiltration.
The patient underwent a laparoscopic exploration and an intrahepatic biopsy. Two specimens from different liver metastases and a blood sample were sent for next generation sequencing panel. A germinal BRCA1 Q858* mutation in both liver metastases was detected and further Sanger sequencing confirmed this result. Based on the gene alteration testing report and the clinical trial studies, the patient was started on olaparib 400 mg twice daily.
There is no report of germline BRCA1 functional mutation in GBC prior to this case. Even further, the GBC with a BRCA1 mutation responded to the poly ADP-ribose polymerase (PARP) inhibitor olaparib.
BRCA1, the first major breast cancer susceptibility gene, has been widely studied in breast and ovarian cancers; their mutation carriers are at a high risk for cancer development. Olaparib, an oral PARP inhibitor (PARPi), has been approved by the Food and Drug Administration and European Commission for the treatment of ovarian cancer with any BRCA1/2 mutations.
This case report describes the response of a germline BRCA1-mutated GBC patient to the PARPi olaparib. While the comprehensive genomic alteration testing may provide novel clinical strategies for personalized therapy in advanced GBC, drug resistance has become a potential issue. More discoveries concerning the mechanisms for chemoresistance will help develop strategies to re-sensitize tumor cells to PARPi and improve the long-term effectiveness.
This is a very interesting case report. In this manuscript, the authors reported a 74-year-old man, with a past history of primary hypertension, atrial fibrillation, coronary disease and cholelithiasis, who presented with epigastric pain.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Charco R, Lee MW, Tsegmed U S- Editor: Gong ZM L- Editor: Logan S E- Editor: Zhang FF
| 1. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 2. | Boutros C, Gary M, Baldwin K, Somasundar P. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Dutta U. Gallbladder cancer: can newer insights improve the outcome? J Gastroenterol Hepatol. 2012;27:642-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 5. | Ferrone CR, Levine DA, Tang LH, Allen PJ, Jarnagin W, Brennan MF, Offit K, Robson ME. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Friedenson B. The BRCA1/2 pathway prevents hematologic cancers in addition to breast and ovarian cancers. BMC Cancer. 2007;7:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7:60. [PubMed] |
| 8. | Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588-5599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1679] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 9. | Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3368] [Cited by in RCA: 3842] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 10. | Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1078] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 11. | Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 910] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 12. | Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1436] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 13. | Bizama C, García P, Espinoza JA, Weber H, Leal P, Nervi B, Roa JC. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. 2015;41:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Foulkes WD, Shuen AY. In brief: BRCA1 and BRCA2. J Pathol. 2013;230:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Cavanagh H, Rogers KM. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Luo G, Lu Y, Jin K, Cheng H, Guo M, Liu Z, Long J, Liu C, Ni Q, Yu X. Pancreatic cancer: BRCA mutation and personalized treatment. Expert Rev Anticancer Ther. 2015;15:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4316] [Cited by in RCA: 4887] [Article Influence: 244.4] [Reference Citation Analysis (0)] |
| 19. | Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899-23903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 442] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med. 2013;19:1381-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 21. | Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas. 2015;81:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Montoni A, Robu M, Pouliot E, Shah GM. Resistance to PARP-Inhibitors in Cancer Therapy. Front Pharmacol. 2013;4:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |