Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9994
Peer-review started: July 12, 2016
First decision: July 29, 2016
Revised: October 2, 2016
Accepted: November 12, 2016
Article in press: November 13, 2016
Published online: December 7, 2016
Processing time: 151 Days and 13.4 Hours
To evaluate the clinical significance of the preoperative fibrinogen plasma level as a prognostic marker after surgery for colorectal cancer.
This retrospective study analysed 652 patients undergoing surgery for stage I-IV colorectal cancer between January 2005 and December 2012, at the Division of General Surgery A, University of Verona Hospital Trust, in whom preoperative fibrinogen plasma values were assessed at baseline. Fibrinogen is involved in tumourigenesis as well as tumour progression in several malignancies. Correlations between preoperative plasma fibrinogen values and clinicopathological characteristics were investigated. Univariate and multivariate survival analyses were performed to identify factors associated with overall and tumour-related survival.
Among the 652 patients, the fibrinogen value was higher than the threshold of 400 mg/dL in 345 patients (53%). The preoperative mean ± SD of fibrinogen was 426.2 ± 23.2 mg/dL (median: 409 mg/dL; range: 143-1045 mg/dL). Preoperative fibrinogen values correlated with age (P = 0.003), completeness of tumour resection, potentially curative vs palliative (P < 0.001), presence of systemic metastasis (P < 0.001), depth of tumour invasion pT (P < 0.001), nodes involvement pN (P = 0.001) and CEA serum level (P < 0.001). The mean fibrinogen value (± SD) was 395.6 ± 120.4 mg/dL in G1 tumours, 424.1 ± 121.4 mg/dL in G2 tumours and 453.4 ± 131.6 mg/dL in G3 tumours (P = 0.045). The overall survival and tumour-related survival were significantly higher in patients with fibrinogen values ≤ 400 mg/dL (P < 0.001). However, hyperfibrinogenemia did not retain statistical significance regarding either overall (P = 0.313) or tumour-related survival (P = 0.355) after controlling for other risk factors in a multivariate analysis.
Preoperative fibrinogen levels correlate with cancer severity but do not help in predicting patient prognosis after colorectal cancer surgery.
Core tip: Fibrinogen is involved in tumourigenesis and in tumour progression in several malignancies. Many studies, particularly from East, have shown a correlation between hyperfibrinogenemia and poor prognosis in patients with colorectal cancer (CRC). This study involves a large cohort of 652 Western patients underwent surgery for CRC. The analysis of our data demonstrates that preoperative fibrinogen plasma levels correlate with leading prognostic factors in patients undergoing surgery for CRC. Although long-term survival and tumour-related survival are worse in patients with hyperfibrinogenemia, these findings are not confirmed in multivariate analysis or after stratification of patients according to completeness of tumour resection and TNM stage.
- Citation: Pedrazzani C, Mantovani G, Salvagno GL, Baldiotti E, Ruzzenente A, Iacono C, Lippi G, Guglielmi A. Elevated fibrinogen plasma level is not an independent predictor of poor prognosis in a large cohort of Western patients undergoing surgery for colorectal cancer. World J Gastroenterol 2016; 22(45): 9994-10001
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/9994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.9994
Colorectal cancer (CRC) is the third most common cancer worldwide, with more than 1 million new cases and 600000 deaths per year[1] . Many haemostatic derangements have been described in cancer progression and prognosis. Solid tumours, both in humans and animal models, entail a considerable amount of fibrinogen-related alterations. In particular, CRC is associated with a large range of fibrinolytic and procoagulant alterations, suggesting that fibrinogen plays an important role in tumour stroma formation[2-4]. In fact, fibrin matrices promote the migration of endothelial cells, fibroblasts and macrophages, as well as neovascularization[5-7]. Moreover, the products of fibrin degradation display chemotactic, angiogenic and immune-modulatory capacities[8,9].
Increased plasma fibrinogen levels are associated with liver and lymph node metastasis, but not with peritoneal metastasis, in gastric cancer[10,11]. Recent studies on CRC support the hypothesis that hyperfibrinogenemia may promote the enhancement of tumour volume[12] and haematogenous metastasis[13,14]. Several studies have demonstrated a significant contribution of high fibrinogen plasma levels in predicting the prognosis in various subsets of CRC patients[15,16].
The aim of this retrospective study was to evaluate the clinical significance of preoperative plasma fibrinogen levels as a prognostic marker after colorectal cancer surgery.
The study population consisted of patients undergoing surgery for colorectal carcinoma at the Division of General Surgery A, University of Verona Hospital Trust, between January 2005 and December 2012. The inclusion criteria were age of 18 years or older, histological diagnosis of cancer, elective or urgent colorectal resection with absence of peritonitis or other acute infectious diseases, no pre-operative chemotherapy or radiotherapy, follow-up available for a minimum period of 30 mo, as well as preoperative assessment of fibrinogen levels. Informed consent was obtained from all the patients, and the study was approved by the local Ethics Committee with ID number: 42763 (CRINF-1034 CESC).
Between January 2005 and December 2012, 969 patients underwent surgery for colorectal carcinoma at the Division of General Surgery A, University of Verona Hospital Trust. Among them, 652 patients met the inclusion criteria and were enrolled in the study.
Before surgery, all elective patients underwent preoperative staging by means of colonoscopy, thoracoabdominal CT scan and tumour markers (CEA, CA 19-9). Abdominal US was performed in selected cases when a CT scan was deemed unnecessary, whilst additional imaging modalities (e.g., MRI, PET-CT, endoluminal US, etc.) were used when indicated (e.g., rectal cancer or liver metastases). The preoperative assessment of urgent cases varied depending on clinical necessities.
All patients underwent preoperative routine laboratory tests, including coagulation profiles with assessment of plasma fibrinogen levels within two weeks of surgery.
The resected specimens were examined using routine histopathological analysis. Tumour staging was assessed according to the criteria established by the 7th Edition of the American Joint Committee on Cancer and the Union International Contre Le Cancer. Tumour differentiation; lymphatic, vascular and neural invasion; and inflammatory reactions were generally reported.
Blood samples were drawn by an expert phlebotomist in evacuated blood tubes containing 0.109 mol/L buffered sodium citrate (Terumo Europe NV, Leuven, Belgium). The blood tubes were left in an upright position at room temperature to allow complete blood stability and then centrifuged at 1500 ×g for 15 min. A second centrifugation was performed to obtain platelet-poor plasma, which was stored in aliquots at -70 °C until measurement. At the time of measurement, the plasma aliquots were thawed in a water bath at 37 °C and then left at room temperature for 1 hour. Fibrinogen was measured on an ACL TOP instrument (Instrumentation Laboratory, Milan, Italy) with the Clauss method and using proprietary reagents (HemosIL® Fibrinogen-C, Instrumentation Laboratory). The reference range of fibrinogen was 200-400 mg/dL
The main goal of the surgery was the complete removal of the tumour (R0 resection), although palliative surgery was carried out in select cases to treat tumour-related complications. The extent of surgery was assessed considering the patient’s performance status, tumour location and stage. Standard colorectal resection (i.e., right hemicolectomy, extended right hemicolectomy, left hemicolectomy, anterior resection, low anterior resection, or abdominoperineal resection) with ligation of vessels at their origin was usually carried out to obtain the optimal management of nodal disease[17], whereas limited colonic resection or stoma formation was performed in select cases (i.e., palliative surgery, high-risk patients).
All clinical and pathological data were retrospectively collected and stored in a digital dataset. The analysed variables included demographic, clinical, surgical and pathological characteristics. Survival and follow-up data were obtained by collecting outpatient clinical records or by contacting the patient or the family physician.
In the preliminary analysis, preoperative fibrinogen levels were normally distributed in the patient cohort. Different cut-off levels were considered to study the potential correlation with clinicopathological factors and survival. The upper limit of the reference range (i.e., 400 mg/dL) was used as the predictive threshold.
To evaluate the significance of difference between cases with values above or below 400 mg/dL, a chi-square test or Fisher’s exact test were used for categorical data and Student’s t-test was used for continuous variables. Survival analysis was computed using the Kaplan-Meier method and compared by the log-rank test, with time of survival measured from the date of surgery to the date of death or most recent follow-up. Multivariate analysis was performed with the Cox regression model by taking into account the following risk factors: age (higher than median vs median or below), gender (male vs female), tumour location (rectum vs colon), type of surgery (urgent vs. elective), presence of residual tumour (R1, R2 vs R0), presence of systemic metastasis (M1a, M1b vs M0), pT category (pT2, pT3, pT4a, pT4b vs pT1), pN category (pN1, pN2 vs pN0), histological type (mucinous vs non-mucinous) and pre-operative fibrinogen plasma levels (fibrinogen value higher than 400 mg/dL vs fibrinogen value lower than this threshold). Statistical analysis was performed using SPSS software version 21.0 (IBM Corporation, Armonk, NY, United States), and the level of statistical significance was set at P < 0.05.
The preoperative mean ± SD of fibrinogen was 426.2 ± 23.2 mg/dL (median: 409 mg/dL; range: 143-1045 mg/dL). Among the 652 patients, the fibrinogen value was higher than the threshold of 400 mg/dL in 345 patients (53%).
Mean ± SD preoperative fibrinogen plasma levels for the 652 patients under study according to their clinicopathological variables are reported in Table 1. Fibrinogen value correlated with age (P = 0.003), type of resection (potentially curative vs palliative) (P < 0.001), presence of systemic metastasis (P < 0.001), CEA serum level (P < 0.001) as well as the pT (P < 0.001) and pN categories (P = 0.001).
| No. of patients | Fibrinogen plasma level | P value | ||
| Mean value | ± SD | |||
| Age (yr) | P = 0.003 | |||
| ≤ 68.8 | 326 | 412.1 | 119.8 | |
| > 68.8 | 326 | 440.3 | 125.1 | |
| Gender | P = 0.479 | |||
| Male | 373 | 429.1 | 128.3 | |
| Female | 279 | 422.2 | 116.2 | |
| Tumour location | P = 0.111 | |||
| Colon | 536 | 429.8 | 125.0 | |
| Rectum | 116 | 409.6 | 113.7 | |
| Type of surgery | P = 0.053 | |||
| Elective | 618 | 424.0 | 120.1 | |
| Urgent | 34 | 466.0 | 167.9 | |
| Resection | P < 0.001 | |||
| Yes | 640 | 423.8 | 121.3 | |
| No | 12 | 551.3 | 162.1 | |
| Type of resection (R) | P < 0.001 | |||
| R0 | 556 | 412.4 | 110.4 | |
| R1 | 7 | 406.0 | 113.1 | |
| R2 | 89 | 513.9 | 159.6 | |
| Depth of invasion (pT)1 | P < 0.001 | |||
| pT1 | 86 | 373.7 | 97.1 | |
| pT2 | 61 | 373.6 | 89.8 | |
| pT3 | 259 | 421.5 | 111.1 | |
| pT4a | 141 | 442.0 | 143.9 | |
| pT4b | 93 | 482.1 | 118.6 | |
| Node involvement (pN)1 | P = 0.001 | |||
| pN0 | 372 | 408.2 | 108.7 | |
| pN1 | 166 | 445.0 | 142.6 | |
| pN2 | 102 | 446.5 | 119.5 | |
| Systemic metastasis (M) | P < 0.001 | |||
| M0 | 534 | 412.1 | 109.6 | |
| M1a | 69 | 466.8 | 144.1 | |
| M1b | 49 | 522.3 | 170.5 | |
| CEA serum level2 | P < 0.001 | |||
| ≤ 5 ng/mL | 284 | 393.4 | 101.0 | |
| > 5 ng/mL | 130 | 485.7 | 129.6 | |
Considering histological characteristics, patients with a mucinous histological type (n = 113) displayed higher fibrinogen values compared to non-mucinous histological types (467.1 ± 135.9 vs 417.6 ± 118.7; P < 0.001). The mean ± SD was 395.6 ± 120.4 mg/dL in G1 tumours, 424.1 ± 121.4 mg/dL in G2 tumours and 453.4 ± 131.6 mg/dL in G3 tumours (P = 0.045). Conversely, vascular invasion (P = 0.204), lymphatic invasion (P = 0.940), neural invasion (P = 0.183) and presence of inflammatory reaction (P = 0.067) were not significantly associated with preoperative fibrinogen plasma levels.
Considering the fibrinogen cut-off value of 400 mg/dL, a significant association was found with age (P = 0.001), type of resection (P < 0.001), depth of tumour invasion (P < 0.001), the presence of systemic metastases (P = 0.001), histological type (P = 0.001) and CEA serum level (P < 0.001). Interestingly, fibrinogen values were found to be > 400 mg/dL in 74.2% of patients with macroscopic residual tumours after resection (R2) compared to 49.5% of potentially curative resections (R0) (P < 0.001). Similarly, fibrinogen values were found to be > 400 mg/dL in 71.4% of M1b patients compared to 65.2% of M1a patients and 49.6% of M0 patients (P = 0.001). Regarding the depth of tumour invasion, fibrinogen values were > 400 mg/dL in 32.6% of pT1 patients, 37.7% of pT2 patients, 54.8% of pT3 patients and 61.1% of pT4 patients (P < 0.001). Conversely, the extent of nodal involvement did not correlate with fibrinogen value. Fibrinogen plasma levels were found to be > 400 mg/dL in 50.3% of pN0 patients, 51.8% of pN1 patients and 61.8% of pN2 patients (P = 0.017).
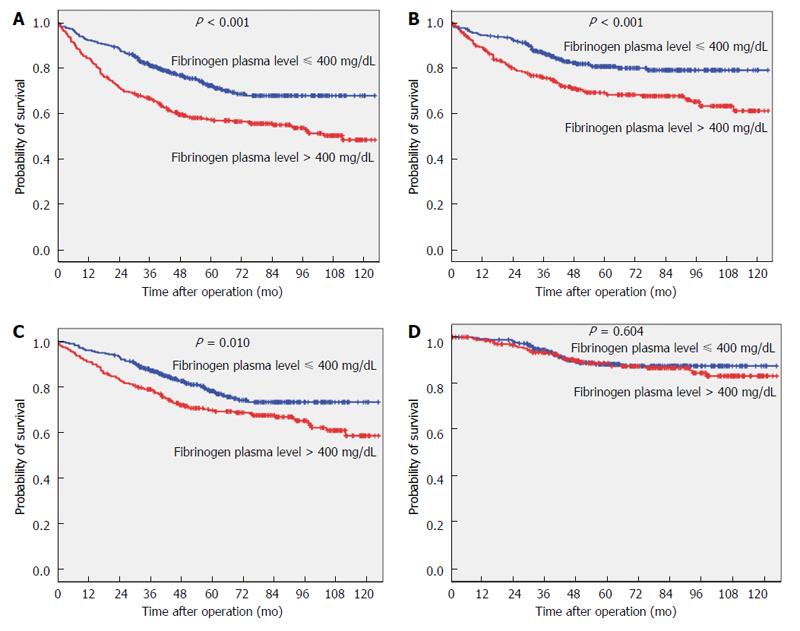
The 5-year survival and 5-year tumour-related survival rates of the study population were 64.4% and 75.2%, respectively. Five-year survival and 5-year tumour-related survival rates according to preoperative fibrinogen plasma levels are shown in Figure 1A. The 5-year survival rate was 72.4% for patients with values ≤ 400 mg/dL and 58.1% for patients with values > 400 mg/dL (P < 0.001). When considering tumour-related mortality, the 5-year survival rate was 81.2% for patients with values ≤ 400 mg/dL and 69.6% for patients with values > 400 mg/dL (P < 0.001).
Survival curves for patients undergoing potentially curative resection (R0) are shown in Figure 1B. Fibrinogen plasma levels were associated with overall survival (P = 0.010), whereas no significant difference was observed when tumour-related survival was considered (P = 0.604). In particular, 5-year tumour-related survival was 88.3% in patients with values ≤ 400 mg/dL and 88.7% for patients with values > 400 mg/dL.
Table 2 shows the multivariate analysis (Cox regression model) adjusted for multiple factors. Age, the presence of systemic metastasis, the presence of residual tumour, pT category and pN category were confirmed as independent predictors of survival, whereas the fibrinogen plasma level was not [hazard ratio (HR) for fibrinogen value > 400 mg/dL compared to ≤ 400 mg/dL: 1.15 (95%CI: 0.86-1.54), P = 0.355]. Similar results were found for tumour-related survival [HR for fibrinogen value > 400 mg/dL compared to ≤ 400 mg/dL: 0.82 (95%CI: 0.54-1.21), P = 0.313]. Table 3 shows 5-year survival and tumour-related survival rates for Stage I, Stage II, Stage III and Stage IV tumours treated by potentially curative resection (R0).
| Survival | Tumour-related survival | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | < 0.001 | < 0.001 | ||
| > 68.8 vs ≤ 68.8 | 2.55 (1.91-3.40) | 2.13 (1.47-3.09) | ||
| Gender | 0.298 | 0.155 | ||
| Male vs female | 1.16 (0.88-1.53) | 1.31 (0.90-1.89) | ||
| Tumour location | 0.368 | 0.928 | ||
| Rectum vs colon | 1.19 (0.82-1.72) | 0.98 (0.59-1.61) | ||
| Type of surgery | 0.180 | 0.805 | ||
| Urgent vs elective | 1.42 (0.85-2.38) | 0.92 (0.49-1.74) | ||
| Type of resection (R) | < 0.001 | < 0.001 | ||
| R1 vs R0 | 1.98 (0.77-5.07) | 4.39 (1.61-11.97) | ||
| R2 vs R0 | 2.93 (1.82-4.71) | 6.46 (3.63-11.49) | ||
| Depth of invasion (pT)1 | < 0.001 | < 0.001 | ||
| pT2 vs pT1 | 1.51 (0.59-3.86) | 7.53 (0.88-64.83) | ||
| pT3 vs pT1 | 1.97 (0.93-4.13) | 6.03 (0.81-44.63) | ||
| pT4a vs pT1 | 4.06 (1.90-8.68) | 14.99 (2.02-111.57) | ||
| pT4b vs pT1 | 2.88 (1.30-6.37) | 15.55 (2.07-117.09) | ||
| Node involvement (pN)1 | < 0.001 | 0.135 | ||
| pN1 vs pN0 | 1.33 (0.94-1.87) | 1.51 (0.97-2.37) | ||
| pN2 vs pN0 | 2.45 (1.69-3.54) | 1.59 (0.94-2.67) | ||
| Systemic metastasis (M) | < 0.001 | < 0.001 | ||
| M1a vs M0 | 2.62 (1.72-3.99) | 4.72 (2.72-8.19) | ||
| M1b vs M0 | 1.88 (1.06-3.30) | 2.59 (1.32-5.06) | ||
| Fibrinogen plasma value | 0.355 | 0.313 | ||
| > 400 vs ≤ 400 | 1.15 (0.86-1.54) | 0.82 (0.54-1.21) | ||
| Survival | Tumour-related survival | |||
| 5-yr rate | P value | 5-yr rate | P value | |
| Stage I | 0.812 | 0.674 | ||
| Fibrinogen ≤ 400 mg/dL | 91.5 | 98.3 | ||
| Fibrinogen > 400 mg/dL | 88.2 | 95.2 | ||
| Stage II | 0.036 | 0.467 | ||
| Fibrinogen ≤ 400 mg/dL | 81.7 | 92.2 | ||
| Fibrinogen > 400 mg/dL | 74.0 | 92.1 | ||
| Stage III | 0.206 | 0.627 | ||
| Fibrinogen ≤ 400 mg/dL | 70.6 | 81.8 | ||
| Fibrinogen > 400 mg/dL | 60.8 | 85.0 | ||
| Stage IV | 0.566 | 0.213 | ||
| Fibrinogen ≤ 400 mg/dL | 30.0 | 31.8 | ||
| Fibrinogen > 400 mg/dL | 40.4 | 61.2 | ||
The main findings of this study are: (1) preoperative fibrinogen plasma levels correlate with the leading prognostic factors in patients undergoing CRC surgery; (2) long-term survival and tumour-related survival appear to be worse in patients with hyperfibrinogenemia; and (2) the prognostic value of the preoperative fibrinogen plasma level is not confirmed by multivariate analysis or by stratification of patients according to completeness of tumour resection (R0) and TNM stage.
It is now widely accepted that the outcome of cancer is mediated by an interaction between tumour-related factors and host factors, with chronic inflammation probably representing the main host-related factor. This explains why the correlation between inflammatory biomarkers and malignancies has been extensively studied[18-20]. Fibrinogen is a protein synthesized by hepatocytes, playing a pivotal role in coagulation, thrombosis, wound healing, and platelet aggregation, as well as in inflammatory states[21,22].
Although an increased plasma fibrinogen level is largely not specific and may occur in many physiological conditions (e.g., pregnancy or intense physical activity) and some pathological conditions (e.g., cardiovascular diseases, trauma and inflammatory diseases), a number of studies have demonstrated the existence of a correlation between high plasma fibrinogen levels and the development and progression of several tumours, including lung, pancreatic, gastric, and colorectal cancer[23-26].
Several mechanisms have been put forward to explain the increase of fibrinogen plasma levels in patients with cancer. First, tumour cells may ectopically produce fibrinogen itself or other cytokines involved in inflammation, such as IL-6, which ultimately trigger the production of fibrinogen in the liver[27]. Tumour growth is also frequently associated with hypercoagulability and hypoxia, with a subsequent increase in plasma fibrinogen levels[28,29]. Finally, cancer-related tissue injury causes a systemic inflammatory response and, consequently, increases the level of plasma fibrinogen.
Several lines of evidence apparently demonstrate that fibrinogen participates in tumourigenesis, although the actual process is not yet completely understood. Fibrinogen may enhance tumour cell proliferation, migration and signalling through interaction with multiple integrin and non-integrin receptors. It may also promote tumour angiogenesis, cooperating with growth factors such as vascular endothelial growth factor and fibroblast growth factors[30]. High levels of fibrinogen receptors, such as α5β1 and ανβ3 integrins, also promote the stable adhesion of tumour cells to the endothelium of target organs and are largely expressed on malignant cells. Notably, a protective role for fibrinogen against natural killer (NK) cells seems to be involved in the haematogenous metastatic potential of tumour cells. Fibrinogen may hence suppress NK cell activity for cancer cell clearance, thus increasing the number of metastatic cells[31]. Conversely, hyperfibrinogenemia does not seem to have a role in the metastatic involvement of lymphatic tissue since the lymphatic fluid does not contain platelets and the lymphatic endothelium has peculiar characteristics compared to the vascular endothelium[32].
A number of studies have shown a correlation between hyperfibrinogenemia and poor prognosis in patients with metastatic and non-metastatic CRC[33,34]. In accord with previous data[16,23], our experience, which represents one of the largest cohorts of CRC patients, confirmed the correlation between fibrinogen plasma levels and the most important prognostic factors, namely completeness of tumour resection (P < 0.001), the presence of systemic metastases (P < 0.001), pT category (P < 0.001), pN category (P = 0.001) and CEA serum level (P < 0.001). Similarly, long-term and tumour-related survival were associated with the presence of preoperative hyperfibrinogenemia. Unlike other studies, however, multivariate analysis and stratification of patients according to completeness of tumour resection (R0) and TNM stage failed to confirm the role of fibrinogen as an independent prognostic factor.
Son et al[33] reported that preoperative hyperfibrinogenemia was significantly associated with shorter survival in 624 patients with non-metastatic CRC when considering stage II and III separately[33]. Similar results were reported by Sun et al[35] in 255 patients with CRC and Tang et al[23] in 341 patients submitted to curative CRC surgery.
In previous studies, different cut-off values for preoperative plasma fibrinogen were used. Some studies identified the mean value as a prognostic threshold[23], others the median value[16] or the 25th percentile[33]. In our study, despite several threshold values being adopted (i.e., mean value, median value, 25th and 75th percentile) to evaluate significance of difference in survival analysis, hyperfibrinogenemia was not found to be an independent prognostic factor in multivariate analysis or after stratification of patients according to completeness of tumour resection and TNM stage (data not shown). In our series, the median preoperative plasma fibrinogen value was 409 mg/dL, which is very close to the upper limit of 400 mg/dL.
In conclusion, this study represents the first analysis of the value of preoperative fibrinogen plasma level in a Western country to the best of our knowledge. The analysis of our data demonstrates that preoperative fibrinogen plasma levels correlate with leading prognostic factors in patients undergoing surgery for CRC. Although long-term survival and tumour-related survival are worse in patients with hyperfibrinogenemia, these findings are not confirmed in multivariate analysis or after stratification of patients according to completeness of tumour resection and TNM stage. It seems reasonable to suggest that evaluation of the preoperative fibrinogen level is not helpful for predicting the prognosis of patients with appropriate TNM staging.
Fibrinogen is involved in tumourigenesis as well as tumour progression in several malignancies. Previous studies have shown hyperfibrinogenemia to be correlated with main clinicopathological characteristics and prognosis after colorectal cancer surgery. Nonetheless, the effective clinical significance of preoperative plasma fibrinogen levels as a prognostic marker after colorectal cancer surgery has not yet been determined.
This study represents the first analysis of the value of preoperative fibrinogen plasma level in a Western population and one of the largest cohort of patients.
This study based on a large Western cohort did not confirm hyperfibrinogenemia to be an independent prognostic factor in colorectal cancer patients.
Evaluation of fibrinogen plasma levels are routinely perform among preoperative blood tests. Its correlation with leading prognostic factors in patients undergoing surgery for colorectal cancer (CRC) is interesting and require further studies.
CRC is the third most common cancer worldwide. CRC is associated with a large range of fibrinolytic and procoagulant alterations and fibrinogen plasma levels could represent the expression of this relationship.
This paper tried to elucidate role of fibrinogen plasma level in the prediction of CRC prognosis. The manuscript is well written and the data and table are clear.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Crea F, Tong WD, Lakatos PL S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2014;59:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 865] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 2. | Costantini V, Zacharski LR. Fibrin and cancer. Thromb Haemost. 1993;69:406-414. [PubMed] |
| 3. | Dvorak HF, Nagy JA, Berse B, Brown LF, Yeo KT, Yeo TK, Dvorak AM, van de Water L, Sioussat TM, Senger DR. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci. 1992;667:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Bárdos H, Molnár P, Csécsei G, Adány R. Fibrin deposition in primary and metastatic human brain tumours. Blood Coagul Fibrinolysis. 1996;7:536-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Altieri DC, Mannucci PM, Capitanio AM. Binding of fibrinogen to human monocytes. J Clin Invest. 1986;78:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Dejana E, Languino LR, Polentarutti N, Balconi G, Ryckewaert JJ, Larrieu MJ, Donati MB, Mantovani A, Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985;75:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 3096] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 8. | Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Gross TJ, Leavell KJ, Peterson MW. CD11b/CD18 mediates the neutrophil chemotactic activity of fibrin degradation product D domain. Thromb Haemost. 1997;77:894-900. [PubMed] |
| 10. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 843] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 11. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 12. | Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 15. | Lee JH, Hyun JH, Kim DY, Yoo BC, Park JW, Kim SY, Chang HJ, Kim BC, Kim TH, Oh JH. The role of fibrinogen as a predictor in preoperative chemoradiation for rectal cancer. Ann Surg Oncol. 2015;22:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Yamashita H, Kitayama J, Taguri M, Nagawa H. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J Surg. 2009;33:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Pedrazzani C, Lauka L, Sforza S, Ruzzenente A, Nifosì F, Delaini G, Guglielmi A. Management of nodal disease from colon cancer in the laparoscopic era. Int J Colorectal Dis. 2015;30:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Pedrazzani C, Cerullo G, Marrelli D, Fernandes E, Carlucci F, Corso G, Bettarini F, De Stefano A, Roviello F. Is circulating D-dimer level a better prognostic indicator than CEA in resectable colorectal cancer? Our experience on 199 cases. Int J Biol Markers. 2010;25:171-176. [PubMed] |
| 19. | Roxburgh CS, Crozier JE, Maxwell F, Foulis AK, Brown J, McKee RF, Anderson JH, Horgan PG, McMillan DC. Comparison of tumour-based (Petersen Index) and inflammation-based (Glasgow Prognostic Score) scoring systems in patients undergoing curative resection for colon cancer. Br J Cancer. 2009;100:701-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, Stift A, Filipits M, Kornek G, Nkenke E. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:960-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96:711-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 674] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 23. | Tang L, Liu K, Wang J, Wang C, Zhao P, Liu J. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J Surg Oncol. 2010;102:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H. Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer. 2006;6:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Jones JM, McGonigle NC, McAnespie M, Cran GW, Graham AN. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006;53:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Yamaguchi T, Yamamoto Y, Yokota S, Nakagawa M, Ito M, Ogura T. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302-3309. [PubMed] |
| 29. | Wenger RH, Rolfs A, Marti HH, Bauer C, Gassmann M. Hypoxia, a novel inducer of acute phase gene expression in a human hepatoma cell line. J Biol Chem. 1995;270:27865-27870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost. 2008;6:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci USA. 2002;99:16069-16074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 33. | Son HJ, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, Park SC, Choi HS, Oh JH. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20:2908-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 34. | Kawai K, Kitayama J, Tsuno NH, Sunami E, Nagawa H. Hyperfibrinogenemia after preoperative chemoradiotherapy predicts poor response and poor prognosis in rectal cancer. Int J Colorectal Dis. 2011;26:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Sun ZQ, Han XN, Wang HJ, Tang Y, Zhao ZL, Qu YL, Xu RW, Liu YY, Yu XB. Prognostic significance of preoperative fibrinogen in patients with colon cancer. World J Gastroenterol. 2014;20:8583-8591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |