Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9623
Peer-review started: May 8, 2016
First decision: June 20, 2016
Revised: July 11, 2016
Accepted: July 31, 2016
Article in press: August 1, 2016
Published online: November 21, 2016
Processing time: 195 Days and 18.5 Hours
To determine the outcomes of partial splenic embolization (PSE) for massive splenomegaly due to idiopathic portal hypertension (IPH).
In this prospective study, we evaluated the characteristics and prognosis of consecutive patients with IPH who underwent PSE for all indications at a single medical center between June 2009 and January 2015. The inclusion criteria were: presence of hypersplenism, massive splenomegaly, and resultant pancytopenia. The exclusion criteria were: presence of other diseases causing portal hypertension. During the post-PSE period, the patients were hospitalized. All patients underwent abdominal computed tomography imaging 4 wk post-PSE to determine total splenic and non-infarcted splenic volumes.
A total of 11 patients, with median age of 33.27 ± 4.8 years, were included in the study. Mean spleen size was 22.9 cm (21-28 cm), and severe hypersplenism was diagnosed in all patients before PSE. Post-PSE, leukocyte and platelet counts increased significantly, reaching peak levels in the second week with gradual decreases thereafter. Liver function tests did not exhibit significant changes during post-intervention follow-up. All patients developed post-embolization syndrome, and one patient experienced serious complications; all complications were successfully treated with conservative therapy and no death occurred.
Our findings showed that PSE has a lower complication rate than previously-reported surgical complication rates, which supports this intervention as a viable alternative for high-risk operable patients with severe hypersplenism.
Core tip: Partial splenic embolization (PSE) for hypersplenism is a novel percutaneous interventional method, has emerged as an alternative to surgery and is a viable approach in high-risk and inoperable patients with portal hypertension. The current trial, which is the largest study in the current literature, confirmed the safety and efficacy of PSE in patients with idiopathic portal hypertension (IPH) regarding complications and morbidity. PSE could be offered to patients with massive splenomegaly due to IPH, with low and manageable complication rates, as this study has confirmed.
- Citation: Ozturk O, Eldem G, Peynircioglu B, Kav T, Görmez A, Cil BE, Balkancı F, Sokmensuer C, Bayraktar Y. Outcomes of partial splenic embolization in patients with massive splenomegaly due to idiopathic portal hypertension. World J Gastroenterol 2016; 22(43): 9623-9630
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9623.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9623
Idiopathic portal hypertension (IPH) is a rare clinical disorder characterized by high portal hypertension (PH) in the absence of cirrhosis or other PH etiologies, such as hepatic or portal vein thrombosis (PVT), hematological disorders, cardiac failure, and parasites of the hepatobiliary system[1-4]. Other diagnostic criteria for IPH include normal liver function tests, the presence of esophageal or gastric varices, and abnormal liver imaging and liver biopsy findings inconclusive of cirrhosis[1,5]. Previously, splenomegaly in IPH was reported as a disease that is distinct from liver cirrhosis[6,7]. IPH is more frequent in Eastern than in Western countries, and in Japan is reported to be a female predominant disease, with rates three times higher than those in males[8]. The most important finding in IPH is the absence of cirrhotic pathology. Pathological examination mostly shows non-specific changes such as phlebosclerosis, aberrant periportal vessels, sinusoidal dilatation, portal fibrosis, and nodular regenerative hyperplasia without fibrosis[1,2]. Many theories have been proposed with potential involvement of immunological disorders, trace metals, recurrent infections, medications, and prothrombotic factors as etiological factors in order to explain the pathogenesis; however, it is still not completely understood. According to a widely accepted hypothesis, IPH is an immunological disorder resulting from continuous antigenic stimulation, which is based on the observation of high serum immunoglobulin levels in patients with IPH[2,8,9].
Patients with IPH mostly present with signs and symptoms of PH, including anemia, leukopenia, thrombocytopenia, splenomegaly, esophageal and fundal varices, and variceal bleeding. Other rarely observed complications are PVT, ascites, and hepatic encephalopathy[1,2,9]. Recurrent variceal bleeding is the most critical IPH complication that is usually fatal if not treated or prevented. Thus, variceal bleeding management is the most important therapeutic goal in these patients. However, repetitive endoscopic sclerotherapy and variceal ligation do not resolve the underlying cause of PH, and thus re-bleeding risk is higher in these patients[9-14].
Hypersplenism, which often develops in patients with IPH as a result of PH, is due to hyperactivity of the spleen and defined as a triad of splenomegaly, pancytopenia, and normocellularity of bone marrow. Cytopenia particularly affects the platelets, and the resulting thrombocytopenia may cause spontaneous bleeding, in particular, difficult-to-control variceal bleeding varices[1,6,15].
Serious cytopenia may be prevented by several approaches aimed at decreasing splenic volume in patients with hypersplenism, which include surgical and percutaneous interventional methods[16-18]. Partial splenic embolization (PSE) for hypersplenism is a novel percutaneous interventional method, has emerged as a viable alternative to surgery[19-21] and is effective in reducing the size of splenic parenchyma by embolization of the major branches of splenic artery[6,20,21]. PSE can be particularly useful in high-risk surgical patients, such as those with co-morbid diseases. Patients with thrombocytopenia, including cancer patients receiving chemotherapy and patients with co-morbidities such as advanced heart and lung disease, are considered inoperable because of a high frequency of complications, even in the setting of simple splenectomy[2,22-25].
PSE has been gaining popularity among patients with hypersplenism and PH due to various reasons. We would like to share our experience with PSE in IPH patients, in terms of efficacy, complications and prognosis.
This study was approved by the local ethics committee (Study No: GO 15/432) of Hacettepe University Hospital. We prospectively reviewed the medical records of 21 consecutive patients who underwent PSE for all indications between June 2009 and January 2015 at Hacettepe University Hospital. Demographic characteristics, laboratory results, endoscopic reports, radiological imaging studies and immunohistochemical analyses were retrospectively collected via the electronic medical records. The inclusion criteria were laboratory and clinical findings of hypersplenism and massive splenomegaly in patients with IPH. According to current literature, massive splenomegaly was accepted as a spleen with the longest axis being > 20 cm, and cytopenia was accepted as a platelet count of ≤ 60 × 10³/mm³ and/or a leukocyte count of ≤ 3.0 × 10³/mm³[26]. Hypersplenism was diagnosed by clinical examination, laboratory findings and radiological imaging methods, such as ultrasonography and computed tomography (CT). The exclusion criteria were presence of other PH etiologies, including cirrhosis, infection, hematological or parasitic disease, or occlusion of the hepatic or portal veins.
Patients were hospitalized before the PSE procedure; full clinical examination, detailed medical history and laboratory tests, including complete blood count (CBC), liver and kidney function tests and serum electrolytes, were performed for all patients. All of the patents underwent endoscopy (to evaluate varices), abdominal ultrasonography and abdominal CT angiography prior to PSE. All patients received Haemophilus influenzae, meningococcal and pneumococcal vaccines at 4 wk before the embolization procedure.
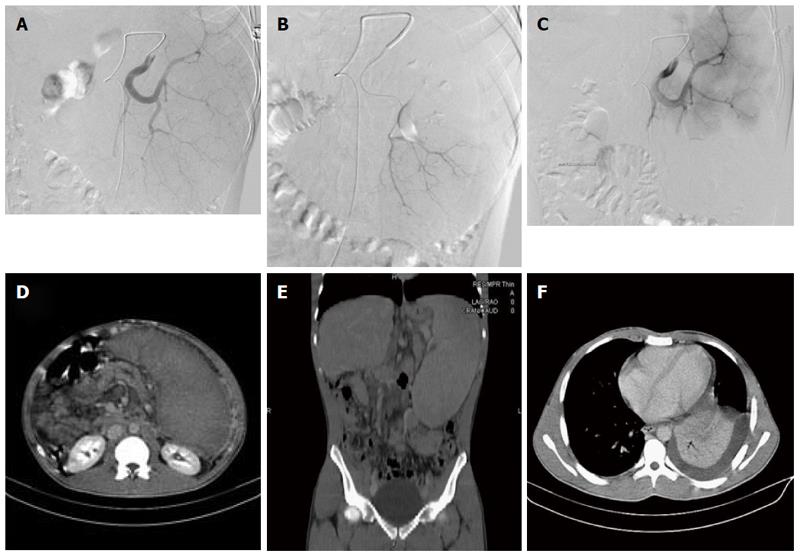
All patients underwent PSE under intravenous conscious sedation with intravenous antibiotic prophylaxis (ampicillin + sulbactam 1 gr, 3 times a day) administered 30 min prior to intervention and continued for 1 wk after the procedure. Under sterilized conditions, abdominal angiogram following common femoral artery access was obtained with a 4 or 5 F pigtail catheter. Next, selective catheterization of the splenic artery was performed with a 4 or 5F C2 catheter to evaluate splenic and pancreatic artery branches. A 2.8F microcatheter (Cantata® Cook Medical) was used to super selectively catheterize distal splenic vascular branches with respect to the vascular anatomy (Figure 1A). Superior pole branches were protected to prevent development of pleural effusion, and middle and lower pole branches were targeted. First, 500-700-μm tris-acryl gelatin microspheres (Embosphere® Merit Medical) were administrated to the targeted branch, followed by infusion of intra-arterial gentamicin sulfate solution. Embolization was continued with larger particles, if needed, to obtain stagnant flow. These steps were repeated for every targeted branch until the targeted volume of two-thirds of the spleen was achieved (Figure 1B and C). Following a final angiogram, manual compression was applied to achieve hemostasis of the femoral access site.
Post-PSE, all patients were hospitalized and followed-up by physical examination, CBC and laboratory tests. Supportive care was provided with hydro-electrolyte infusion, and paracetamol, nonsteroidal anti-inflammatory drug or morphine was prescribed for analgesia. Appropriate medical treatment was given if complications arose. At 4 wk post-PSE, all patients underwent triphasic abdominal CT imaging to calculate total splenic and noninfarcted splenic volumes (Figure 1D).
The statistical methods of this study were reviewed by Kav T and Ozturk O. Data were analyzed with the SPSS 23 software. Data were expressed as mean± standard deviation. Categorical variables were compared with the χ2 test. Spearman’s rank correlation test was used for correlation analysis. Statistical significance was set at a P-value of less than 0.05.
A total of 21 patients underwent PSE between June 2009 and January 2015 at our hospital, of which 10 patients were excluded from the study (6 patients with splenic artery aneurysm, 2 patients with cirrhosis, 1 patient with autoimmune hemolytic anemia, and 1 patient with splenic hemorrhage, following the drainage of a massive hydatid cyst). Thus, 11 patients (7 males and 4 females; mean age: 33.27 years; range: 18-48 years) with hypersplenism and massive splenomegaly caused by IPH who underwent PSE were included in the final analysis. Past medical history revealed 1 patient with Hodgkin’s lymphoma in remission, 2 patients with concomitant splenic artery aneurysm, and 1 patient with thalassemia intermedia with no need for transfusions; the other patients did not have a remarkable medical history.
PSE was successfully performed on all 11 patients. The mean duration of follow-up after IPH diagnosis was 6.5 years (ranging from 4 mo to 12 years). Liver function test results were normal in all patients, and none of the patients had ascites. Massive splenomegaly (mean spleen size: 22.9 cm; range: 21-28 cm) (Figure 1E) and signs of hypersplenism, such as anemia, leukopenia and thrombocytopenia, were detected in all patients. Esophageal varices were present in all patients; two patients also had fundal varices. Six patients had no history of variceal bleeding, whereas the remaining 5 patients had a history of esophageal variceal bleeding. In addition, 1 patient had experienced bleeding from both esophageal and fundal varices and received appropriate endoscopic interventions like variceal ligation and sclerotherapy. Demographic and clinical characteristics of the patients are summarized in Table 1.
| Patient No. | Age | Sex, male/female | Concomitant diseases | Spleen size, cm | Esophageal varices | Gastric varices | Variceal bleeding | Variceal therapy |
| 1 | 29 | M | - | 28 | + | + | Esophageal | Band ligation |
| 2 | 33 | M | - | 22 | + | + | Esophageal and gastric | Band ligation and glue injection |
| 3 | 48 | F | Concomitant 3-cm splenic artery aneurysm | 21 | + | + | - | - |
| 4 | 43 | F | - | 25 | + | - | Esophageal | Band ligation |
| 5 | 40 | F | Concomitant 4-cm splenic artery aneurysm | 20 | + | - | - | - |
| 6 | 25 | F | - | 24 | + | - | - | - |
| 7 | 42 | M | Hodgkin's lymphoma | 21 | + | - | - | - |
| 8 | 36 | M | - | 21 | + | - | - | - |
| 9 | 18 | M | Thalassemia intermedia | 23 | + | - | Esophageal | Sclerotherapy and band ligation |
| 10 | 23 | M | - | 24 | + | - | - | - |
| 11 | 29 | M | - | 23 | + | - | Esophageal | Band ligation |
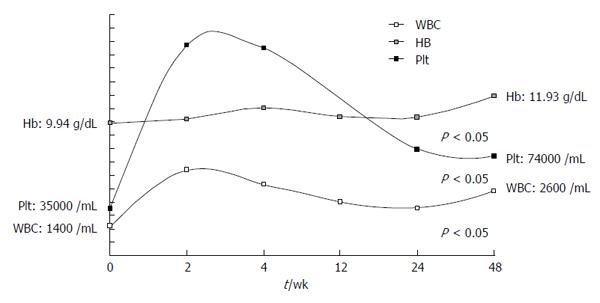
Mean white blood cell (WBC) count at postoperative weeks 2, 4 and 48 was significantly higher than the preoperative values (P < 0.05). Mean hemoglobin values were not statistically significant compared to preoperative values for the first two visits at weeks 2 and 4, but values progressively increased and were significantly higher at the end of follow-up at week 48 (P = 0.028). Platelet values were also significantly higher compared to preoperative values, and this was maintained at week 48 (P < 0.05). CBC values for post-PSE follow-up weeks are summarized in Figure 2.
No significant changes were detected in liver function parameters, including alanine aminotransferase, aspartate aminotransferase, albumin, bilirubin and prothrombin time, in both short- and long-term follow-up.
Triphasic CT imaging of the abdomen obtained at post-PSE week 4 detected splenic infarction at rates of 60%-70%, > 70% and complete in 7, 3 and 1 patients, respectively. In our study, we failed to observe positive correlations between the leukocyte or platelet counts and splenic infarction rate during follow-up (P > 0.05).
Post-embolization syndrome (defined by the presence of abdominal pain, fever and vomiting) developed in all patients (100%), all of whom were managed with administration of subcutaneous morphine and per oral acetaminophen, if needed. Transient pleural effusion developed in 3 patients, but none of the patients required drainage of effusion. Asymptomatic small and subsegmental left atelectasis occurred in 2 patients (Figure 1F). Hematoma of the femoral artery puncture site was observed in 1 patient. Antibiotic-associated gastroenteritis developed in 1 patient. Recently developed ascites were observed in 2 patients, which spontaneously resolved. All of the above-mentioned PSE-associated complications were successfully treated with conservative therapy. Complications experienced by patients with IPH undergoing PSE during the study period are summarized in Table 2. Other serious complications were: large-volume pleural effusion with dyspnea and chest pain, which developed in 1 patient; later in the follow-up period, the same patient developed pneumonia and syndrome of inappropriate antidiuretic hormone secretion (SIADH) and was successfully treated with drainage of effusion, intensive antibiotic and conservative therapy.
| Patient No. | Hospitalization (d) | Splenic infarction ratio | Post-embolization syndrome | Other complications |
| 1 | 16 | 60-70 | + | Atelectasis and pleural effusion |
| 2 | 8 | 100 | + | Subileus and diarrhea due to antibiotics |
| 3 | 21 | 60-70 | + | Development of ascites |
| 4 | 45 | 60-70 | + | Pneumonia, pleural effusion, SIADH |
| 5 | 14 | 60-70 | + | - |
| 6 | 21 | 75 | + | - |
| 7 | 18 | 75 | + | - |
| 8 | 9 | 80 | + | - |
| 9 | 6 | 60-70 | + | Hematoma placing femoral catheter |
| 10 | 10 | 60-70 | + | Atelectasis, pleural effusion, and development of ascites |
| 11 | 8 | 60-70 | + | - |
To the best of our knowledge, this is the largest study that has evaluated the outcomes of PSE in patients with IPH. The majority of previous reports on patients with IPH undergoing PSE are case series. The largest one, reported by Romano et al[2], had only 6 patients; otherwise, PSE has been performed mostly in cirrhotic patients[6,14,24,26].
The spleen is a multi-functional organ that serves as a defensive barrier against infections and a reservoir for circulating blood volume. It also has critical functions in hematopoiesis and protection against malignancy[24,27]. The mean craniocaudal length (CCL) of a normal spleen is 11-12 cm and its approximate weight is 150 g[28]. Classification of splenomegaly by Poulin et al[29] defines a spleen with a CCL between 11-20 cm as having moderate splenomegaly and a CCL of > 20 cm as having massive splenomegaly. The latter is an uncommon clinical presentation, with florid hematological manifestations resulting from hypersplenism[2,6,14,24]. In our study, all patients had massive splenomegaly causing severe hypersplenism.
Thrombocytopenia is the most common symptom of hypersplenism and can cause spontaneous bleeding, complicating the successful control of variceal bleeding. Thrombocytopenia prevention can be achieved by decreasing splenic volume in patients with hypersplenism. In addition, PSE decreases the incidence of gastrointestinal bleeding caused by esophageal and fundal variceal rupture in patients with cirrhosis and IPH[2,6,14]. In several studies, PSE was reported as an effective approach for bleeding from esophageal and fundal varices[30,31]. In the current series, none of the patients experienced gastrointestinal bleeding due to esophageal or fundal varices throughout the follow-up period. Furthermore, Romano et al[2] reported that the severity of esophageal and fundal varices decreased in patients with IPH post-PSE; in some cases, they even disappeared. They also reported that variceal bleeding did not recur after intervention. Our results are in agreement with these previously published reports; none of the patients had variceal bleeding during the post-PSE follow-up period.
Serious cytopenia caused by hypersplenism may be prevented by a number of interventions. Several approaches, including surgery, PSE, total splenic arterial embolization, placement of a narrow stent into the splenic artery, transjugular intrahepatic portosystemic shunt, and ligation or banding of the splenic artery, have been suggested[2,14,16-18]. Patients with hypersplenism are at a higher risk of developing complications if they undergo surgery, with a rate ranging from 9% to 27%, including postoperative PVT, sepsis and multi-organ failure, abscess and death. In addition, PSE requires several days of hospitalization and is considered as an alternative method to treat hypersplenism[26,32-34].
PSE was first described in a male patient with cirrhosis that was complicated by recurrent gastrointestinal bleeding from esophageal varices by Maddison in 1973[7]; PSE was successful in preventing the bleeding. Since then, multiple studies demonstrated the technical feasibility and efficacy of PSE for improving cytopenia. PSE has favorable outcomes in a variety of splenectomy indications, such as hypersplenism, splenic artery aneurysms, gastric variceal hemorrhage due to splenic vein thrombosis or PH, intra-operative blood loss during splenectomy and in hematological disorders, including idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia and hereditary spherocytosis[2,6,14,23-26].
Occlusion of the splenic arterial supply during PSE leads to a decrease in splenic size secondary to ischemic necrosis. The splenic infarction rate appears to be an important indicator of PSE efficacy for the treatment of IPH-associated hypersplenism[2,24,26]. Studies showed that embolization of < 50% of the spleen was associated with shorter hypersplenism relapse times, suggesting that PSE should cover a minimum of 50% of the spleen to be effective[6,9,14]. Zhu et al[14] and Noguchi et al[31] reported that the success of PSE positively correlated with the rate of splenic infarction. A study by Hayashi et al[35] concluded that, infarction volume was the best predictor of increases in leukocyte and platelet counts in patients with hypersplenism due to segmental PVT. Furthermore, Zhu et al[14] demonstrated that, the long-term efficacy of PSE and improvement in cytopenia directly correlated with the rate of splenic infarction[14]. Consistent with previous reports, we found that platelet counts increased post-PSE, peaking at 2 wk, and followed by a gradual decrease during the follow-up period. Splenic volume has been shown to increase with the regeneration of residual splenic tissue after PSE[2,6,14,26]. In cases with the re-emergence of cytopenia, cell counts are much higher than the levels prior to the procedure, and a repeat PSE may be required in these patients[2,14,26].
In the current study, no significant changes were observed in hepatic enzyme levels post-PSE. Similarly, Tajiri et al[20] reported that hepatic enzyme levels did not change over an 8-year follow-up period post-PSE.
PSE is associated with various complications, such as post-embolization syndrome, decompensation, ascites, edema, abscess, sepsis, bleeding and respiratory problems. Most of these can be treated by conservative therapy and supportive care[2,6,14,26,36].
In concordance with findings by other groups, post-embolization syndrome was the most frequent side effect post-PSE in our study; all patients developed post-embolization syndrome and were successfully treated with conservative therapy. Post-embolization syndrome is the most frequent side effect of all of solid organ embolizations[35-38]. One patient in whom a large-volume pleural effusion developed during the follow-up period had pneumonia and SIADH post-PSE; she was treated with drainage of effusion, antibiotics and conservative therapy. To the best of our knowledge, SIADH in patients with IPH undergoing PSE has not been reported previously; as such, we believe that the SIADH observed in our patient was due to pneumonia. Although major complications are not frequently observed in patients with IPH post-PSE, they occur more frequently and are associated with increased mortality in cirrhotic patients. In comparison with PSE, splenectomy is associated with more frequent major and minor complications[32-34,36]. Several studies reported a positive correlation between the splenic infarction rate and the complication rates. Because complications were observed less in patients with a splenic infarction region of ≤ 50%, compared with those with a splenic infarction region of ≥ 70%, it was recommended that the splenic infarction rate should not exceed 70% in order to decrease complications[14,37,38]. However, our patients with 100% splenic infarction region did not develop serious complications. Therefore, a high splenic infarction rate in IPH may not be as dangerous as reported for those with cirrhosis.
PSE continues to be associated with a lower complication rate than that of surgery and is a viable alternative approach in high-risk and inoperable patients. In addition, the functional residual spleen with PSE provides protection against infections, a significant post-splenectomy complication.
Idiopathic portal hypertension (IPH) is a rare clinical disorder characterized by high portal hypertension (PH) in the absence of cirrhosis or other PH etiologies, such as hepatic or portal vein thrombosis, hematological disorders, cardiac failure and parasites of the hepatobiliary system. Patients with IPH mostly present with signs and symptoms of PH, including cytopenia and hypersplenism variceal bleeding. PSE has been recognized more commonly in patients with hypersplenism and PH due to reasons other than cirrhosis. The current trial was reported with the aim of sharing our experience with partial splenic embolization (PSE) in IPH patients, in terms of efficacy, complications and prognosis.
PSE continues to be associated with a lower complication rate than that of surgery. In addition, the functional residual spleen with PSE provides protection against infections, a significant post-splenectomy complication.
PSE for hypersplenism is a novel percutaneous interventional method, has emerged as an alternative to surgery and is a viable approach in high-risk and inoperable patients. The current trial, which is the largest study to date, confirmed the safety and efficacy of PSE in patients with IPH regarding complications and morbidity.
All of the patients in this study were experiencing complications of deep pancytopenia and were not candidates for splenectomy. PSE could be offered to patients with massive splenomegaly due to idiopathic portal hypertension, with low and manageable complication rates, as this study has confirmed.
PSE for hypersplenism, due to massive splenomegaly of non-cirrhotic portal hypertension, is a percutaneous interventional method for reducing the size of splenic parenchyma effectively by embolization of the major branches of the splenic artery and is a minimal invasive alternative to surgery.
The authors have described 11 patients with IPH who underwent partial splenic embolization.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bihari C, Manenti A S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Harmanci O, Bayraktar Y. Clinical characteristics of idiopathic portal hypertension. World J Gastroenterol. 2007;13:1906-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Romano M, Giojelli A, Capuano G, Pomponi D, Salvatore M. Partial splenic embolization in patients with idiopathic portal hypertension. Eur J Radiol. 2004;49:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Boyer JL, Sen Gupta KP, Biswas SK, Pal NC, Basu Mallick KC, Iber FL, Basu AK. Idiopathic portal hypertension. Comparison with the portal hypertension of cirrhosis and extrahepatic portal vein obstruction. Ann Intern Med. 1967;66:41-68. [PubMed] |
| 4. | Okuda K, Nakashima T, Okudaira M, Kage M, Aida Y, Omata M, Musha H, Futagawa S, Sugiura M, Kameda H. Anatomical basis of hepatic venographic alterations in idiopathic portal hypertension. Liver. 1981;1:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Tanaka T, Sugawara Y, Kokudo N. The current clinical aspects of idiopathic portal hypertension. Intractable Rare Dis Res. 2013;2:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Petermann A, Chabrot P, Cassagnes L, Dumousset E, Alfidja A, Gageanu C, Ravel A, Abergel A, Boyer L. Hypersplenism due to portal hypertension: retrospective evaluation of 17 patients treated by splenic embolization. Diagn Interv Imaging. 2012;93:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Maddison F. Embolic therapy for hypersplenism. Invest Radiol. 1973;8:280-283. |
| 8. | Okudaira M, Ohbu M, Okuda K. Idiopathic portal hypertension and its pathology. Semin Liver Dis. 2002;22:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, Turon F, Hernandez-Gea V, Bosch J, Garcia-Pagán JC. Idiopathic portal hypertension: natural history and long-term outcome. Hepatology. 2014;59:2276-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Schouten JN, Van der Ende ME, Koëter T, Rossing HH, Komuta M, Verheij J, van der Valk M, Hansen BE, Janssen HL. Risk factors and outcome of HIV-associated idiopathic noncirrhotic portal hypertension. Aliment Pharmacol Ther. 2012;36:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 12. | Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, Lesmana LA, Guha Mazumder D, Omata M, Qureshi H. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Cazals-Hatem D, Hillaire S, Rudler M, Plessier A, Paradis V, Condat B, Francoz C, Denninger MH, Durand F, Bedossa P. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011;54:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Zhu K, Meng X, Qian J, Huang M, Li Z, Guan S, Jiang Z, Shan H. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig Liver Dis. 2009;41:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Ohmoto K, Yamamoto S. Prevention of variceal recurrence, bleeding, and death in cirrhosis patients with hypersplenism, especially those with severe thrombocytopenia. Hepatogastroenterology. 2003;50:1766-1769. [PubMed] |
| 16. | Owman T, Lunderquist A, Alwmark A, Borjesson B. Embolization of the spleen for treatment of splenomegaly and hypersplenism in patients with portal hypertension. Invest Radiol. 1979;14:457-464. [PubMed] |
| 17. | Miyake Y, Ando M, Kaji E, Toyokawa T, Nakatsu M, Hirohata M. Partial splenic embolization prior to combination therapy of interferon and ribavirin in chronic hepatitis C patients with thrombocytopenia. Hepatol Res. 2008;38:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kimura F, Ito H, Shimizu H, Togawa A, Otsuka M, Yoshidome H, Shimamura F, Kato A, Nukui Y, Ambiru S. Partial splenic embolization for the treatment of hereditary spherocytosis. AJR Am J Roentgenol. 2003;181:1021-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Liu JD, Chang CC, Chen YY. Evaluation of the effect of partial splenic embolization on platelet values for liver cirrhosis patients with thrombocytopenia. World J Gastroenterol. 2007;13:619-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Tajiri T, Onda M, Yoshida H, Mamada Y, Taniai N, Kumazaki T. Long-term hematological and biochemical effects of partial splenic embolization in hepatic cirrhosis. Hepatogastroenterology. 2002;49:1445-1448. [PubMed] |
| 21. | Yoshida H, Mamada Y, Taniai N, Tajiri T. Partial splenic embolization. Hepatol Res. 2008;38:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Kauffman CR, Mahvash A, Kopetz S, Wolff RA, Ensor J, Wallace MJ. Partial splenic embolization for cancer patients with thrombocytopenia requiring systemic chemotherapy. Cancer. 2008;112:2283-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Pålsson B, Hallén M, Forsberg AM, Alwmark A. Partial splenic embolization: long-term outcome. Langenbecks Arch Surg. 2003;387:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Firat A, Boyvat F, Moray G, Aytekin C, Karakayali H, Haberal M. Comparison of two different percutaneous splenic artery interventions in the treatment of hypersplenism: preliminary report. Transplant Proc. 2005;37:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Durusu Tanrıöver M, Peynircioğlu B, Ergan Arsava B, Topeli İskit A. Splenic artery embolization: An alternative approach in a critically ill patient with autoimmune hemolytic anemia. Turk J Haematol. 2011;28:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Elmonem SA, Tantawy HI, Ragheb AS, Matar NEH, Tantawi I. The outcome of partial splenic embolization for hypersplenism in the cirrhotic patients. The Egyptian J Radio a Nuc Med. 2011;42:35-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | McCormick PA, Murphy KM. Splenomegaly, hypersplenism and coagulation abnormalities in liver disease. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:1009-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Elmakkı E. Hypersplenism: Review article. J Biol Agric Healthc. 2012;2:89-97. |
| 29. | Poulin EC, Mamazza J, Schlachta CM. Splenic artery embolization before laparoscopic splenectomy. An update. Surg Endosc. 1998;12:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Koconis KG, Singh H, Soares G. Partial splenic embolization in the treatment of patients with portal hypertension: a review of the english language literature. J Vasc Interv Radiol. 2007;18:463-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Noguchi H, Hirai K, Aoki Y, Sakata K, Tanikawa K. Changes in platelet kinetics after a partial splenic arterial embolization in cirrhotic patients with hypersplenism. Hepatology. 1995;22:1682-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Winslow ER, Brunt LM. Perioperative outcomes of laparoscopic versus open splenectomy: a meta-analysis with an emphasis on complications. Surgery. 2003;134:647-653; discussion 654-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Watanabe Y, Horiuchi A, Yoshida M, Yamamoto Y, Sugishita H, Kumagi T, Hiasa Y, Kawachi K. Significance of laparoscopic splenectomy in patients with hypersplenism. World J Surg. 2007;31:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Hayashi H, Beppu T, Masuda T, Mizumoto T, Takahashi M, Ishiko T, Takamori H, Kanemitsu K, Hirota M, Baba H. Predictive factors for platelet increase after partial splenic embolization in liver cirrhosis patients. J Gastroenterol Hepatol. 2007;22:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Guan YS, Hu Y. Clinical application of partial splenic embolization. ScientificWorldJournal. 2014;2014:961345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Matsumoto T, Yamagami T, Terayama K, Kato T, Hirota T, Yoshimatsu R, Miura H, Ito H, Okanoue T, Nishimura T. Risk factors and clinical course of portal and/or splenic vein thrombosis after partial splenic embolization. Acta Radiol. 2009;50:617-623. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | N’Kontchou G, Seror O, Bourcier V, Mohand D, Ajavon Y, Castera L, Grando-Lemaire V, Ganne-Carrie N, Sellier N, Trinchet JC. Partial splenic embolization in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol. 2005;17:179-184. [PubMed] |