Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10249
Peer-review started: August 2, 2016
First decision: August 29, 2016
Revised: September 10, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: December 14, 2016
Processing time: 132 Days and 22.3 Hours
There are diverse protocols to manage patients with recurrent disease after primary cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis. We describe a case of metachronous liver metastasis after CRS and HIPEC for colorectal cancer, successfully treated with a selective metastectomy and partial graft of the inferior vena cava. A 35-year-old female presented with a large tumour in the cecum and consequent colonic stenosis. After an emergency right colectomy, the patient received adjuvant chemotherapy. One year later she was diagnosed with peritoneal carcinomatosis, and it was decided to carry out a CRS/HIPEC. After 2 years of total remission, an isolated metachronous liver metastasis was detected by magnetic resonance imaging surveillance. The patient underwent a third procedure including a caudate lobe and partial inferior vena cava resection with a prosthetic graft interposition, achieving an R0 situation. The postoperative course was uneventful and the patient was discharged on postoperative day 17 after the liver resection. At 18-mo follow-up after the liver resection the patient remained free of recurrence. In selected patients, the option of re-operation due to recurrent disease should be discussed. Even liver resection of a metachronous metastasis and an extended vascular resection are acceptable after CRS/HIPEC and can be considered as a potential treatment option to remove all macroscopic lesions.
Core tip: Treatment of liver recurrence after a cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is a great challenge. We report here the case of a young patient with metachronous liver metastases who was treated with a limited resection of segment I of the liver and vascular graft interposition of the inferior vena cava achieving a long-term survival. The surgical approach of these patients is extremely complicated and often requires complex major surgical procedures.
- Citation: Sánchez-Velázquez P, Moosmann N, Töpel I, Piso P. “En bloc” caudate lobe and inferior vena cava resection following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal and liver metastasis of colorectal cancer. World J Gastroenterol 2016; 22(46): 10249-10253
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10249
Peritoneal carcinomatosis (PC) is the second most common presentation of metastatic colorectal cancer and is diagnosed in up to 4%-6% of these cases. Twenty five percent of these patients have the peritoneum as the only site of disseminated disease[1]. PC was traditionally considered the last stage of the disease and was associated with a poor prognosis so that patients were often relegated to palliative systemic therapies. In recent decades multimodal PC treatment has made great advances; cytoreductive surgery (CRS), hyperthermic intraperitoneal chemotherapy (HIPEC) and systemic chemotherapy have shown promising results and have become standard therapy for PC patients in several countries[2-7]. Various studies have reported that patients undergoing CRS with total macroscopic cytoreduction and HIPEC may achieve prolonged overall survival and potentially even a complete cure in selected patients[4,8,9].
Up to 80% of patients with PC of colorectal origin treated with CRS and HIPEC are likely to recur[10,11]. In selected cases the possibility of a reoperation due to recurrence or even an extensive abdominal surgical procedure can be individually assessed. The data available on this approach show favourable long-term outcomes with similar morbidity and mortality to that of initial CRS/HIPEC[12-15]. Simultaneous or staged combined CRS and liver resections have also been performed with comparable morbidity and long-term results[16]. In this report we describe the case of a young female patient with metachronous liver metastasis after CRS and HIPEC for colorectal cancer successfully treated by a selective liver and vascular resection.
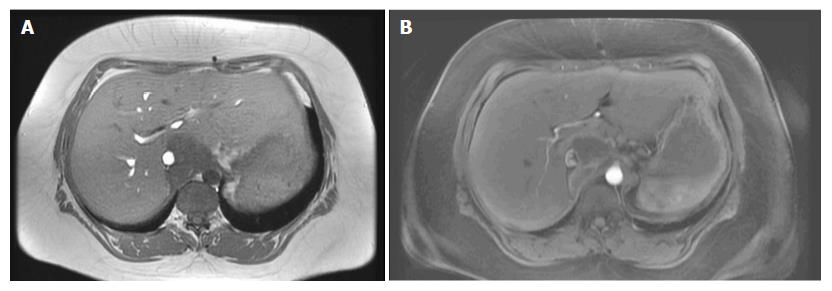
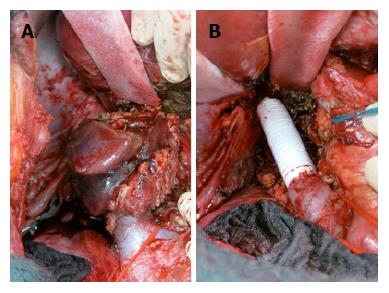
A 35-year-old female was referred to our emergency unit in February 2011, presenting with abdominal distension, pain, and vomiting for 3 d. Her medical history was only remarkable for asthma. Abdominal computed tomography (CT) was performed and revealed a large tumor in the cecum with consecutive colonic stenosis. The patient underwent an emergency right colectomy, and an R0 situation was achieved. Pathologic examination showed pT4a pN2a (4/22) cM0 poorly differentiated adenocarcinoma. Between March and August 2011 she received 12 cycles of adjuvant FOLFOX (folinic acid, 5-fluorouracil, and oxaliplatin). In February 2012 a CT scan identified lesions in the peritoneum with suspicions of peritoneal carcinomatosis. Our multidisciplinary tumour board decided on pursuing CRS with HIPEC. Abdominal exploration revealed widespread peritoneal carcinomatosis, especially in the pelvis and a large tumor mass in the left sub-diaphragmatic region with a peritoneal cancer index (PCI) of 14. Total parietal and diaphragmatic peritonectomy, proctocolectomy with an end ileostomy, terminal ileum resection, splenectomy, omentectomy, hysterectomy and bilateral salpingo-oophorectomy (CCR-0) were performed to remove the macroscopic tumor. HIPEC was carried out to treat the microscopic residual with mitomycin C, according to the closed-abdomen technique. The postoperative course was uneventful and the patient was duly discharged from hospital. At this time, no further chemotherapy was recommended as the patient had completed adjuvant chemotherapy after the first operation and a R0 situation was achieved. Adjuvant systemic chemotherapy, according to our institution protocols, is performed only in chemo naïve patients. From February 2012 until June 2014 the patient stayed free from recurrence. In July 2014 abdominal magnetic resonance imaging (MRI) surveillance revealed a solid tumor in segment 1 of the liver (Figure 1) so the patient underwent explorative laparotomy. During the procedure no evidence of a peritoneal recurrence was shown but many adhesions were found from the previous operations. A 4 cm tumor was identified in the caudate hepatic lobe infiltrating the inferior vena cava (IVC). The distal cava was mobilized towards the left renal vein and was divided at a level free of tumor (Figure 2A). Liver segment 1 was transected following the clamp-crushing technique and ICV cranial to the tumor was dissected and divided under the major suprahepatic trunks. Vascular continuity was restored using a prosthetic graft interposition sutured with a running suture of prolene 5/0 onto the proximal and distal IVC (Figure 2B). The postoperative course was mainly uncomplicated except for right pleural effusion, initially managed conservatively with diuretic therapy, and later by thoracocentesis. The patient was finally discharged on the 17th postoperative day. Pathological examination revealed a poorly differentiated adenocarcinoma with identical immunohistochemical phenotype as previously. At this point it was decided not to continue with chemotherapy, as a CCR-0 situation had been surgically achieved and the patient had completed the 12-cycle adjuvant treatment in the past (Table 1). Eighteen months after metastasis resection of the liver and 4 years after CRS and HIPEC, the patient shows no evidence of neoplastic disease.
| Time point | Diagnosis | Procedure |
| 2011 | Stenotic tumour of the cecum | Right Colectomy Adjuvant chemotherapy (12 cycles with folinic acid, 5-fluorouracil, and oxaliplatin) |
| 2012 | Peritoneal carcinomatosis PCI = 14 | CRS (CCR-0) Total parietal and diaphragmatic Peritonectomy Proctocolectomy with end ileostomy Terminal ileum resection Splenectomy Omentectomy Hysterectomy Bilateral salpingo-oophorectomy HIPEC 43.8 mg Mitomycin C intraperitoneal (1 h) |
| 2014 | Liver metastases segment I with IVC infiltration | Resection of liver segment I Partial resection of IVC with prosthetic graft interposition Cholecystectomy Partial adrenalectomy |
| 2016 | No evidence of neoplastic disease |
After initial CRS and HIPEC, recurrences are mostly intra-abdominal, even if complete cytoreduction is achieved[17]. Protocols differ among the different institutions, thus a number of different treatments are applied in high-volume centres, including chemotherapy, tumour debulking or re-do surgery for intraadominal metastasis.
The presence of synchronous liver metastases (LM) and PC was traditionally a contraindication for cytoreductive surgery. However, it has been shown that selected patients with low PCI and three or fewer LM can achieve prolonged survival if a liver resection is performed simultaneously[16,18]. A recent meta-analysis by Cuba et al[19] shows improved overall survival (OS) in patients who were treated with CRS and HIPEC and curative treatment of LM as compared to patients treated with modern systemic chemotherapy alone.
Nevertheless, it remains unclear which approach should be used in patients with isolated metachronous LM, as in our case report. Iterative cytoreductive surgery is feasible in cases of recurrence and appears to be worthwhile in terms of long-term outcomes[20]. The study by Sugarbaker and colleagues was on one of the largest series and included 70 patients with PC of colorectal origin[15]. This study showed that 53% of the patients had at least one reoperation after the initial cytoreduction. The overall survival of patients with repeated surgery approach was significantly longer (39 mo vs 20 mo). Brouquet et al[13] reported on a cohort of 20 patients with repeat CRS + intraperitoneal chemotherapy (IPC) for isolated peritoneal tumour recurrence of all origins. Five- and 10-year overall survival (OS) rates were 72.5% and 58.1% respectively.
Even though they studied selected groups of patients, the studies by Sugarbaker and Brouquet underline the possibility of highly favourable outcomes and even long-term survival in palliative patients with recurrence of peritoneal carcinomatosis.
In this context, the study by Kianmanesh et al[12] included 43 patients with PC of colorectal cancer origin who underwent CRS/HIPEC and specifically evaluated the role of simultaneous liver resection. They concluded that patients with colorectal PC, iterative CRS and HIPEC achieved appreciable long-term survival and that liver metastasis resection did not negatively influence the postoperative outcomes. However, they did not specify whether an extended vascular resection was performed or if liver resections were performed in cases of metachronous LM.
Achieving complete cytoreduction in most cases is challenging and implies an aggressive approach combining major surgical procedures not exempt from complications. In selected cases, as in the one presented here, the option of re-do surgery for liver metastasis is feasible. Even extended vascular resection is acceptable after CRS/HIPEC and can be considered as a potentially curative treatment option. Early detection of tumour recurrence through a close follow-up is essential, as well as a multidisciplinary assessment of patient selection. The patients should then be referred to a centre specialized in the treatment of peritoneal and liver metastases. In the present case, staged resection of both metastatic sites achieved long-term survival for a young female patient. To our knowledge, this is this first report on liver resection due to metachronous liver metastases following CRS and HIPEC.
A 35-year-old patient with liver recurrent disease after an extended cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) procedure.
Abdominal magnetic resonance imaging (MRI) diagnosed the liver recurrence in the follow-up.
There is no possible differential diagnosis in this case.
All labs were within normal limits.
MRI showed a solid tumor in segment 1 of the liver.
pT4a pN2a (4/22) cM0 poorly differentiated adenocarcinoma.
Complete surgical excision of lesion.
There are currently no other reports of surgical excision of a liver metastasis in the caudate lobe two years after a cytoreductive surgery.
The report is good example of patient tailored treatment in cases where guidelines are missing or suggest only palliative or best supportive care. It is also novel to perform such an extensive surgery after cytoreductive surgery and HIPEC.
The paper is well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hoskovec D, Levine EA, Peng SY, Tentes AA, van Oudheusden TR S- Editor: Qi Y L- Editor:A E- Editor: Liu WX
| 1. | Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128:2717-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 2. | Esquivel J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: survival outcomes and patient selection. J Gastrointest Oncol. 2016;7:72-78. [PubMed] |
| 3. | Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1506] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 5. | Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756-3762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Piso P, Arnold D, Glockzin G. Challenges in the multidisciplinary management of stage IV colon and rectal cancer. Expert Rev Gastroenterol Hepatol. 2015;9:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Piso P, Arnold D. Multimodal treatment approaches for peritoneal carcinosis in colorectal cancer. Dtsch Ärzteblatt Int. 2011;108:802-808. |
| 8. | Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 731] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 9. | Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 401] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 10. | Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, Garofalo A, Di Filippo F, Giannarelli D, Vaira M, Valle M. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Mirnezami R, Moran BJ, Harvey K, Cecil T, Chandrakumaran K, Carr N, Mohamed F, Mirnezami AH. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases. World J Gastroenterol. 2014;20:14018-14032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, Hay JM, Flamant Y, Msika S. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Brouquet A, Goéré D, Lefèvre JH, Bonnet S, Dumont F, Raynard B, Elias D. The second procedure combining complete cytoreductive surgery and intraperitoneal chemotherapy for isolated peritoneal recurrence: postoperative course and long-term outcome. Ann Surg Oncol. 2009;16:2744-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Vassos N, Förtsch T, Aladashvili A, Hohenberger W, Croner RS. Repeated cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with recurrent peritoneal carcinomatosis. World J Surg Oncol. 2016;14:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol. 2008;98:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Maggiori L, Goéré D, Viana B, Tzanis D, Dumont F, Honoré C, Eveno C, Elias D. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Verwaal VJ, Boot H, Aleman BM, van Tinteren H, Zoetmulder FA. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: location, treatment, and outcome. Ann Surg Oncol. 2004;11:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Elias D, Faron M, Iuga BS, Honoré C, Dumont F, Bourgain JL, Dartigues P, Ducreux M, Goéré D. Prognostic similarities and differences in optimally resected liver metastases and peritoneal metastases from colorectal cancers. Ann Surg. 2015;261:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | de Cuba EM, Kwakman R, Knol DL, Bonjer HJ, Meijer GA, Te Velde EA. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev. 2013;39:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Esquivel J, Sugarbaker PH. Second-look surgery in patients with peritoneal dissemination from appendiceal malignancy: analysis of prognostic factors in 98 patients. Ann Surg. 2001;234:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |