Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9534
Peer-review started: August 24, 2016
First decision: September 6, 2016
Revised: September 20, 2016
Accepted: October 19, 2016
Article in press: October 19, 2016
Published online: November 21, 2016
Processing time: 86 Days and 5.8 Hours
To assess the effects of hepatitis B virus (HBV) on the expression of host α-1,2-mannosidases and determine the underlying mechanisms.
We measured the expression levels of MAN1A1, MAN1A2, MAN1B1, and MAN1C1 in cell lines HepG2.2.15, HepN10, HepAD38 and HepG2 by Western blot. Viral antigens (HBsAg and HBeAg) in the culture medium were measured using the chemiluminescence method. HBV DNA quantification assays were performed using a commercial real-time PCR kit. Protein levels of human liver tissue α-1,2-mannosidases were also evaluated by Western blot. Plasmids containing seven individual viral genes of HBV (PTT22-HBx, PTT22-HBs, PTT22-preS2, PTT22-preS1, PTT22-HBc, PTT22-HBe, and PTT22-HBp) or control plasmids (PTT22-vector) were transfected into HepG2 cells. MK886 (PPARα) and GW9662 (PPARγ) inhibitors were used to explore the effects of HBV on α-1,2-mannosidase expression after the PPARα and PPARγ pathways were blocked.
We showed that the expression of α-1,2-mannosidases was higher in stably transfected HBV cells than in controls. The expression levels of α-1,2-mannosidase were higher in AD38 cells than those in ND10 cells, which were in turn greater than those in G2.2.15 cells, and positively correlated with the expression of HBsAg in all the cell lines. Levels of α-1,2-mannosidase in non-tumorous liver tissues of HBV-related HCC patients were also higher than in the tissues from non-HBV-related HCC patients. Moreover, transfecting HepG2 cells with a component of the HBV viral envelope also increased the expression of α-1,2-mannosidases. However, this envelope protein component could not induce MAN1C1 expression in the presence of a PPARα inhibitor, MK886. We also found that MK886 did not affect the expression of MAN1C1 in AD38 cells without tetracycline in the culture medium. This phenomenon was not observed in the case of GW9662.
Our results indicate that HBV increases the expression of α-mannosidases both in vitro and in vivo via activation of the PPARα pathway by its envelope protein.
Core tip: To date, few studies have investigated whether hepatitis B virus (HBV) hijacks the host hepatocyte’s demannosylation system to trim the mannose oligosaccharides found on its viral envelope, thereby evading DC-SIGN recognition. Our study showed that HBV could increase the expression of α-mannosidase I both in vitro and in vivo. Moreover, the HBV envelope protein increases the expression of α-mannosidase I via the PPARα pathway. Therefore, α-mannosidase I may be a novel drug target to inhibit the demannosylation of HBV, and prevent viral escape.
- Citation: Hu S, Jiang LB, Zou XJ, Yi W, Tian DY. Hepatitis B virus upregulates host expression of α-1,2-mannosidases via the PPARα pathway. World J Gastroenterol 2016; 22(43): 9534-9543
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9534
Hepatitis B virus (HBV) infection is the most common chronic viral infection in the world. An estimated 2 billion people are infected, and more than 350 million are chronic carriers of the virus[1]. Due to an insufficient immune response, some individuals with HBV infection can develop chronic hepatitis, which can eventually result in liver cirrhosis and hepatocellular carcinoma (HCC). While the underlying mechanisms for HBV-induced chronic hepatitis remain unclear, several studies indicate that dendritic cell (DC) function is impaired in patients with chronic hepatitis B[2,3]. DCs are potent antigen-presenting cells (APCs) that can present antigen to T cells and activate naive T cells. Multiple receptor molecules on the surface of DCs, including Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), participate in the recognition and uptake of pathogens, and can regulate the expression of co-stimulatory molecules[4]. In particular, DC-specific ICAM-3 grabbing non-integrin (DC-SIGN) is an important CLR that is mainly expressed on the surface of mature and immature DCs[5].
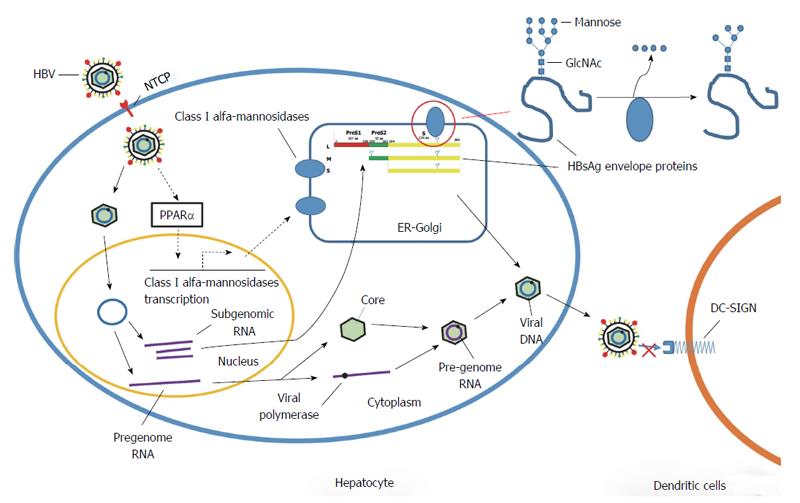
DC-SIGN plays an important role in the recognition of pathogen-associated molecular patterns (PAMPs)[5]. Moreover, previous studies have shown that DC-SIGN is involved in the immune escape of multiple pathogenic microorganisms, including HIV-1, Ebola virus, hepatitis C virus (HCV), Dengue fever virus, cytomegalovirus (CMV), SARS-coronavirus, mycobacterium tuberculosis, Helicobacter pylori, fungus, and some parasites[6,7]. Unfortunately, the mechanisms behind immune escape mediated by DC-SIGN remain unclear. It may be that DC-SIGN directly recognizes mannose oligosaccharides found on the viral envelope of HBV. Through modifications to these mannose oligosaccharides on its viral envelope, HBV could potential evade the host immune system. Indeed, it has been shown that DC-SIGN does not recognize wild-type HBV but does recognize a high-mannose type HBV produced by the application of a class I α-mannosidase inhibitor, kifunensine[8,9]. Moreover, in our previous studies, we found that the high-mannose type HBV could promote the maturation of DCs, increase IL-12 secretion, and effectively stimulate the proliferation of allogeneic lymphocytes, and that these effects were blocked by specific anti-DC-SIGN antibodies[10]. Therefore, demannosylation appears to be beneficial to HBV to escape DC-SIGN recognition and avoid the consequent immune elimination.
Demannosylation of glycoproteins occurs through a family of mannosidases that trim mannan in the N-oligosaccharide. Human Class I α-mannosidases include the ER α-1, 2-mannosidase I (MAN1B1), and three Golgi α-1,2-mannosidases, namely α-mannosidase IA (MAN1A1), α-mannosidase IB (MAN1A2), and α-mannosidase IC (MAN1C1). Generally, MAN1B1 firstly trims α-1,2-mannose from Man9GlcNAc2-Asn, and then MAN1A1, MAN1A2, and MAN1C1 trim the residual α-1,2-mannose, to generate Man5GlcNAc2-Asn. These processes are dependent on the varying levels of the mannosidase subtypes in different cell types[11]. There are glycosylation sites on the surface of HBV envelope proteins, which could be glycosylated by the host[12]. Therefore, it is not surprising that HBV infection has previously been shown to upregulate the expression of ER degradation-enhancing α-mannosidases (EDEMs) in order to increase demannosylation[13]. However, few studies have investigated the effects of HBV on the expression of class I α-mannosidases.
Peroxisome proliferator activated-receptors (PPARs), a group of ligand-activated nuclear transcription factors, may be involved in α-mannosidase expression during HBV infection. Indeed, tumor necrosis factor-α (TNF-α) has been shown to downregulate the expression of MAN1C1 to increase the expression of high-mannose type proteoglycans, and this can be reversed by the activation of the PPAR signaling pathway[14]. Based on the results of these previous studies, we hypothesized that HBV could promote the demannosylation of the viral protein by increasing the expression of α-mannosidase I in the host cells. In turn, DC-SIGN can no longer recognize the HBV glycoprotein coat, allowing the virus to escape from immune attack. In the present study, we investigated the effects of HBV infection on the expression of α-mannosidase I, and also determined the mechanisms driving the altered expression. We show for the first time that HBV infection increases the expression of α-mannosidase I via the PPARα signaling pathway.
Human hepatocellular carcinoma cells (HepG2, HepG2.2.15, AD38, and N10) were cultured in DMEM at 37 °C in a 5% CO2 incubator. The medium was supplemented with 10% FBS, 100 IU/mL penicillin, and 100 IU/mL streptomycin. Cells were changed into fresh medium every third day, and split by trypsinization at a confluence of about 90%[15]. AD38 cells, which are a variant of HepG2 cells, express the HBV genome under the control of a tetracycline (Tet)-off promoter. Therefore, the AD38 cell culture medium also contained tetracycline (1 μg/mL) when not requiring the expression of HBV genes[16]. HepG2.2.15 and N10 cells are secretory HBV cell lines derived from G2[17]. Viral antigens (HBsAg and HBeAg) in the culture medium were measured using the chemiluminescence method with commercial assay kits (Wantai, Beijing, China). HBV DNA quantification assays were performed using a commercial real-time PCR kit (Kehua, Shanghai, China).
Plasmids containing seven individual viral genes of HBV (i.e., Ptt22-HBx, Ptt22-HBs, Ptt22-preS2, Ptt22-preS1, Ptt22-HBc, Ptt22-HBe, and Ptt22-HBp) and the control plasmid (Ptt22-vector) were a kind gift from Dr. Quan Yuan and Tianying Zhang (National Institute of Diagnostics and Vaccine Development in Infectious Diseases, Xiamen University, China). Transient transfections with plasmids were performed using Lipofectamine 2000 Reagent (Invitrogen, United States) according to the manufacturer’s protocol. HepG2 cell lines only express PPARα and PPARγ but not PPARβ[18]. Therefore, PPARα (MK886) and PPARγ (GW9662) inhibitors were used at a concentration of 10 μmol/L, unless otherwise noted. MK886 and GW9662 (Sigma-Aldrich) were added to the cells 6 h after transfection.
Human liver tissues were obtained from patients who were treated at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between July 2014 and December 2014, and included 12 non-tumorous samples from HBV-related HCC patients and 12 samples from non-HBV-related HCC patients. All tissue samples were collected and stored at -80 °C before investigation.
Cells were lysed 72 h after transfection. Cell and tissue samples were lysed in RIRA (Sigma-Aldrich) buffer with PMSF (Sigma-Aldrich). Lysates were centrifuged for 10 min at 12000 ×g, and protein in the supernatant was quantified using the BCA method (Pierce). SDS-PAGE and western blot analysis were performed according to standard procedures. Mouse anti-HBs, anti-HBx, anti-HBp, and anti-HBc antibodies were kindly provided by Dr. Quan Yuan from Xiamen University. Goat anti-MAN1A1 (Santa Cruz; 1:500 dilution), rabbit anti-MAN1A2 (Proteintech; 1:200 dilution), rabbit anti-MAN1B1 (GeneTex; 1:500 dilution), mouse anti-MAN1C1 (Abcam; 1:500 dilution) antibodies were used as the primary antibodies. Mouse anti-β-actin (Proteintech; 1:1000 dilution) was used as a reference for protein quantification. Goat anti-mouse IgG (Proteintech; 1:10000 dilution), goat anti-rabbit IgG (Proteintech; 1:10000 dilution), and donkey anti-goat IgG (Santa Cruz; 1:10000 dilution) were used as the secondary antibodies followed by enhanced chemiluminescence (ECL; ThermoFisher Scientific) detection.
The Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology authorized the study protocol. Written informed consent was obtained from all participants regarding the aims and objectives of the study. IRB ID:TJ-C20140411. The Valid Date was from 05/2014 to 05/2015.
Data are mean ± SD of at least three replicates. The expression of the α-1,2-mannosidase levels were compared between groups using the Student t test. Statistical analysis was performed using SPSS software. Differences were considered statistically significant at a value of P < 0.05.
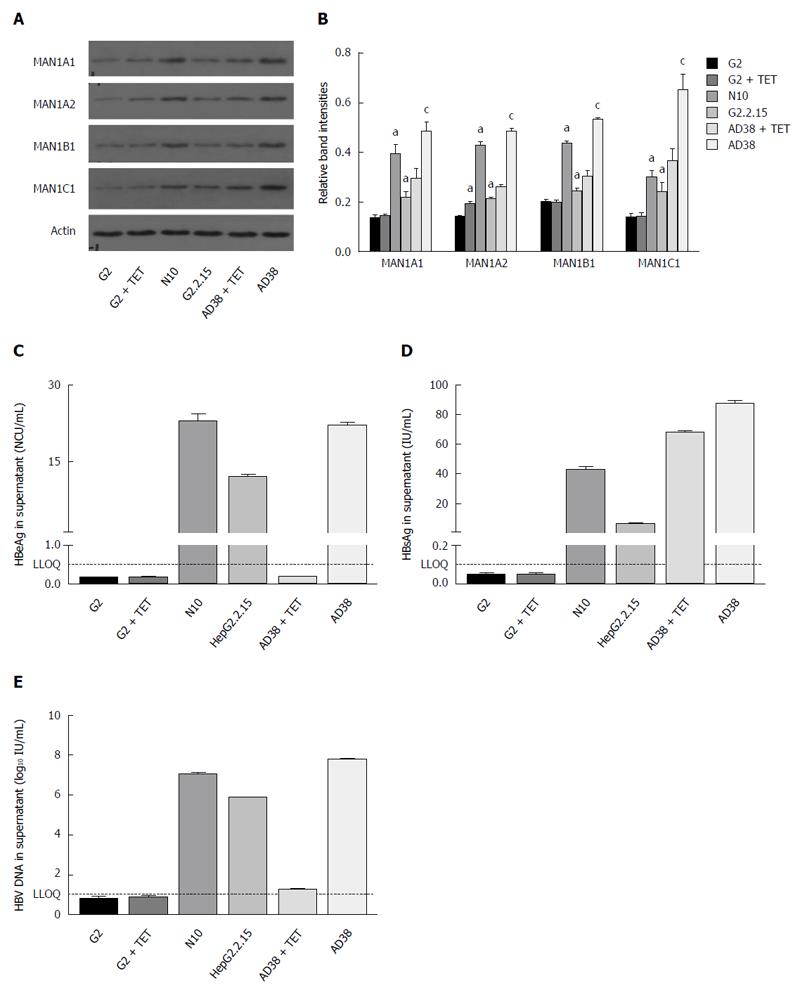
In order to investigate whether HBV could upregulate the expression of α-1, 2-mannosidases, we measured the expression of MAN1A1, MAN1A2, MAN1B1, and MAN1C1 in hepatoma cells with or without HBV transfection. MAN1A1, MANA2, MAN1B1, and MAN1C1 protein levels in the HepG2.2.15 and N10 cell lines with stable HBV-transfection were higher than in HepG2 cells (Figure 1). To confirm whether HBV infection was the cause of the upregulation, AD38 cells, which express the HBV genome under the control of a tetracycline (Tet)-off promoter, were further investigated. The expression of HBV genes in the AD38 cell line was restricted in the presence of Tet. When Tet was absent, these cells produced 3.5 kb HBV pregenomic RNA and secreted virus-like particles into the supernatant. We found that α-1,2-mannosidase expression in the AD38 cells without Tet was higher than in cells with Tet (Figure 1A and B).
The expression levels of mannosidases were higher in AD38 cells than those in ND10 cells, which were in turn greater than those in G2.2.15 cells. To investigate the association between the expression of α-1,2-mannosidase and virus secretion, we assessed virus secretion in the cell culture supernatants of various HBV cell lines. Among the three cell lines, AD38 without Tet showed the highest secretion of HBV-DNA (107.8± 0.1 IU/mL), HBeAg (21.9 ± 0.8 NCU/mL), and HBsAg (87.5 ± 2.4 IU/mL) (Figure 1C-E). Further, the expression of α-1,2-mannosidase in all the cell lines was positively correlated with the expression of HBsAg (Figure 1D).
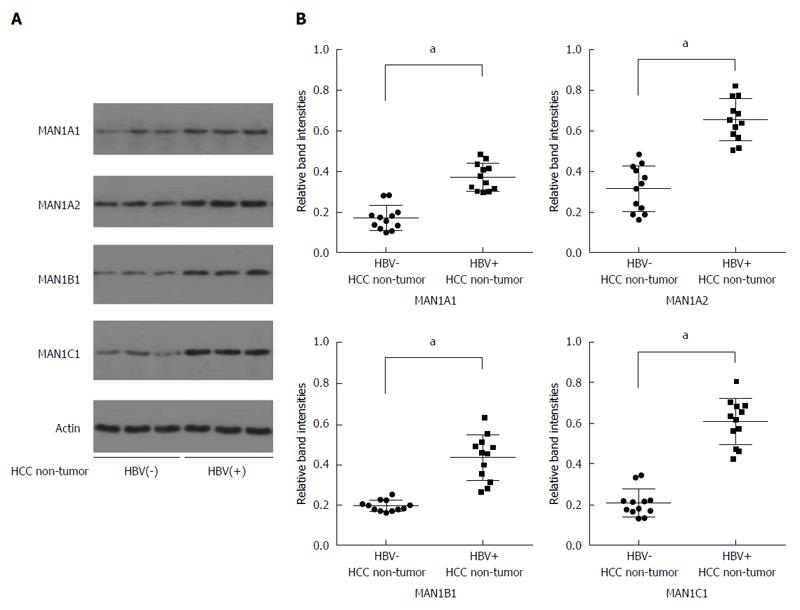
Next, we investigated whether HBV could upregulate the expression of α-1, 2-mannosidases (MAN1A1, MAN1A2, MAN1B1, and MAN1C1) in human liver samples. Levels of α-1,2-mannosidase in non-tumorous liver tissues of the HBV-related HCC patients were higher than in the tissues from non-HBV-related HCC patients (Figure 2). These findings suggest that HBV increases the expression of α-1,2-mannosidases both in vivo and in vitro. Since previous studies have shown that MAN1C1 plays a vital role in glycosylation[14], subsequent experiments focused on the role of this protein, as a representative of the α-1,2-mannosidases.
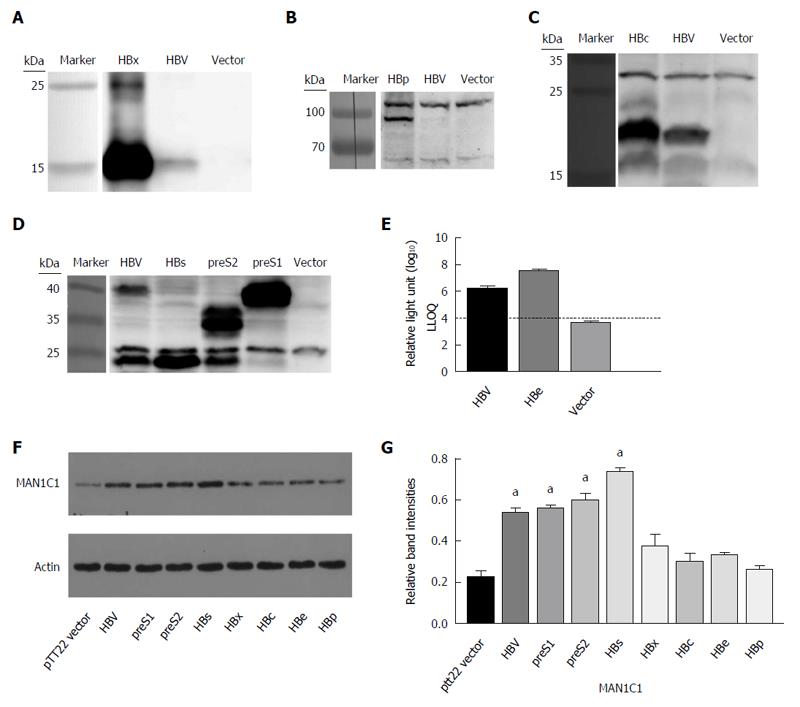
To further confirm the effect of HBV on α-1,2-mannosidase expression, plasmids containing seven individual viral genes of HBV (PTT22-HBx, PTT22-HBs, PTT22-preS2, PTT22-preS1, PTT22-HBc, PTT22-HBe, and PTT22-HBp) or control plasmids (PTT22-vector) were transfected into HepG2 cells. Western blot was used to determine the expression of HBx (Figure 3A), HBp (Figure 3B), HBc (Figure 3C), HBs, preS2, and preS1 (Figure 3D); whereas ELISA was used to estimate the expression of HBe (Figure 3E). The MAN1C1 protein levels increased in cells transfected with HBs, preS2, and preS1; however, the other viral proteins (HBc, HBx, HBe, and HBp) had no significant effects on α-1,2-mannosidase expression (Figure 3F and G). HBV has the genes for three envelope proteins: SHB, MHB (PreS2 + S) and LHB (PreS1 + PreS2 + S)[19]. Because of the similarity in the SHB motifs for PTT22-HBs (SHB), PTT22-preS2 (MHB), and PTT22-preS1 (LHB), we focused on PTT22-HBs for further study.
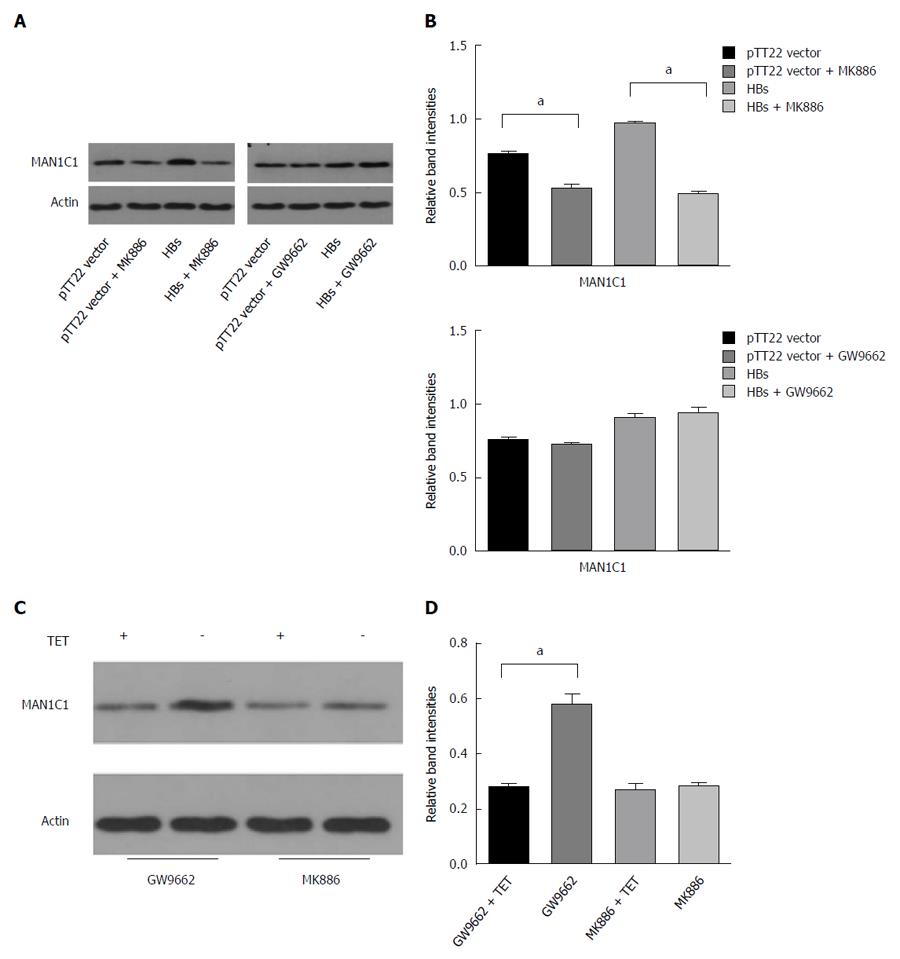
As the downregulation of MAN1C1 expression can be reversed by activation of the PPAR signaling pathway[14], we investigated whether the HBV-induced increase in α-1,2-mannosidase expression is associated with the PPAR signaling pathway. As HepG2 cells only express PPARα and PPARγ[18], MK886 (PPARα) and GW9662 (PPARγ) inhibitors were used to explore the effects of HBV on α-1,2-mannosidase expression after the PPARα and PPARγ pathways were blocked. The results showed that after the application of the PPARα inhibitor MK886, the effects of HBV on α-1,2-mannosidase expression were neutralized; however, no such effects were found when a PPARγ inhibitor was applied (Figure 4A). In order to further confirm whether the PPAR signaling pathway plays a role in the HBV-mediated increase in α-1,2-mannosidase levels, we tested the effect of GW9662 and MK886 on the expression of α-1,2-mannosidase in AD38 cells. We found that MK886 did not affect the expression of MAN1C1 in AD38 cells without Tet, indicating that MK886 inhibited the increase in α-1,2-mannosidase levels caused by the Tet withdrawal. However, this phenomenon was not observed in the case of GW9662 (Figure 4C).
Most enveloped viruses, including HBV, contain envelope protein polysaccharides, which are extensively glycosylated[20]. Protein glycosylation has multiple functions, and sometimes assists in evading immune surveillance[21]. Viruses use the host cell glycosylation system to synthesize and modify their envelope proteoglycans, and thus, the structures of viral envelope glycoproteins can be affected by the glycosylation mechanisms in the host[22]. Indeed, previous studies have shown that applying low-doses of glucosidase inhibitors to host cells changes the phenotype of the viral envelope proteins[23]. This, in turn, can reduce viral infection and affect the virus assembly and/or particle release[23]. For example, the α-glucosidase inhibitor NB-DNJ was previously shown to prevent the HBV from correct folding and the release of viral envelope molecules, and thus, could dose-dependently reduce the virus level[9]. On the other hand, while the α-mannosidase I inhibitor kifunensine did not affect the production or secretion of HBV virus particles, it did increase its recognition by DC-SIGN, resulting in activation of the immune response[8,9]. Moreover, in our previous studies, we found that demannosylation is beneficial to HBV to escape DC-SIGN recognition[10]. Therefore, the upregulation of host α-mannosidases by HBV, as observed in this study, appears to contribute to viral escape[10].
HBV contains three envelope proteins made up of the preS1, preS2, and S domains: the large (L, preS1/preS2/S), middle (M, preS2/S), and small (S) envelope proteins[24]. The L and S proteins can only be singly glycosylated (P39 and GP42 for the L protein, and P24 and GP27 for S protein). There is also a N-glycosylation site (NXS/T) at N146 of the S region. On the other hand, the M protein can be bi-glycosylated (at the GP33 and GP36 sites)[25]. We used plasmids encoding different HBV proteins, including the L, M, and S proteins, to transfect HepG2 cells. Surprisingly, we found that only the L, M, and S proteins could increase the expression of MAN1C1; however, such an effect was not found for the other HBV proteins. As all the L, M, and S proteins include the S domain, we hypothesized that it was this region that induced the increase of MAN1C1. Therefore, the S protein was used to represent the envelope protein in the consequent experiments.
As it was unclear whether this envelope protein increased MAN1C1 expression via a direct or indirect effect, we further investigated the exact mechanisms involved in HBV-induced MAN1C1 expression. We examined whether the HBV envelope protein activated the PPARγ pathway to increase the expression of MAN1C1 protein. However, we found that inhibition of the PPARγ pathway with GW9662 did not affect the HBV envelope protein-induced upregulation of MAN1C1 expression. On the other hand, when the PPARα pathway was inhibited with MK886, the envelope protein could not upregulate MAN1C1 expression. Our results indicate that MAN1C1 expression is associated with the PPARα pathway, whereas previous studies have shown that MAN1C1 expression in endothelial cells is associated with the PPARγ pathway[14]. We speculate that the expression of PPAR subtypes could be altered in different cell types, which could be at least partially responsible for the difference between our findings and the previous studies. We also tested the effect of MK886 on the expression of α-1,2-mannosidases in AD38 cells. Increases in the levels of MAN1C1 were inhibited by MK886 in AD38 cells. Therefore, we speculate that the effects of the HBV envelope protein in upregulating the expression of class I α-mannosidases are closely associated with the PPARα pathway.
We only focused on MAN1C1 in this study. Furthermore, the measurement of HBV glycosylation status together with the effect on HBV glycosylation upon knock-down of the different mannosidase members in HBV expressing or not cells might better explain the correlation between HBV production, mannosidase level and HBV glycosylation status. We will consider all the mannosidase members and explain the correlation between HBV production, mannosidase level and HBV glycosylation status in future experiments.
In conclusion, the findings of the present study showed that HBV could increase the expression of class I α-mannosidases both in vitro and in vivo. Moreover, the HBV envelope protein increases the expression of class I α-mannosidases via the PPARα pathway (Figure 5). These findings suggest that the class I α-mannosidases could be used as drug targets to inhibit the demannosylation of HBV, thereby improving the binding of the virus to DC-SIGN and disrupting the immune tolerance to prevent and treat viral infection.
We would like to thank Professor Xiao-Ping Chen from the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for providing the liver samples, and Mr. Quan Yuan and Tian-Ying Zhang from the Xiamen University for providing the cells and plasmids.
Hepatitis B virus (HBV) infection is the most common chronic viral infection in the world. An estimated 2 billion people are infected, and more than 350 million are chronic carriers of the virus.
Few studies have investigated the effects of HBV on the expression of class I α-mannosidases.
Tumor necrosis factor-α has been shown to downregulate the expression of MAN1C1 to increase the expression of high-mannose type proteoglycans, and this can be reversed by the activation of the PPAR signaling pathway. Based on the results of these previous studies, the authors hypothesized that HBV could promote the demannosylation of the viral protein by increasing the expression of α-mannosidase I in the host cells.
Class I α-mannosidases could be used as drug targets to inhibit the demannosylation of HBV and prevent immune tolerance of the virus. The authors will consider all the mannosidase members and explain the correlation between HBV production, mannosidase level and HBV glycosylation status in future experiments.
HBV infection is the most common chronic viral infection worldwide. Because of insufficient immune response, some HBV-infected patients will develop chronic hepatitis and possibly liver cirrhosis and hepatocellular carcinoma. In the present study, the authors aimed to explore one of the mechanisms able to impair dendritic cell function in patients suffering from chronic hepatitis B; they hypothesized that HBV could promote the demannosylation of HBV glycoprotein coat by increasing the expression of α-mannosidase 1 allowing HBV to escape from host immunity.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Schvoerer E S- Editor: Qi Y L- Editor: Logan S E- Editor: Wang CH
| 1. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1155] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 2. | Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 3. | van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HL. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Cloonan SM, Choi AM. Mitochondria: sensors and mediators of innate immune receptor signaling. Curr Opin Microbiol. 2013;16:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587-597. [PubMed] |
| 6. | Varga N, Sutkeviciute I, Ribeiro-Viana R, Berzi A, Ramdasi R, Daghetti A, Vettoretti G, Amara A, Clerici M, Rojo J. A multivalent inhibitor of the DC-SIGN dependent uptake of HIV-1 and Dengue virus. Biomaterials. 2014;35:4175-4184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | de Witte L, de Jong MA, den Dunnen J, van Kooyk Y, Geijtenbeek TB. Identification of pathogen receptors on dendritic cells to understand their function and to identify new drug targets. Methods Mol Biol. 2009;531:267-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Op den Brouw ML, de Jong MA, Ludwig IS, van der Molen RG, Janssen HL, Geijtenbeek TB, Woltman AM. Branched oligosaccharide structures on HBV prevent interaction with both DC-SIGN and L-SIGN. J Viral Hepat. 2008;15:675-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Garcia T, Li J, Sureau C, Ito K, Qin Y, Wands J, Tong S. Drastic reduction in the production of subviral particles does not impair hepatitis B virus virion secretion. J Virol. 2009;83:11152-11165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Wang M, Zou X, Tian D, Hu S, Jiang L. Role of Dendritic Cell-Specific ICAM-3-Grabbing Nonintegrin on Dendritic Cells in the Recognition of Hepatitis B Virus. Viral Immunol. 2015;28:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Moremen KW, Nairn AV. Mannosidase, Alpha, Class 1 [MAN1A1 (Golgi Alpha-Mannnosidase IA), Man1A2 (Golgi Alpha-Mannosidase IB), MAN1B1(ER Alpha-Mannosidase I), MAN1C1 (Golgi Alpha-Mannosidase IC)]. Japan: Springer 2014; . |
| 12. | Simsek E, Mehta A, Zhou T, Dwek RA, Block T. Hepatitis B virus large and middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme. J Virol. 2005;79:12914-12920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Lazar C, Macovei A, Petrescu S, Branza-Nichita N. Activation of ERAD pathway by human hepatitis B virus modulates viral and subviral particle production. PLoS One. 2012;7:e34169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Chacko BK, Scott DW, Chandler RT, Patel RP. Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor γ ligands. J Biol Chem. 2011;286:38738-38747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Wang HW, Gao HL, Wei XX, Wang XH. Up-regulation of IL-12 expression in patients with chronic hepatitis B is mediated by the PI3K/Akt pathway. Mol Cell Biochem. 2015;407:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Ladner SK, Otto MJ, Barker CS, Zaifert K, Wang GH, Guo JT, Seeger C, King RW. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother. 1997;41:1715-1720. [PubMed] |
| 17. | Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005-1009. [PubMed] |
| 18. | Han C, Demetris AJ, Michalopoulos G, Shelhamer JH, Wu T. 85-kDa cPLA(2) plays a critical role in PPAR-mediated gene transcription in human hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G586-G597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Wounderlich G, Bruss V. Characterization of early hepatitis B virus surface protein oligomers. Arch Virol. 1996;141:1191-1205. [PubMed] |
| 20. | Geijtenbeek TB, den Dunnen J, Gringhuis SI. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009;4:879-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 22. | Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107-151. [PubMed] |
| 23. | Lazar C, Durantel D, Macovei A, Zitzmann N, Zoulim F, Dwek RA, Branza-Nichita N. Treatment of hepatitis B virus-infected cells with alpha-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antiviral Res. 2007;76:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013;35:39-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Mehta A, Lu X, Block TM, Blumberg BS, Dwek RA. Hepatitis B virus (HBV) envelope glycoproteins vary drastically in their sensitivity to glycan processing: evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion. Proc Natl Acad Sci USA. 1997;94:1822-1827. [PubMed] |