Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9571
Peer-review started: June 17, 2016
First decision: July 12, 2016
Revised: August 2, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: November 21, 2016
Processing time: 160 Days and 2 Hours
To determine whether diabetes mellitus (DM) affects prognosis/recurrence after liver transplantation (LT) for hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).
A retrospective study was conducted between January 2000 and August 2013 on 1631 patients with HBV-related HCC who underwent LT with antiviral prophylaxis. Patient data were obtained from the China Liver Transplant Registry (https://www.cltr.org/). To compare the outcomes and tumor recurrence in the HBV-related HCC patients with or without DM, statistical analyses were conducted using χ2 tests, Mann-Whitney tests, the Kaplan-Meier method, log-rank tests and multivariate step-wise Cox regression analysis.
Univariate analysis of 1631 patients who underwent LT found overall 1-, 3- and 5-year survival rates of 79%, 73% and 71% respectively in the DM patients, and 84%, 78% and 76% in the non-DM patients respectively. Overall survival rate differences after LT between the two groups were significant (P = 0.041), but recurrence-free survival rates were not (P = 0.096). By stratified analysis, the overall survival rates in DM patients for age > 50 years (P = 0.002), the presence of vascular invasion (P = 0.096), tumors ≤ 3 cm (P = 0.047), two to three tumor nodules (P = 0.007), Child-Pugh grade B (P = 0.018), and pre-LT alanine aminotransferase levels between 40 and 80 IU/L (P = 0.017) were significantly lower than in non-DM patients. Additionally, serum α-fetoprotein level > 2000 ng/mL (P = 0.052) was associated with a significant survival difference trend between DM and non-DM patients. Multivariate analysis showed that the presence of DM (P < 0.001, HR = 1.591; 95%CI: 1.239-2.041) was an independent predictor associated with poor survival after LT.
HBV-related HCC patients with DM have decreased long-term overall survival and poor LT outcomes. Prevention strategies for HCC patients with DM are recommended.
Core tip: Diabetes mellitus (DM) may be associated with hepatocellular carcinoma (HCC) pathological features, old age and poor liver function, which may influence post-liver transplantation (LT) survival in hepatitis B virus (HBV)-related HCC patients. However, no direct relation between DM and HBV infection or HBV load were observed in HCC patients. The current data represent a multi-center study involving a larger number of samples, and suggest that DM may affect the long-term post-LT survival of HBV-related HCC patients, but not of tumor recurrence. Thus, HCC patients with DM undergoing LT should be closely followed to optimize outcomes. Follow-up examinations are especially important among DM patients who are elderly or who have the presence of vascular invasion and serum α-fetoprotein levels > 2000 ng/mL pre-LT.
- Citation: Zhang Q, Deng YL, Liu C, Huang LH, Shang L, Chen XG, Wang LT, Du JZ, Wang Y, Wang PX, Zhang H, Shen ZY. Diabetes mellitus may affect the long-term survival of hepatitis B virus-related hepatocellular carcinoma patients after liver transplantation. World J Gastroenterol 2016; 22(43): 9571-9585
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9571
Of the 350 million individuals infected with hepatitis B virus (HBV) worldwide, there are 130 million HBV carriers and 30 million chronically HBV-infected people in China[1,2]. The incidence of hepatocellular carcinoma (HCC) in China is dramatically increased by HBV infection, which is the main cause of HCC[3,4]. Over 70% of cases of HCC in China have been associated with HBV infection[5].
Diabetes mellitus (DM) is the sixth leading cause of death in China, and its prevalence in the adult Chinese population increased to 11.7% in 2010[6]. Recent studies have shown that DM may be a potential risk factor for HCC[5,7,8], and this hypothesis has been supported by several case control[9-12] and cohort studies[13-17]. Additionally, increased HCC prevalence in the diabetic population has been reported, possibly due to steatohepatitis[7,9]. However, it is unknown whether HBV infection acts directly or additively with steatohepatitis on the incidence of HCC in DM patients. Liver transplantation (LT) has been established as the only curative method for simultaneous treatment of HCC and cirrhosis[18,19], and is generally accepted as superior for early HCC with cirrhosis compared to hepatic resection[20,21].
There has been recent debate about DM as an independent risk factor for post-treatment HCC recurrence and poor outcomes. Some studies have suggested that DM increases the risk of HCC recurrence and poor patient outcomes in patients undergoing hepatic resection[22]. However, other studies reported that DM does not worsen the prognosis of HCC patients after resection[23,24]. Few studies have investigated the association between HBV, DM, tumor recurrence and long-term survival of HCC patients who underwent LT. The aim of this study was to investigate the potential associations between pre-existing DM with HBV-induced HCC, HCC recurrence and long-term survival after LT in a large cohort.
Between January 2000 and August 2013, 2128 patients were diagnosed with primary HCC and underwent LT at the General Hospital of Chinese People’s Armed Police Force, Tianjin First Central Hospital and First Affiliated Hospital, School of Medicine, Xi’an Jiaotong University (data from the China Liver Transplant Registry: https://http://www.cltr.org/). Among the 2128 HCC patients who underwent LT, 1631 HBV-related cirrhosis patients with antiviral prophylaxis and complete follow-up information were studied retrospectively.
Inclusion criteria were: (1) adults ≥ 18-year-old; (2) fulfillment of the UCSF criteria[25] or Hangzhou criteria (China)[26]; (3) diagnosis of cirrhosis and HCC; and (4) diagnosis of pre-LT HBV infection. Exclusion criteria were: (1) pre-LT imaging diagnosis of HCC complicated by inferior vena cava tumor thrombus or extrahepatic tumor metastasis (including lymph node metastasis); (2) hepatitis C co-infection; (3) any history of alcohol abuse; and (4) HIV or HDV infection without adequate social support.
This study enrolled 1496 men and 135 women, with ages ranging between 25 and 80 years and a median age of 52.42 years. Of the 1631 patients, at the time of LT, 1336 (81.9%) were DM-negative and 295 (18.1%) had DM. Among the DM patients, 17 (5.8%) had type 1 diabetes and 278 (94.2%) had type 2 diabetes. Among the 295 DM cases, 29 (9.8%) were controlled by diet and exercise alone, 71 (24.1%) used oral sulfonylurea medications, 92 (31.2%) used oral sulfonylureas combined with insulin, and 103 (34.9%) used insulin alone. There were 101 (34.2%) patients with fasting blood glucose ≤ 7.0 mmol/L and 194 (65.8%) patients with fasting blood glucose > 7.0 mmol/L, out of the total of 295 DM patients. The baseline characteristics of the 1631 HBV-induced HCC patients with or without DM are summarized in Table 1.
| Category | Diabetic (n = 295) | Non-diabetic (n = 1336) | Value | df | P value |
| Sex | 2.999 | 1 | 0.083 | ||
| Male | 278 (94.2) | 1218 (91.2) | |||
| Female | 17 (5.8) | 118 (8.8) | |||
| Age, yr | 23.690 | 1 | 0.000 | ||
| ≤ 50 | 96 (32.5) | 643 (48.1) | |||
| > 50 | 199 (67.5) | 693 (51.9) | |||
| Tumor size | 4.333 | 3 | 0.228 | ||
| ≤ 3 | 163 (55.3) | 704 (52.7) | |||
| 3-5 | 85 (28.8) | 350 (26.2) | |||
| 5-7 | 28 (9.5) | 157 (11.8) | |||
| > 7 | 19 (6.4) | 125 (9.4) | |||
| Number of tumor nodules | 0.245 | 2 | 0.885 | ||
| Singer | 147 (49.8) | 666 (49.9) | |||
| ≥ 2, ≤ 3 | 72 (24.4) | 341 (25.5) | |||
| ≥ 4 | 76 (25.8) | 329 (24.6) | |||
| Venous invasion | 0.641 | 1 | 0.423 | ||
| Absent | 256 (86.8) | 1135 (85.0) | |||
| Present | 39 (13.2) | 201 (15.0) | |||
| Lymph node invasion | 1.272 | 1 | 0.304 | ||
| Absent | 284 (96.3) | 1265 (94.7) | |||
| Present | 11 (3.7) | 71 (5.3) | |||
| Capsular invasion | 0.005 | 1 | 0.942 | ||
| Absent | 259 (87.8) | 1175 (87.9) | |||
| Present | 36 (12.2) | 161 (12.1) | |||
| Microsatellite lesions | 0.430 | 1 | 0.512 | ||
| Absent | 234 (79.3) | 1082 (81.0) | |||
| Present | 61 (20.7) | 254 (19.0) | |||
| Edmondson | 4.359 | 2 | 0.108 | ||
| 1 | 12 (4.1) | 27 (2.0) | |||
| 2 | 168 (56.9) | 797 (59.7) | |||
| 3 + 4 + 5 | 115 (39.0) | 512 (38.3) | |||
| Serum AFP level, in ng/mL | 5.518 | 4 | 0.236 | ||
| < 200 | 231 (78.3) | 966 (72.3) | |||
| 200-400 | 18 (6.1) | 87 (6.5) | |||
| 400-1000 | 17 (5.8) | 122 (9.1) | |||
| 1000-2000 | 13 (4.4) | 65 (4.9) | |||
| > 2000 | 16 (5.4) | 96 (7.2) | |||
| MELD score | 14.184 | 2 | 0.001 | ||
| < 10 | 97 (32.9) | 582 (43.6) | |||
| 10-15 | 94 (31.9) | 406 (30.4) | |||
| > 15 | 104 (35.3) | 348 (26.0) | |||
| Child-Pugh score | 12.456 | 2 | 0.002 | ||
| A | 125 (42.4) | 715 (53.5) | |||
| B | 104 (35.3) | 396 (29.6) | |||
| C | 66 (22.4) | 225 (16.8) | |||
| Fasting blood glucose, in mmol/L | 678.412 | 1 | 0.000 | ||
| < 7 | 101 (34.2) | 1273 (95.3) | |||
| ≥ 7 | 194 (65.8) | 63 (4.7) | |||
| BMI, in kg/m2 | 7.937 | 3 | 0.047 | ||
| < 18.5 | 19 (6.4) | 69 (5.2) | |||
| 18.5-24 | 164 (55.6) | 856 (64.1) | |||
| 24-28 | 84 (28.5) | 295 (22.1) | |||
| > 28 | 28 (9.5) | 116 (8.7) | |||
| ALP, in IU/L | 1.546 | 1 | 0.214 | ||
| ≤ 112 | 179 (60.7) | 862 (64.5) | |||
| > 112 | 116 (39.3) | 474 (35.5) | |||
| GGT, in IU/L | 2.196 | 1 | 0.138 | ||
| ≤ 54 | 107 (36.3) | 547 (40.9) | |||
| > 54 | 188 (63.7) | 789 (59.1) | |||
| AST, in IU/L | 2.682 | 2 | 0.262 | ||
| ≤ 40 | 102 (34.6) | 481 (36.0) | |||
| 40-80 | 107 (36.3) | 526 (39.4) | |||
| > 80 | 86 (29.2) | 329 (24.6) | |||
| ALT, in IU/L | 3.142 | 2 | 0.208 | ||
| ≤ 40 | 121 (41.0) | 566 (42.4) | |||
| 40-80 | 105 (35.6) | 517 (38.7) | |||
| > 80 | 69 (23.4) | 253 (18.9) | |||
| TC, in mmol/L | 22.536 | 1 | 0.000 | ||
| ≤ 1.7 | 250 (84.7) | 1245 (93.2) | |||
| > 1.7 | 45 (15.3) | 91 (6.8) | |||
| CH, in mmol/L | 3.840 | 2 | 0.147 | ||
| < 3.88 | 185 (62.7) | 865 (64.7) | |||
| 3.88-5.2 | 67 (22.7) | 329 (24.6) | |||
| > 5.2 | 43 (14.6) | 142 (10.6) | |||
| Pre-LT antitumor therapy | 0.451 | 2 | 0.798 | ||
| No | 219 (74.5) | 1016 (76.2) | |||
| local therapy | 48 (16.3) | 209 (15.7) | |||
| Resection | 27 (9.2) | 109 (8.2) | |||
| HBeAg | 0.670 | 1 | 0.413 | ||
| Negative | 232 (78.6) | 1021 (76.4) | |||
| Positive | 63 (21.4) | 315 (23.6) | |||
| HBV DNA, in IU/mL | 6.140 | 1 | 0.013 | ||
| Negative, ≤ 103 | 191 (64.7) | 760 (56.9) | |||
| Positive, ≥ 103 | 104 (35.3) | 576 (43.1) | |||
| Post-LT HBV recurrence | 0.217 | 1 | 0.740 | ||
| No | 285 (96.6) | 1283 (96.0) | |||
| Yes | 10 (3.4) | 53 (4.0) |
For HBV-related patients, the pre-LT HBV infection status was checked routinely using the following viral markers: hepatitis B surface antigen (HBsAg); HB surface antibody (HBsAb), HB e antigen (HBeAg); hepatitis B core antibody (HBcAb); and HBV deoxyribonucleic acid (DNA) levels. HBV infection was defined by the presence of serum HBsAg or HBV DNA positivity. Before and after LT, HBV status was assessed using HBV markers and PCR for HBV DNA. In order to identify lamivudine resistance, viral mutation tests were also conducted[27]. Percutaneous or transjugular liver biopsies were performed to prove HBV re-infection. HBV genotyping was not performed in the majority of patients, and, therefore, was not included in the final analysis. All patients were routinely given nucleoside analogues (lamivudine, adefovir or entecavir) pre- and post-LT, and hepatitis B immunoglobulins post-LT (Table 2).
| Patients with high risk of HBV reinfection[HBV-DNA ≥ 105 copies/mL or HBeAg(+)] | Patients with low risk of HBV reinfection[HBV-DNA < 105 copies/mL or HBeAg(-)] |
| Pre-LT: nucleoside analogues, qd 2-4 w | Pre-LT: nucleoside analogues, qd 0-2 w |
| Intraoperative: HBIG 4000 IU, iv | Intraoperative: HBIG 2000 IU, iv |
| Post-LT: HBIG 1000 IU, iv, qd, 1-7 d | Post-LT: HBIG 1000 IU, iv, qd, 1-7d |
| After 7 d, HBIG 1000 IU, iv, once a week; or HBIG 400 IU, im, qd or qod or twice a week. Adjust frequency of HBIG administration to reach target therapeutic concentration | After 7 d, HBIG 1000 IU, iv, once a week; or HBIG 400 IU, im, qd or qod or twice a week. Adjust frequency of HBIG administration to reach target therapeutic concentration |
| Target therapeutic concentration post-LT | Target therapeutic concentration post-LT |
| ≤ 6 mo post-LT: anti-HBs titer ≥ 500 IU/L | ≤ 6 mo post-LT: anti-HBs titer ≥ 300 IU/L |
| 6-12 mo post-LT: anti-HBs titer ≥ 200 IU/L | 6-12 mo post-LT: anti-HBs titer ≥ 200 IU/L |
| ≥ 12 mo post-LT: anti-HBs titer ≥ 100 IU/L | ≥ 12 mo post-LT: anti-HBs titer ≥ 100 IU/L |
Pre-LT HCC diagnosis was confirmed by a combination of two abdominal imaging techniques [ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI)], and by measuring serum α-fetoprotein (AFP) levels, or positron emission tomography (PET), if necessary, to exclude extrahepatic tumor metastasis. HCC and cirrhosis were diagnosed by pathological examination post- LT.
During the LT follow-up, routine examinations included X-ray imaging, abdominal ultrasonography, whole-body CT scans and measurement of serial serum AFP levels. Early tumor recurrence or metastasis was assessed by monthly monitoring of AFP levels and abdominal ultrasonography, and by whole-body CT, MRI examinations and bone scintigraphy every 3 to 6 mo. Histological confirmation of recurrence was conducted using patient biopsies, if necessary. Liver function tests were regularly checked before and after LT.
Pre-LT DM status was determined by retrospective review and laboratory tests, and assessed using the DM history. Medical history included medical records, recent history and physical examination records of use of oral hypoglycemic agents or insulin. Patients were also asked if they had a history of physician-diagnosed DM. The diagnostic criteria for DM consisted of fasting (≥ 8 h) plasma glucose levels ≥ 126 mg/dL and 2-h oral glucose tolerance test results showing ≥ 200 mg/dL[28]. Post-LT DM status was not included in this study. The treatment method for early post-LT DM was mainly insulin alone or oral sulfonylureas combined with insulin, according to fasting plasma glucose levels and 2-h oral glucose tolerance test results.
Variables analyzed included sex, age, body mass index (BMI), DM, HBV markers, pathologic tumor characteristics (including number of tumor nodules, tumor size, venous invasion, lymph node invasion, capsular invasion and microsatellite lesions), serum AFP levels, Child-Pugh scores, model for end-stage liver disease (MELD) scores, and alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), triglyceride (TG), and cholesterol (CH) levels. Pre-LT antitumor therapy and post LT follow-up data included post-LT HBsAg reinfection, tumor recurrence, metastasis and survival time. These variables were collected and recorded in the databases of the China Liver Transplant Registry (https://http://www.cltr.org/).
Patient height and weight were recorded prior to LT, as well as BMI for all patients. BMI was defined as weight in kilograms divided by height2 (m2). All included patients tested positive for HBsAg and showed detectable HBV DNA levels within the 3 mo prior to LT. Liver function levels and blood fat levels were measured at the time of transplant. The tumor nodule number, tumor nodule size (maximum diameter), vascular invasion, perihepatic lymph node invasion, capsular invasion, microsatellite lesions and tumor differentiation were determined by experienced pathologists.
Categorical variables and non-normal distribution quantitative data were analyzed to compare the differences between non-diabetic and diabetic groups using χ2 tests and Mann-Whitney tests, respectively. From the date of LT to the date of death or to the most recent follow-up, survival time was calculated. The Kaplan-Meier method was used to analyze overall and recurrence-free survival. The log-rank test (Mantel-Cox) was used to compare group survival curves. Additionally, multivariate step-wise Cox regression was performed to analyze survival and tumor recurrence risk factors among LT patients. Statistical calculations were carried out using SPSS 17.0 software (SPSS Inc., Chicago, IL, United States). Two-sided P of < 0.05 was considered statistically significant.
The mean ages in patients with or without DM were 54.1 or 50.8, respectively (t = 6.597, P < 0.001). Mean patient BMI with or without DM was 24.2 for both (t = 0.036, P = 0.971). Mean fasting plasma glucose levels were 9.23 in DM patients, and 5.20 in non-DM patients (t = 16.849, P < 0.001). The mean TC level (t = 2.342, P = 0.019) and CH level (t = 1.106, P = 0.269) were 1.12 or 4.46 in DM patients and 0.92 or 3.82 in non-DM patients, respectively.
In the current study, patients with DM exhibited a higher proportion of individuals with age > 50-years-old (67.5% vs 51.9%, χ2 = 23.690, P < 0.001), Child-Pugh grade B (35.3% vs 29.6%, χ2 = 13.456, P = 0.002) and C (22.4% vs 16.8%, χ2 = 13.456, P = 0.002), MELD scores > 15 (35.3% vs 26.0%, χ2 = 14.184, P = 0.001), BMI of 24-28 (28.5% vs 22.1%, χ2 = 7.937, P = 0.047), TC > 1.7 mmol/L (15.3% vs 6.8%, χ2 = 22.536, P < 0.001) and HBV DNA ≤ 103 IU/mL (64.7% vs 56.9%, χ2 = 6.140, P = 0.013), when compared with non-DM patients (Table 1).
Analysis of DM and non-DM patients showed significant differences in age (P < 0.001), MELD scores (P = 0.001), Child-Pugh grade (P = 0.002), BMI (P = 0.047), TC levels (P < 0.001) and HBV DNA (P = 0.013; Table 1). No significant differences were observed between patients with and without DM for other factors analyzed (Table 1).
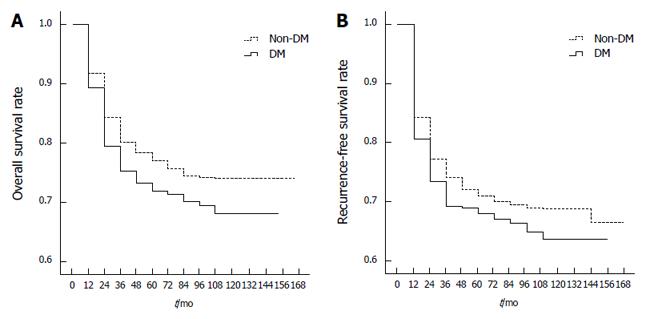
Complete follow-up monitoring of the 1631 HBV-associated HCC patients who underwent LT was conducted. The median survival time was 62.0 ± 39.1 mo (0 to 152 mo) in DM patients and 67.0 ± 38.9 mo (0 to 165 mo) in non-DM patients. Univariate analysis of 1631 patients showed that 1-, 3- and 5-year overall survival rates post-LT were 79%, 73% and 71% in DM patients, respectively, and 84%, 78% and 76% in non-DM patients, respectively. Overall survival rates post-LT were significantly different between the HCC patients with DM and those without DM (P = 0.041; Figure 1A).
The median tumor recurrence-free survival time was 55.0 ± 40.9 mo in DM patients and 62.0 ± 41.3 mo (0 to 165 mo) in non-DM patients. Univariate analysis of 1631 patients found that post-LT 1-, 3- and 5-year recurrence-free survival rates were 73%, 69% and 67% in DM patients, respectively, and 77%, 72% and 70% in non-DM patients, respectively. However, there were no significant differences in tumor recurrence-free survival rates between the two groups (P = 0.096; Figure 1B).
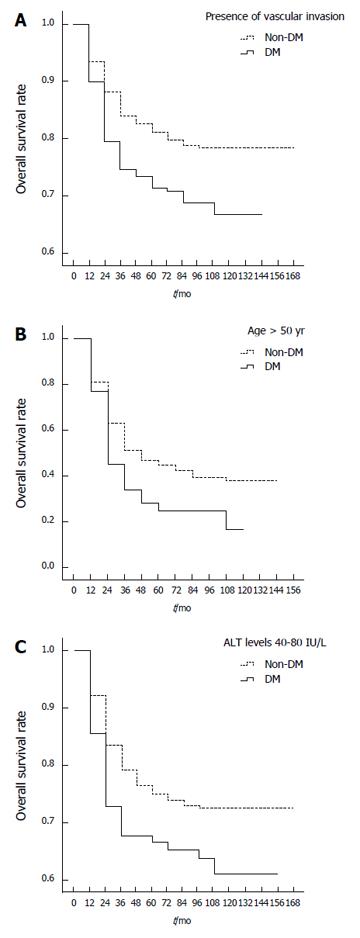
Table 3 shows the results of a stratified analysis of risk factors for overall survival of HBV-related HCC patients with and without DM. Significant survival differences were found between DM and non-DM patients in relation to age > 50 years (P = 0.002; Figure 2A), tumor size ≤ 3 cm (P = 0.047), number of tumor nodules between two and three (P = 0.007), vascular invasion (P = 0.033; Figure 2B), Child-Pugh grade B (P = 0.018), BMI of 18.5-24 (P = 0.048), ALT levels of 40-80 IU/L (P = 0.017; Figure 2C), AST levels of 40-80 IU/L (P = 0.038), TC levels ≤ 1.7 mmol/L (P = 0.021), no pre-LT antitumor therapy (P = 0.020), and absence of post-LT HBsAg reinfection (P = 0.045). When compared with non-DM patients, DM patients with these factors had poorer overall 1-, 3- and 5-year post-LT survival rates. Analysis of patients with vascular invasion showed that the 1-, 3- and 5-year post-LT survival rates were 45%, 28% and 25% in DM patients, respectively, compared to 63%, 47% and 42% in non-DM patients, respectively. DM patients > 50 years of age had 1-, 3- and 5-year post-LT survival rates of 79%, 73% and 71%, respectively, compared to 88%, 83% and 80% in non-DM patients, respectively. DM patients with ALT levels of 40 to 80 IU/L had overall 1-, 3- and 5-year post-LT survival rates of 76%, 71% and 70%, respectively, compared to 85%, 78% and 76% in non-DM patients, respectively.
| Variables | DM, % | Non-DM, % | P value | ||||
| 1 yr | 3 yr | 5 yr | 1 yr | 3 yr | 5 yr | ||
| Sex | |||||||
| Male | 80 | 74 | 72 | 84 | 77 | 75 | 0.140 |
| Female | 76 | 62 | 62 | 90 | 88 | 86 | 0.016 |
| Age, yr | |||||||
| ≤ 50 | 80 | 73 | 73 | 80 | 74 | 71 | 0.970 |
| > 50 | 79 | 73 | 71 | 88 | 83 | 80 | 0.002 |
| Tumor size, in cm | |||||||
| ≤ 3 | 83 | 79 | 79 | 90 | 86 | 83 | 0.047 |
| 3-5 | 86 | 79 | 74 | 87 | 79 | 77 | 0.689 |
| 5-7 | 66 | 49 | 43 | 71 | 65 | 63 | 0.171 |
| > 7 | 36 | 30 | 30 | 63 | 49 | 48 | 0.055 |
| Number of tumor nodules | |||||||
| Single | 85 | 82 | 79 | 90 | 84 | 82 | 0.325 |
| 2-3 | 73 | 68 | 68 | 86 | 81 | 79 | 0.007 |
| ≥ 4 | 76 | 62 | 60 | 72 | 64 | 61 | 0.961 |
| Venous invasion | |||||||
| Absent | 85 | 80 | 79 | 88 | 84 | 82 | 0.113 |
| Present | 45 | 28 | 25 | 63 | 47 | 42 | 0.033 |
| Lymph node invasion | |||||||
| Absent | 80 | 74 | 72 | 85 | 79 | 76 | 0.032 |
| Present | 73 | 64 | 64 | 78 | 70 | 66 | 0.979 |
| Capsular invasion | |||||||
| Absent | 80 | 74 | 72 | 85 | 79 | 77 | 0.050 |
| Present | 75 | 61 | 61 | 79 | 72 | 70 | 0.554 |
| Edmondson | |||||||
| 1 | 91 | 91 | 91 | 100 | 100 | 100 | 0.134 |
| 2 | 82 | 77 | 75 | 87 | 81 | 78 | 0.124 |
| 3 + 4 + 5 | 74 | 66 | 64 | 79 | 73 | 71 | 0.229 |
| Serum AFP level, in ng/mL | |||||||
| < 200 | 85 | 81 | 79 | 88 | 83 | 82 | 0.161 |
| 200-400 | 64 | 64 | 64 | 78 | 68 | 66 | 0.502 |
| 400-1000 | 71 | 51 | 42 | 82 | 72 | 66 | 0.189 |
| 1000-2000 | 68 | 41 | 41 | 73 | 61 | 55 | 0.454 |
| > 2000 | 34 | 26 | 26 | 62 | 56 | 51 | 0.052 |
| MELD score | |||||||
| < 10 | 79 | 68 | 68 | 84 | 77 | 74 | 0.101 |
| 10-15 | 78 | 70 | 70 | 84 | 78 | 76 | 0.197 |
| > 15 | 82 | 80 | 75 | 85 | 80 | 78 | 0.423 |
| Child-Pugh class | |||||||
| A (5-6) | 83 | 78 | 78 | 85 | 79 | 76 | 0.792 |
| B (7-9) | 75 | 67 | 63 | 83 | 78 | 75 | 0.018 |
| C (≥ 9) | 79 | 74 | 72 | 82 | 77 | 75 | 0.479 |
| Fasting blood glucose, in mmol/L | |||||||
| ≤ 7 | 77 | 73 | 71 | 85 | 79 | 76 | 0.145 |
| > 7 | 80 | 73 | 71 | 80 | 73 | 67 | 0.955 |
| BMI, in kg/m2 | |||||||
| < 18.5 | 68 | 62 | 62 | 81 | 79 | 76 | 0.156 |
| 18.5-24 | 77 | 72 | 70 | 84 | 78 | 76 | 0.048 |
| 24-28 | 86 | 78 | 76 | 85 | 79 | 76 | 0.951 |
| > 28 | 81 | 72 | 72 | 85 | 80 | 74 | 0.656 |
| ALP | |||||||
| ≤ 112 | 83 | 78 | 76 | 87 | 81 | 78 | 0.281 |
| > 112 | 74 | 66 | 65 | 80 | 74 | 72 | 0.102 |
| GGT | |||||||
| ≤ 54 | 87 | 86 | 85 | 92 | 88 | 85 | 0.559 |
| > 54 | 75 | 66 | 63 | 79 | 72 | 69 | 0.104 |
| ALT, in IU/L | |||||||
| ≤ 40 | 86 | 81 | 80 | 89 | 85 | 83 | 0.339 |
| 40-80 | 73 | 68 | 65 | 83 | 77 | 74 | 0.017 |
| > 80 | 77 | 68 | 66 | 75 | 68 | 64 | 0.633 |
| AST, in IU/L | |||||||
| ≤ 40 | 88 | 82 | 80 | 91 | 86 | 84 | 0.307 |
| 40-80 | 76 | 71 | 70 | 85 | 78 | 76 | 0.038 |
| > 80 | 74 | 67 | 64 | 74 | 68 | 64 | 0.872 |
| TC, in mmol/L | |||||||
| ≤ 1.7 | 79 | 72 | 71 | 85 | 79 | 77 | 0.021 |
| > 1.7 | 82 | 79 | 76 | 79 | 71 | 64 | 0.473 |
| CH, in mmol/L | |||||||
| ≤ 3.88 | 81 | 75 | 73 | 85 | 80 | 77 | 0.151 |
| 3.88-5.2 | 74 | 69 | 67 | 86 | 78 | 75 | 0.058 |
| > 5.2 | 83 | 71 | 71 | 77 | 71 | 68 | 0.737 |
| Pre-LT antitumor therapy | |||||||
| No | 77 | 73 | 71 | 85 | 79 | 76 | 0.020 |
| local therapy | 83 | 71 | 71 | 79 | 73 | 71 | 0.910 |
| Resection | 92 | 80 | 72 | 88 | 84 | 80 | 0.809 |
| HBeAg | |||||||
| Negative | 79 | 73 | 71 | 85 | 78 | 76 | 0.055 |
| Positive | 82 | 75 | 73 | 83 | 79 | 76 | 0.446 |
| HBV-DNA, in × 103 U/mL | |||||||
| < 1 | 80 | 73 | 71 | 85 | 79 | 78 | 0.072 |
| > 1 | 77 | 73 | 72 | 84 | 77 | 74 | 0.236 |
| Post-LT HBsAg reinfection | |||||||
| Negative | 79 | 73 | 72 | 84 | 79 | 76 | 0.045 |
| Positive | 80 | 70 | 60 | 87 | 73 | 69 | 0.678 |
Additionally, a trend was observed in survival differences between DM and non-DM patients in relation to tumor size > 7 cm (P = 0.055), no capsular invasion (P = 0.050), serum AFP levels > 2000 ng/mL (P = 0.052) and HBeAg negativity (P = 0.055).
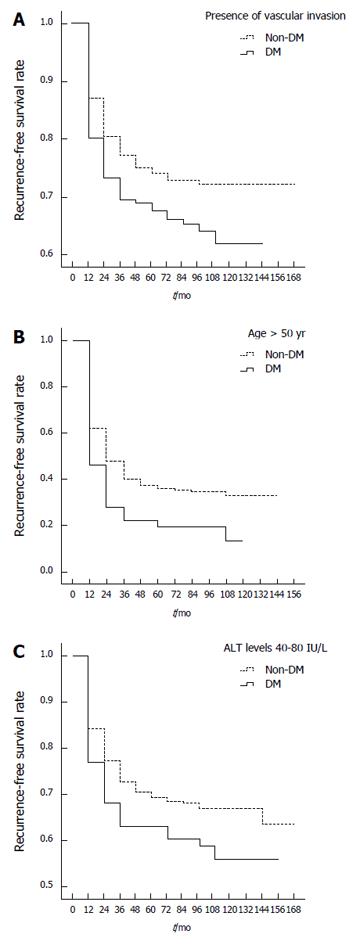
Stratified analysis of risk factors for the tumor recurrence-free survival between DM and non-DM patients (Table 4) showed that age > 50 years (P = 0.015; Figure 3A), two to three tumor nodules (P = 0.031), presence of vascular invasion (P = 0.005; Figure 3B), presence of microsatellite lesions (P = 0.039) and ALT levels of 40-80 IU/L (P = 0.033; Figure 3C) were associated with significant tumor recurrence-free survival differences. DM patients with these factors had poorer 1-, 3- and 5-year recurrence-free survival rates post-LT when compared with non-DM patients (Table 4). Analysis of the presence of vascular invasion in DM patients found that 1-, 3- and 5-year recurrence-free post-LT survival rates were 28%, 22% and 19%, respectively, but 48%, 37% and 35% in non-DM patients, respectively. DM patients > 50 years of age had 1-, 3- and 5-year post-LT survival rates of 73%, 69% and 66%, respectively, while non-DM patients had post-LT survival rates of 81%, 75% and 73%, respectively. DM patients with ALT levels between 40 and 80 IU/L had 1-, 3- and 5-year post-LT survival rates of 68%, 63% and 60%, respectively, compared to post-LT survival rates of 77%, 70% and 68% in non-DM patients, respectively.
| Variables | DM, % | Non-DM, % | P value | ||||
| 1 yr | 3 yr | 5 yr | 1 yr | 3 yr | 5 yr | ||
| Sex | |||||||
| Male | 73 | 69 | 67 | 77 | 71 | 69 | 0.204 |
| Female | 76 | 63 | 63 | 83 | 82 | 80 | 0.110 |
| Age, yr | |||||||
| ≤ 50 | 73 | 68 | 68 | 73 | 69 | 67 | 0.940 |
| > 50 | 73 | 69 | 66 | 81 | 75 | 73 | 0.015 |
| Tumor size, in cm | |||||||
| ≤ 3 | 78 | 75 | 75 | 85 | 81 | 79 | 0.076 |
| 3-5 | 82 | 75 | 71 | 80 | 73 | 71 | 0.823 |
| 5-7 | 52 | 43 | 31 | 58 | 54 | 53 | 0.174 |
| > 7 | 31 | 25 | 25 | 47 | 39 | 39 | 0.087 |
| Number of tumor nodules | |||||||
| Single | 81 | 77 | 75 | 83 | 79 | 77 | 0.475 |
| 2-3 | 67 | 64 | 64 | 79 | 75 | 73 | 0.031 |
| ≥ 4 | 64 | 57 | 55 | 63 | 55 | 53 | 0.825 |
| Venous invasion | |||||||
| Absent | 80 | 76 | 74 | 82 | 78 | 76 | 0.301 |
| Present | 28 | 22 | 19 | 48 | 37 | 35 | 0.005 |
| Lymph node invasion | |||||||
| Absent | 74 | 69 | 67 | 78 | 73 | 71 | 0.058 |
| Present | 64 | 64 | 64 | 67 | 59 | 55 | 0.580 |
| Capsular invasion | |||||||
| Absent | 75 | 71 | 69 | 78 | 73 | 71 | 0.201 |
| Present | 60 | 55 | 55 | 69 | 67 | 62 | 0.192 |
| Microsatellite lesions | |||||||
| Absent | 79 | 74 | 72 | 80 | 75 | 73 | 0.542 |
| Present | 52 | 49 | 46 | 63 | 60 | 58 | 0.039 |
| Edmonton | |||||||
| 1 | 91 | 91 | 91 | 100 | 100 | 100 | 0.134 |
| 2 | 77 | 73 | 72 | 80 | 74 | 72 | 0.375 |
| 3 + 4 + 5 | 67 | 59 | 57 | 72 | 67 | 66 | 0.175 |
| Serum AFP level, in ng/mL | |||||||
| < 200 | 80 | 76 | 75 | 82 | 78 | 76 | 0.272 |
| 200-400 | 59 | 59 | 59 | 72 | 63 | 62 | 0.484 |
| 400-1000 | 65 | 46 | 37 | 77 | 65 | 63 | 0.251 |
| 1000-2000 | 61 | 43 | 43 | 62 | 55 | 49 | 0.682 |
| > 2000 | 17 | 17 | 17 | 44 | 42 | 39 | 0.055 |
| MELD score | |||||||
| < 10 | 68 | 60 | 58 | 75 | 69 | 67 | 0.058 |
| 10-15 | 72 | 70 | 70 | 78 | 73 | 70 | 0.412 |
| > 15 | 79 | 76 | 72 | 79 | 76 | 74 | 0.534 |
| Child-Pugh class | |||||||
| A | 75 | 71 | 69 | 78 | 72 | 70 | 0.672 |
| B | 69 | 65 | 61 | 77 | 72 | 70 | 0.052 |
| C | 76 | 71 | 71 | 76 | 73 | 71 | 0.610 |
| Fasting blood glucose, in mmol/L | |||||||
| ≤ 7 | 72 | 68 | 67 | 77 | 72 | 70 | 0.154 |
| > 7 | 74 | 69 | 67 | 70 | 68 | 63 | 0.914 |
| BMI, in kg/m2 | |||||||
| < 18.5 | 63 | 57 | 57 | 80 | 72 | 69 | 0.223 |
| 18.5-24 | 73 | 70 | 68 | 77 | 72 | 70 | 0.216 |
| 24-28 | 76 | 69 | 69 | 78 | 73 | 71 | 0.524 |
| > 28 | 77 | 69 | 63 | 76 | 71 | 67 | 0.774 |
| ALP, in IU/L | |||||||
| ≤ 112 | 80 | 75 | 73 | 81 | 75 | 73 | 0.747 |
| > 112 | 63 | 60 | 57 | 71 | 67 | 65 | 0.056 |
| GGT, in IU/L | |||||||
| ≤ 54 | 85 | 84 | 82 | 87 | 82 | 80 | 0.957 |
| > 54 | 67 | 60 | 58 | 70 | 65 | 63 | 0.101 |
| AST, in IU/L | |||||||
| ≤ 40 | 81 | 76 | 73 | 84 | 79 | 78 | 0.201 |
| 40-80 | 69 | 65 | 65 | 78 | 72 | 70 | 0.103 |
| > 80 | 70 | 65 | 62 | 66 | 61 | 58 | 0.767 |
| ALT, in IU/L | |||||||
| ≤ 40 | 79 | 76 | 75 | 82 | 79 | 77 | 0.318 |
| 40-80 | 68 | 63 | 60 | 77 | 70 | 68 | 0.033 |
| > 80 | 72 | 65 | 64 | 65 | 60 | 58 | 0.376 |
| TC, in mmol/L | |||||||
| ≤ 1.7 | 74 | 69 | 67 | 78 | 73 | 71 | 0.113 |
| > 1.7 | 71 | 68 | 65 | 66 | 63 | 60 | 0.894 |
| CH, in mmol/L | |||||||
| < 3.88 | 75 | 70 | 68 | 78 | 74 | 72 | 0.175 |
| 3.88-5.2 | 68 | 66 | 64 | 79 | 72 | 71 | 0.092 |
| > 5.2 | 76 | 68 | 68 | 66 | 60 | 57 | 0.415 |
| Pre-LT antitumor therapy | |||||||
| No | 73 | 68 | 67 | 78 | 72 | 70 | 0.061 |
| local therapy | 68 | 66 | 66 | 70 | 67 | 64 | 0.939 |
| Resection | 81 | 77 | 70 | 83 | 79 | 76 | 0.758 |
| HBeAg | |||||||
| Negative | 72 | 69 | 66 | 78 | 71 | 70 | 0.095 |
| Positive | 77 | 69 | 69 | 75 | 74 | 71 | 0.633 |
| HBV-DNA, in × 103 IU/mL | |||||||
| < 1 | 75 | 69 | 68 | 78 | 74 | 72 | 0.165 |
| > 1 | 71 | 68 | 65 | 76 | 70 | 67 | 0.256 |
| Post-LT HBsAg reinfection | |||||||
| Negative | 73 | 69 | 67 | 77 | 73 | 70 | 0.092 |
| Positive | 70 | 60 | 60 | 72 | 60 | 58 | 0.899 |
Trends were observed in tumor recurrence-free survival differences between DM and non-DM patients with AFP levels > 2000 ng/mL (P = 0.055) and with AST levels >112 IU/L (P = 0.056).
The multivariate Cox modeling for overall survival included sex, age, BMI, DM, number of tumor nodules, tumor size, venous invasion, lymph node invasion, capsular invasion, microsatellite lesions, Edmondson grade, AFP level, Child-Pugh score, MELD score, ALP, GGT, AST ALT, TC, CH, HBeAg, HBV DNA, pre-LT antitumor therapy and post-LT HBsAg reinfection. Multivariate analysis (Table 5) revealed that DM [hazard rate (HR) = 1.591; 95% confidence interval (CI): 1.239-2.041, P < 0.001] was an independent predictor of poor survival, and that age (HR = 0.781, P = 0.017), vascular invasion (P < 0.001), number of tumor nodules ≥ 2 (HR = 1.695-2.342, P < 0.001), tumor size > 5 cm (HR = 2.093-3.496, P < 0.00), AFP level pre-LT > 1000 ng/mL (HR = 1.860-2.065, P≤ 0.001), ALT level > 40 IU/L (HR = 1.103-1.246, P≤ 0.006), GGT level > 54 IU/L (HR = 1.394, P = 0.010) and ALP level > 112 IU/L (HR = 0.618, P = 0.018) were independent predictors of poor survival. Additionally, antitumor local therapy pre-LT was an independent predictor of good survival.
| Variables | B | SE | Wald | P value | OR (95%CI) |
| Venous invasion, absent vs present | 0.893 | 0.117 | 57.882 | 0.000 | 2.442 (1.940-3.074) |
| Pre-LT antitumor therapy | 7.129 | 0.032 | |||
| Local therapy | -0.319 | 0.138 | 5.368 | 0.021 | 1.375 (1.050-1.801) |
| Resection | -0.227 | 0.212 | 1.146 | 0.284 | 0.797 (0.526-1.208) |
| Age, ≤ 50 vs > 50 | -0.248 | 0.104 | 5.655 | 0.017 | 0.781 (0.636-0.957) |
| ALP, ≤ 112 IU/L vs > 112 IU/L | -0.264 | 0.111 | 5.630 | 0.018 | 0.768 (0.618-0.955) |
| GGT, ≤ 54 IU/L vs > 54 IU/L | 0.332 | 0.128 | 6.681 | 0.010 | 1.394 (1.084-1.793) |
| ALT | 13.416 | 0.001 | |||
| ≤ 40 IU/L vs 40-80 IU/L | 0.340 | 0.123 | 7.575 | 0.006 | 1.404 (1.103-1.789) |
| ≤ 40 IU/L vs > 80 IU/L | 0.496 | 0.141 | 12.396 | 0.000 | 1.642 (1.246-2.164) |
| Serum AFP level, in ng/mL | 30.829 | 0.000 | |||
| < 200 vs 200-400 | 0.525 | 0.185 | 8.097 | 0.004 | 1.691 (1.178-2.427) |
| < 200 vs 400-1000 | 0.399 | 0.163 | 6.012 | 0.014 | 1.491 (1.083-2.052) |
| < 200 vs 1000-2000 | 0.621 | 0.189 | 10.804 | 0.001 | 1.860 (1.285-2.693) |
| < 200 vs > 2000 | 0.725 | 0.160 | 20.569 | 0.000 | 2.065 (1.509-2.825) |
| Number of tumor nodules | 47.936 | 0.000 | |||
| Single vs 2-3 | 0.528 | 0.138 | 14.631 | 0.000 | 1.695 (1.293-2.221) |
| Single vs≥ 4 | 0.851 | 0.124 | 47.265 | 0.000 | 2.342 (1.837-2.984) |
| Tumor size, in cm | 78.643 | 0.000 | |||
| ≤ 3 vs 3-5 | 0.152 | 0.132 | 1.314 | 0.252 | 1.164 (0.898-1.509) |
| ≤ 3 vs 5-7 | 0.739 | 0.149 | 24.596 | 0.000 | 2.093 (1.563-2.802) |
| ≤ 3 vs > 7 | 1.252 | 0.152 | 67.571 | 0.000 | 3.496 (2.594-4.711) |
| DM, yes vs no | 0.464 | 0.128 | 13.230 | 0.000 | 1.591 (1.239-2.043) |
In multivariate Cox modeling for tumor recurrence, the variables analyzed were the same as those used for multivariate analysis for overall survival. The multivariate analysis (Table 6) indicated that tumor size > 5 cm (HR = 3.042-4.065, P < 0.001), tumor nodule number ≥ 2 (HR = 1.457-2.144, P≤ 0.009), presence of vascular invasion (HR = 2.182, P < 0.001), AFP level > 2000 ng/mL (HR = 2.213, P < 0.001), GGT level > 54 IU/L (HR = 1.433, P = 0.006) and TC >1.7 mmol/L (HR = 1.530, P = 0.010) were independent predictors of tumor recurrence post-LT. However, multivariate analysis indicated that DM was not an independent predictor of post-LT tumor recurrence (P > 0.05).
| Variables | B | SE | Wald | P value | OR (95%CI) |
| Venous invasion, absent vs present | 0.780 | 0.124 | 39.825 | 0.000 | 2.182 (1.712-2.780) |
| Pre-LT antitumor therapy | 11.093 | 0.005 | |||
| Local therapy | -0.404 | 0.138 | 8.555 | 0.003 | 1.498 (1.143-1.964) |
| Resection | -0.285 | 0.230 | 1.536 | 0.215 | 0.752 (0.480-1.180) |
| GGT, ≤ 54 IU/L vs > 54 IU/L | 0.360 | 0.131 | 7.559 | 0.006 | 1.433 (1.109-1.851) |
| TC, ≤ 1.7 mmol/L vs > 1.7 mmol/L | 0.425 | 0.165 | 6.682 | 0.010 | 1.530 (1.108-2.113) |
| Serum AFP level, in ng/mL | 27.634 | 0.000 | |||
| < 200 vs 200-400 | 0.330 | 0.198 | 2.767 | 0.096 | 1.391 (0.943-2.051) |
| < 200 vs 400-1000 | 0.031 | 0.193 | 0.026 | 0.872 | 1.032 (0.707-1.506) |
| < 200 vs 1000-2000 | 0.378 | 0.212 | 3.161 | 0.075 | 1.459 (0.962-2.212) |
| < 200 vs > 2000 | 0.794 | 0.158 | 25.372 | 0.000 | 2.213 (1.625-3.015) |
| Number of tumor nodules | 34.680 | 0.000 | |||
| Single vs 2-3 | 0.377 | 0.144 | 6.885 | 0.009 | 1.457 (1.100-1.931) |
| Single vs≥ 4 | 0.763 | 0.130 | 34.674 | 0.000 | 2.144 (1.664-2.764) |
| Tumor size, in cm | 110.647 | 0.000 | |||
| ≤ 3 vs 3-5 | 0.143 | 0.146 | 0.953 | 0.329 | 1.154 (0.866-1.537) |
| ≤ 3 vs 5-7 | 1.112 | 0.150 | 55.206 | 0.000 | 3.042 (2.268-4.079) |
| ≤ 3 vs > 7 | 1.402 | 0.158 | 78.831 | 0.000 | 4.065 (2.983-5.540) |
Among the 1631 HCC patients enrolled in this study with HBV-induced cirrhosis who underwent LT in multiple institutions, univariate analysis indicated that patients with DM had worse overall survival rates than patients without DM. There was no significant difference in tumor recurrence-free survival between patients with and without DM. Multivariate analysis also indicated that DM was a significant independent predictor of poor survival, but not of HCC recurrence post-LT. This finding is consistent with previous studies that reported decreased post-operative survival rates in HCC patients with DM[23,24,29-32].
To our knowledge, only two studies have compared the impact of pre-LT DM on survival and tumor recurrence in HCC patients after LT[23,24]. Connolly et al[23] studied 191 LT-treated HCC patients (12 HBV-related, 104 HCV-related and 81 other HCCs) and determined that the median survival was 78.3 mo for the non-DM patients, but only 31.1 mo for the DM patients. However, no significant differences were observed in survival between the two groups. Siegel et al[24] studied 342 patients with HBV-related HCC (16%) and HCV-related (70%) HCC. They reported that DM patients had worse overall survival than those without diabetes. The reason for the different outcomes in these studies is not clear, but the differences may be partly attributable to the smaller patient populations with HBV-related HCC in the Connolly et al[23] study. Nonetheless, both studies determined that there was no difference in tumor recurrence between DM and non-DM patients. This finding is consistent with the results of the current study.
Additionally, some studies have investigated the post-surgical impact of DM on HBV- and HCV-related HCC patient outcomes[33-35]. Huo et al[34] showed that DM patients with HBV (but not HCV) had poor long-term outcomes. However, Komura et al[33] reported the opposite results: DM patients with HCV (but not HBV) exhibited poor long-term outcomes. Other studies reported that DM had a similar impact on HCC patients with HBV- and HCV-infection after surgical resection[35]. The reason for these different results is not clear. Our current report is a multi-center study involving a larger number of samples, and we reported that DM had a significant effect on long-term survival post-LT in HBV-related HCC patients, but not on HCC recurrence.
Vascular invasion, tumor size > 5 cm, and tumor nodule number > 1 were also demonstrated by the current study to be independent prognostic factors of poor survival. These findings are also consistent with previous studies which reported that vascular invasion, tumor size (≤ 3 cm or ≤ 5 cm, or > 3 cm) and the number of tumor lesions appears to affect prognosis in DM patients compared with non-DM patients[23,29,31,33]. The current findings suggest that DM may be related to pathological features of tumors that affect long-term survival post-LT in HBV-related HCC patients.
DM, obesity and metabolic syndrome (MS) are related to each other, and are key pathogenetic factors for non-alcoholic fatty liver disease (NAFLD)[36]. It has been reported that “metabolic epidemics” are probably involved in the rise in incidence of HCC, and NAFLD has been cited as the major causative factor[36]. However, although MS has been shown to predispose to NAFLD, and the latter has been associated with an increased incidence of HCC, it has not been shown that MS is a direct etiological factor for development of HCC. While impaired glucose metabolism may be involved in hepato-carcinogenesis, its role in the development of HCC has not been proven[37,38]. In the current study, DM patients with vascular invasion or microsatellite lesions had worse tumor recurrence-free survival rates when compared with non-DM patients. Connolly et al[23] analyzed 191 HCC patients treated with LT, in which 84 (44%) of the patients had DM, and found macrovascular invasion in 20.2% of the DM patients and 9.3% of the non-DM patients. By multivariate analysis, they also found that DM was associated with macrovascular invasion in HCC patients who underwent LT. This supports the current findings that DM may impact the risk of vascular invasion in HCC patients treated with LT.
The current study suggests that DM may be related to older age (> 50 years) and poor liver function (ALT or AST levels 40-80 IU/L, Child-Pugh score B), which may affect outcomes post-LT. A previous study of 389 patients with HCC and cirrhosis by Ting et al[31] reported that DM, Child-Pugh scores (B and C) and AST levels were significant risk factors affecting HCC prognosis after surgical resection. Huo et al[32] reported that elderly and DM patients were associated with hepatic decompensation. Some previous studies have suggested that DM patients with HCC have an increased cumulative risk of hepatic decompensation after partial hepatectomy[24]. However, unlike partial liver resection, LT offers a more radical oncologic treatment: the complete replacement of a poorly functioning liver with a normal one. Moreover, large doses of hormones and calcineurin inhibitor immunosuppressant drugs (used as anti-rejection therapy post-LT) can increase blood glucose and may induce or aggravate DM or make DM difficult to control. This treatment regimen and its associated side effects may contribute to an elevated incidence of diabetic complications, and decrease survival post-LT for HCC patients with DM (especially among older patients). Thus, we suggest that factors contributing to poor outcomes post-LT in DM patients compared with non-DM patients may include advanced age, reduced liver function and an increase in diabetes-associated complications.
Additionally, the current study observed a survival difference trend between DM and non-DM patients with pre-LT AFP levels > 2000 ng/mL. The exact cause of this result is not clear. To our knowledge, no previous studies have reported this relationship between DM and elevated serum AFP levels. Some data suggest that hepatocyte damage or regeneration play a role in increasing AFP levels[39,40]. Elevated serum AFP may reflect both HCC progress and the severity of chronic liver damage[40-42]. Chronic liver disease has been reported to predispose patients to DM[40,41]. In our study, pre-LT DM patients had a higher proportion of Child-Pugh grades B and C, and higher MELD scores compared to non-DM patients. These findings suggest that DM may be related to liver necrosis severity or poor liver function, and that HBV-related HCC patients with both DM and AFP levels > 2000 ng/mL may have worse survival post-LT.
Several limitations of this study must be considered. First, this study was retrospective. Second, DM was determined based on medical history, fasting glucose, medication use, oral glucose tolerance test results or glycohemoglobin level. However, there was no analysis of glycohemoglobin levels because glycohemoglobin levels were not tested in some pre-LT patients. Third, the HBV DNA assay kits used varied between the hospitals in this study and, therefore, the specific kits used in each institution may have had variable sensitivity and detection limits. However, these results represent a large multi-center sample that suggests that DM is a risk predictor for decreased long-term survival after LT in HBV-related HCC patients.
In conclusion, compared with non-DM patients, DM patients exhibited decreased post-LT survival rates. DM may be associated with various tumor pathological features, older age and poor liver function, and these factors may influence post-LT survival in HBV-related HCC patients. However, no direct relation between DM and HBV infection or HBV load were observed in HCC patients. The current data suggest that DM may affect the long-term post-LT survival of HBV-related HCC patients, but not tumor recurrence. Thus, HBV-related HCC patients with DM undergoing LT should be closely followed to optimize postoperative outcomes. Follow-up examinations are especially important among elderly DM patients, or those who have vascular invasion and AFP levels > 2000 ng/mL pre-LT. These factors need to be carefully considered when selecting HCC patients for LT. Furthermore, a preventive strategy pre- and post-LT for HCC patients with DM should be established. A future prospective cohort study with larger a sample size should be conducted to confirm these observations.
We thank the staff of the China Liver Transplant Registry for medical data assistance and the patients who participated in this study.
In China, the major risk factor for the development of hepatocellular carcinoma (HCC) is chronic hepatitis B virus (HBV) infection. Recently, an increased incidence of HCC in the diabetic population has been reported, possibly due to an increased incidence steatohepatitis. However, it is unknown whether diabetes mellitus (DM) acts directly in the development of HCC in HBV- related cirrhosis patients. Few studies have investigated the association between HBV, DM, tumor recurrence and survival after liver transplantation (LT) in HCC patients.
Recently, it has been confirmed that non-alcoholic fatty liver disease (NAFLD) is related to diabetes, obesity and metabolic syndrome, and has been cited as a causative factor for the development of HCC. DM may be a potential risk factor for tumor development.
This is the first report of a multi-center study in a large cohort to investigate the potential associations between pre-existing DM with HBV-induced HCC, tumor recurrence and long-term survival post-LT in patients without alcoholic fatty liver disease.
DM may be associated with tumor pathological features, old age and poor liver function, and may be a predictor of poor survival post-LT in HBV-related HCC patients. The current data suggest that DM may affect the long-term survival post-LT for HBV-related HCC patients, but not tumor recurrence. HCC patients with DM undergoing LT should be closely monitored to optimize postoperative outcomes during follow-up.
NAFLD, the abnormal accumulation of fat in the liver, is related to obesity, diabetes and metabolic syndrome. NAFLD has been linked clinically and pathophysiologically to obesity, insulin resistance and type 2 DM, and can be considered a new criterion of metabolic syndrome.
In this study, the authors investigated the potential associations between DM with HBV-induced HCC, HCC recurrence and long-term survival after LT in a large cohort. It is an interesting study and well-written manuscript. The results can support the conclusion.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu SH, Tarantino G S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38:S158-S168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 556] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 4. | Xu J, Lin Y, Wang YP, Chen YX, Shi B, Lu J, Xie WF. Hepatitis B virus DNA in patients with hepatitis B-related liver cirrhosis with or without hepatocellular carcinomas: a matched case-control study. J Dig Dis. 2009;10:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Zheng Z, Zhang C, Yan J, Ruan Y, Zhao X, San X, Mao Y, Sun Q, Zhang K, Fan Z. Diabetes mellitus is associated with hepatocellular carcinoma: a retrospective case-control study in hepatitis endemic area. PLoS One. 2013;8:e84776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 2166] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 7. | Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128:635-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Adami HO, Chow WH, Nyrén O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 256] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Li Q, Li WW, Yang X, Fan WB, Yu JH, Xie SS, Liu L, Ma LX, Chen SJ, Kato N. Type 2 diabetes and hepatocellular carcinoma: a case-control study in patients with chronic hepatitis B. Int J Cancer. 2012;131:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Koh WP, Wang R, Jin A, Yu MC, Yuan JM. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Ko WH, Chiu SY, Yang KC, Chen HH. Diabetes, hepatitis virus infection and hepatocellular carcinoma: A case-control study in hepatitis endemic area. Hepatol Res. 2012;42:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Kamangar F. Confounding variables in epidemiologic studies: basics and beyond. Arch Iran Med. 2012;15:508-516. [PubMed] |
| 14. | Yu MC, Tong MJ, Govindarajan S, Henderson BE. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J Natl Cancer Inst. 1991;83:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 590] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 16. | Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18:2054-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Demir M, Serin E, Göktürk S, Ozturk NA, Kulaksizoglu S, Ylmaz U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eur J Gastroenterol Hepatol. 2008;20:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5299] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 19. | Zhang Q, Chen X, Zang Y, Zhang L, Chen H, Wang L, Niu Y, Ren X, Shen Z, Shang L. The survival benefit of liver transplantation for hepatocellular carcinoma patients with hepatitis B virus infection and cirrhosis. PLoS One. 2012;7:e50919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Sutcliffe R, Maguire D, Portmann B, Rela M, Heaton N. Selection of patients with hepatocellular carcinoma for liver transplantation. Br J Surg. 2006;93:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Kaneda K, Uenishi T, Takemura S, Shinkawa H, Urata Y, Sakae M, Yamamoto T, Kubo S. The influence of postoperative glycemic control on recurrence after curative resection in diabetics with hepatitis C virus-related hepatocellular carcinoma. J Surg Oncol. 2012;105:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Connolly GC, Safadjou S, Kashyap R, Chen R, Orloff MS, Hezel AF. Diabetes mellitus impacts risk of macrovascular invasion in patients undergoing transplantation for hepatocellular carcinoma. BMC Gastroenterol. 2013;13:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, Jacobson JS, Hershman DL, Verna EC, Zaretsky J. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1691] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 26. | Chen J, Xu X, Wu J, Ling Q, Wang K, Wang W, Zhang M, Shen Y, Zhou L, Xie H. The stratifying value of Hangzhou criteria in liver transplantation for hepatocellular carcinoma. PLoS One. 2014;9:e93128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Xu B, Cheng J, Xu DP, Li XD, Mao YL, Wang HB, Ma HB, Hu JH, Wang YD. Analysis of mutations at hepatitis B virus P gene in lamivudine-refractory patients with chronic hepatitis B. Gan Zang. 2008;13:104-107. |
| 28. | American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 Suppl 1:S11-S63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 1365] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 29. | Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Tanikawa M, Sone Y, Hisanaga Y. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer. 2001;91:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Ikeda Y, Shimada M, Hasegawa H, Gion T, Kajiyama K, Shirabe K, Yanaga K, Takenaka K, Sugimachi K. Prognosis of hepatocellular carcinoma with diabetes mellitus after hepatic resection. Hepatology. 1998;27:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Ting CT, Chen RC, Chen CC, Liu MH, Chu D, Kuo NW. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med. 2012;227:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Huo TI, Lui WY, Huang YH, Chau GY, Wu JC, Lee PC, Chang FY, Lee SD. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol. 2003;98:2293-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, Yamashita T, Ohta T, Shimizu K, Nakamoto Y. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Huo TI, Wu JC, Lui WY, Lee PC, Huang YH, Chau GY, Tsay SH, Chang FY, Lee SD. Diabetes mellitus is a recurrence-independent risk factor in patients with hepatitis B virus-related hepatocellular carcinoma undergoing resection. Eur J Gastroenterol Hepatol. 2003;15:1203-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Miyagawa S, Kawasaki S, Makuuchi M. Comparison of the characteristics of hepatocellular carcinoma between hepatitis B and C viral infection: tumor multicentricity in cirrhotic liver with hepatitis C. Hepatology. 1996;24:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217-9228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (1)] |
| 37. | Howell J, Yiu M, Gibson R, Thomson B, Stella D, Gorelik A, Prichard PJ, Nicoll AJ. Type 2 diabetes does not worsen prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Wang WM, Xu Y, Yang XR, Wang YH, Sun HX, Fan J. Prognostic role of diabetes mellitus in hepatocellular carcinoma patients after curative treatments: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2011;10:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Collazos J, Rodríguez J, de Miguel J. Serum alpha-fetoprotein in liver cirrhosis. Rev Clin Esp. 1993;192:214-216. [PubMed] |
| 40. | Liaw YF, Chen JJ, Chen TJ. Acute exacerbation in patients with liver cirrhosis: a clinicopathological study. Liver. 1990;10:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Kingston ME, Ali MA, Atiyeh M, Donnelly RJ. Diabetes mellitus in chronic active hepatitis and cirrhosis. Gastroenterology. 1984;87:688-694. [PubMed] |
| 42. | Del Vecchio Blanco C, Gentile S, Marmo R, Carbone L, Coltorti M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res Clin Pract. 1990;8:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |