INTRODUCTION
Splanchnic circulation is the main region primarily responsible for regulating circulating blood volume and systemic blood pressure in patients with liver cirrhosis accompanied by portal hypertension[1]. A persistent reduction in splanchnic blood flow is associated multi-organ failure as well as an increased rate of mortality among critically ill patients. However, increased splanchnic blood flow is associated with increased portal venous inflow, which worsens pre-existing portal hypertension in patients with liver cirrhosis[2]. Splanchnic and systemic circulation closely interact with each other. For instance, an increase in portal hypertension is associated with the overproduction of vasodilatory molecules, and consequent arterial vasodilatation and decreased central blood volume[2]. This review highlights some potential benefits derived from modulating splanchnic circulation during the perioperative management of patients undergoing liver transplantation.
OPTIMIZATION OF BLOOD VOLUME MANAGEMENT DURING LIVER TRANSPLANTATION
Blood volume in patients with liver cirrhosis
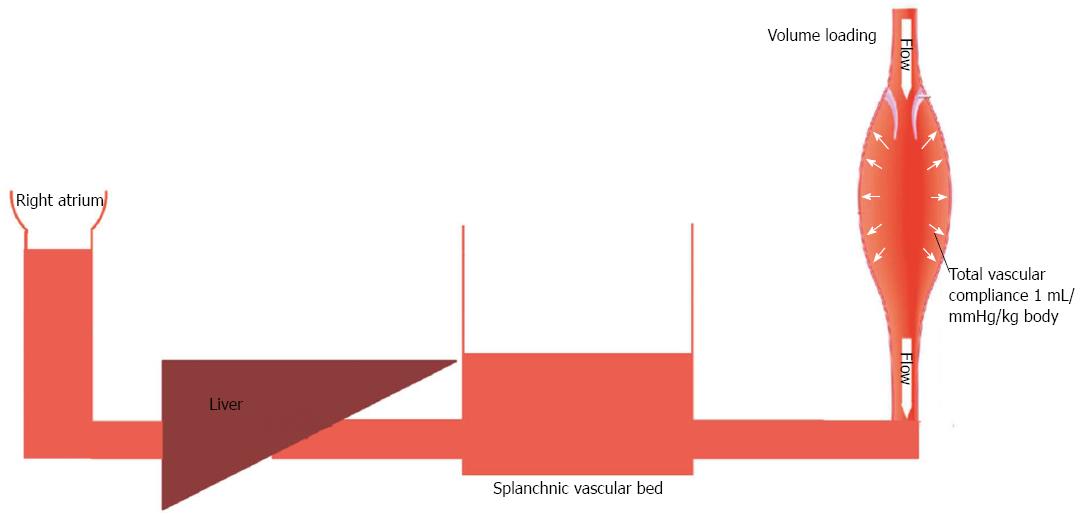
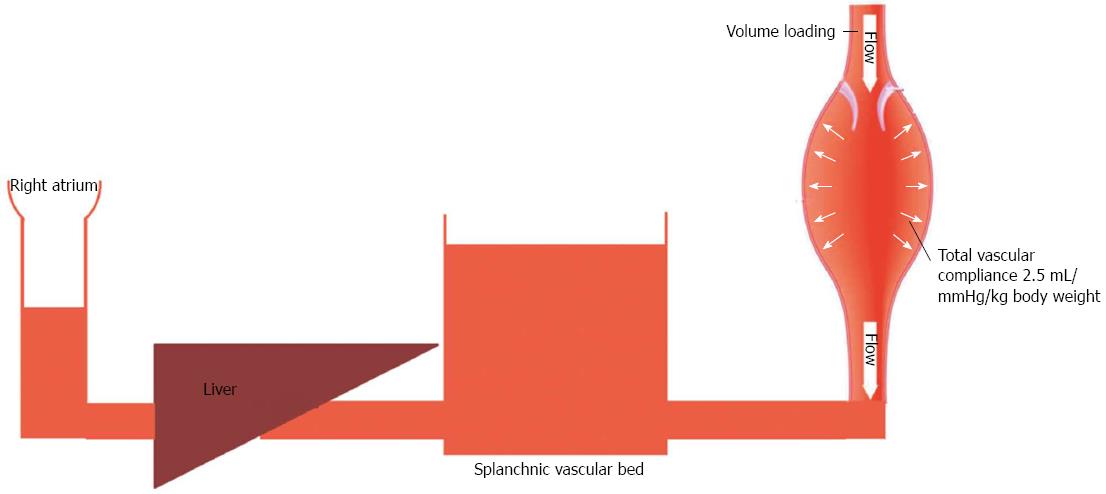
Patients with liver cirrhosis experience a substantial increase in their total blood volume[1]. Kiszka-Kanowitz et al[1] reported that patients with end-stage liver disease not only have an abnormally high volume of blood, but also an abnormal distribution of their blood volume. Such patients typically have > 37% of their total blood volume located in the abdomen, which is significantly higher when compared to healthy subjects, in whom abdominal organs contain < 30% of the total blood volume. Healthy individuals and patients with end-stage liver disease also differ in their response to blood volume expansion. In healthy individuals, the value for total vascular compliance is 0.5-1 mL/mmHg/kg body weight[3]. Thus each 500 mL of fluid loading produces a 5 mmHg increase in blood pressure (Figure 1). However, total vascular compliance is significantly increased (values of 1.5-2.5 mL/mmHg/kg body weight)[4] in patients with liver cirrhosis (Figure 2). Thus it is questionable whether the traditional approach of maintaining systemic arterial pressure by optimizing cardiac output via aggressive volume resuscitation is the best strategy to employ during the intraoperative management of liver transplant patients[2]. Moreover, extensive fluid loading may result in increased blood loss due to aggravation of portal hyperemia and increased splanchnic venous congestion, but have minimal effect on cardiac output[1]. Therefore, when performing the dissection phase of a liver transplant, restrictive volume management has been suggested as the preferred method for minimizing venous congestion and reducing blood loss[5]. However, volume restriction is associated with increased risks for both postoperative acute renal failure and mortality[6]. Pharmacologic modulation of splanchnic circulation by use of vasoconstrictors might produce the same response as obtained when using a restrictive volume strategy (minimization of venous congestion and replenishment of central blood volume). However, the question regarding which pharmacologic agent to use has not been answered. To help answer this question, we have provided an overview of the distribution patterns of catecholamine and vasopressin receptors in the splanchnic area.
Figure 1 Fluid loading in a healthy subject.
Low vascular compliance combined with decreased pooling of blood in splanchnic circulation is associated with increased central venous pressure.
Figure 2 Fluid loading in a cirrhotic patient.
High vascular compliance combined with increased pooling of blood in splanchnic circulation is associated with a minimal increase in central venous pressure.
Anatomy and regulation of splanchnic blood flow
It is estimated that 25% percent of total cardiac output enters the splanchnic circulation via three large arteries (celiac, superior, and inferior mesenteric arteries). The liver receives 25% of its arterial blood supply via the hepatic artery, and the remaining 75% via the portal vein. Venous drainage through the hepatic vein accounts for the entire volume of hepatosplanchnic blood flow (Figure 3).
Figure 3 Anatomy of splanchnic blood flow.
The liver receives 25% of its arterial blood supply via the hepatic artery and the remaining 75% via the portal vein.
Distribution of catecholamine receptors in the splanchnic area
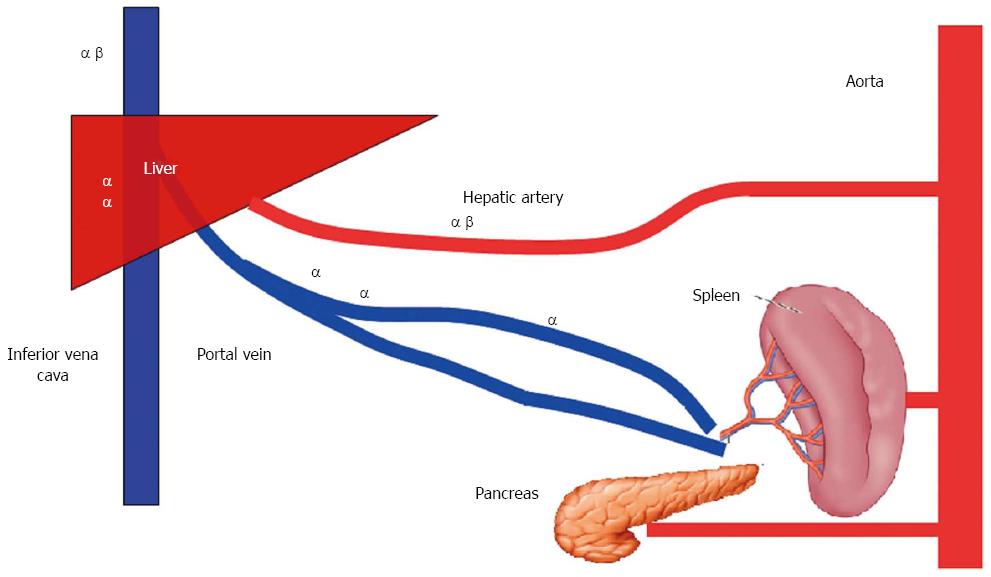
While the hepatic artery contains α1-, α2-, and β2-adrenergic receptors, the preportal (intestinal capacitance) and portal veins contain α-adrenergic, but not β2-adrenergic receptors[7]. Capacitance vessels located inside the liver contain only α-adrenergic receptors, while the hepatic veins contain both α- and β2-adrenergic receptors[7] (Figure 4).
Figure 4 Distribution of catecholamine receptors in the splanchnic area.
The hepatic artery contains α1-, α2-, and β2-adrenergic receptors; the preportal (intestinal capacitance) and portal veins contain α-adrenergic, but not β2-adrenergic receptors. Capacitance vessels located inside the liver contain only α-adrenergic receptors, while the hepatic veins contain both α- and β2-adrenergic receptors.
Effect of catecholamines on shift of blood volume from splanchnic to systemic circulation
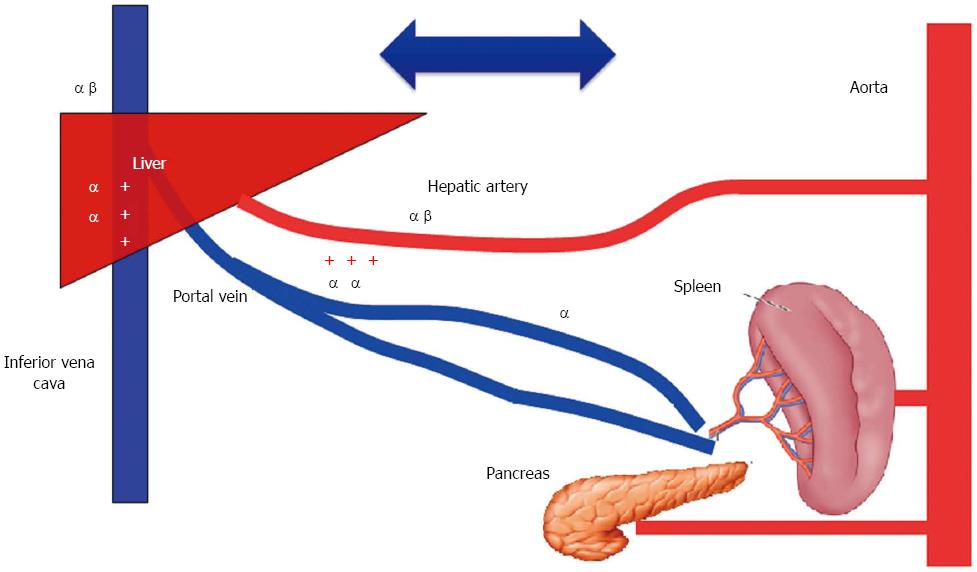
A shift of blood volume from splanchnic to systemic circulation requires formation of a pressure gradient between prehepatic (splanchnic) and intrahepatic vascular resistance. A stimulation of prehepatic vascular resistance induces splanchnic venoconstriction which expels blood from splanchnic to systemic circulation[8], while an increase in intrahepatic vascular resistance can cause blood to pool within the splanchnic area and worsen any pre-existing systemic hypovolemia[9]. The effect of any specific catecholamine-like agent that alters systemic circulating blood volume by manipulating splanchnic circulation depends on three factors: (1) the respective densities of α- and β-adrenergic receptors throughout the splanchnic circulatory system; (2) the effect of the catecholamine agent on various subtypes of adrenoceptors; and (3) the volume of blood within the splanchnic circulatory system. Thus, while a pure α-adrenergic receptor agonist such as phenylephrine might increase venous return in normovolemic patients, it may decrease it in hypovolemic patients[8]. In contrast, epinephrine-like agents have a high affinity for both α- and β-adrenoceptors in the splanchnic vasculature; as a result, their effect on splanchnic circulation is complex, and can be dose-related[8]. Recently, Massicotte et al[10] examined the effects of phlebotomy and a subsequent phenylephrine infusion on portal venous pressure and intravascular blood volume in patients undergoing orthotopic liver transplantation. Intravascular blood volumes were assessed by measuring early peak velocities of transmitral flow, pulmonary artery occlusion pressure, and cardiac output. The results showed that phlebotomy decreased both portal venous pressure and intravascular blood volume, and an infusion of phenylephrine following phlebotomy failed to restore the previous intravascular blood volume. Moreover, an infusion of phenylephrine did not further reduce portal venous pressure. Those study results can be explained by the pure stimulatory effect of phenylephrine on α-adrenergic receptors located in the splanchnic vasculature. Stimulation of α-receptors in pre- and post-portal capacitance vessels failed to create a gradient sufficient to shift blood from splanchnic to systemic circulation, and especially in the presence of hypovolemia (Figure 5).
Figure 5 Effect of stimulation of catecholamine receptors within the splanchnic area on blood flow.
Stimulation of α receptors in the pre- and post-portal capacitance vessels failed to create a gradient sufficient to shift blood from splanchnic to systemic circulation.
No data is available regarding the effect of other catecholamines on splanchnic circulation during a liver transplant. However, a recent study used Doppler ultrasound to examine the effect of low-dose epinephrine infusion on spleen size and hepatic vein blood velocity in healthy volunteers[11]. Those results showed that spleen volume began to decrease following an epinephrine infusion, and this decrease was accompanied by an increase in hepatic blood flow, which was primarily due to activation of -adrenergic receptors and decreased vascular resistance.
Distribution of vasopressin receptors in the splanchnic area
Three main and distinct types of vasopressin receptors have been identified in the body. V1 receptors (the main vasopressin receptors) facilitate vasoconstriction, and are primarily found on vascular smooth muscle tissue.
Types of vasopressin agents
Arginine-vasopressin (AVP) is a non-peptide molecule that acts by stimulating V1 and V2 receptors. AVP has a short duration of action (half-life approximately 6 min) because it is rapidly eliminated by circulating vasopressinase[12]. Terlipressin (triglycyl-lysine-vasopressin) is a synthetic analogue of arginine vasopressin, and has a half-life of approximately 6 h[13]. Terlipressin has a similar pharmacodynamic profile but displays different pharmacokinetic properties compared to its parent molecule. Terlipressin is rapidly metabolized by endopeptidases to form vasoactive lysine vasopressin, which is characterized by its greater selectivity for V1 vs V2 receptors (2.2:1 compared to 1:1 for arginine vasopressin)[13].
Effect of vasopressin agents on the shift of blood volume from splanchnic to systemic circulation
Several reports have described the effect of AVP on splanchnic vasculature in animal models of portal hypertension[14,15]. In those studies, AVP was shown to be a powerful splanchnic vasoconstrictive agent capable of reducing portosystemic collateral blood flow. In another study, AVP displayed this same effect in cirrhotic patients without variceal bleeding[16]. However, in that study, AVP increased intrahepatic vascular resistance and decreased hepatic blood flow by 30%. Terlipressin has demonstrated splanchnic and systemic hemodynamic effects similar to those produced by AVP when examined in animal models[17], and also patients with liver cirrhosis[18]. However, terlipressin and AVP showed different effects on intrahepatic hemodynamics. Terlipressin reduced intrahepatic vascular resistance, resulting in a concomitant increase in hepatic arterial blood flow[18]. Kiszka-Kanowitz et al[19] reported that when administered to cirrhotic patients, terlipressin increased the volumes of blood in the liver and thoracic regions by 12% and 6%, respectively, resulting in an increase in mean arterial pressure and reduction in hyperdynamic circulation. Our group reported the effect of intraoperative terlipressin infusion on splanchnic and systemic hemodynamics in patients undergoing a living donor liver transplant (LDLT)[20]. We found that when compared to patients in a control group, patients receiving terlipressin required a lower volume of colloid fluid therapy to maintain their intravascular blood volume. Thus, terlipressin might benefit central blood volume by increasing effective blood volume via stimulating arteriolar vasoconstriction in the splanchnic area, and redistributing blood into systemic circulation.
MINIMIZATION OF HIGH PORTAL FLOW AFTER LIVER RESECTION AND LIVER TRANSPLANT
Portal hemodynamics during liver resection and LDLT
Portal blood flow has a significant impact on liver regeneration following liver resection, as well as the outcomes of patients undergoing LDLT. High portal flow impairs liver regeneration, and thus adversely affects the postoperative recovery of transplant recipients. A major liver resection (> 75% the total liver) can produce a significant increase in portal pressure, and the subsequent development of small for size syndrome (SFSS), which is associated with a poor clinical outcome[21]. Furthermore, the increased number of LDLTs performed in adults during the last decade has increased the incidence of SFSS[22]. SFSS occurring after a living donation is defined as two or more of the following findings being recorded on three consecutive days within the first week after transplantation of an undersized graft (graft weight/recipient weight ratio < 0.8%): a bilirubin level > 100 μmol/L; an international normalized ratio > 2.0; grade 3-4 encephalopathy[23]. While the pathogenesis of SFSS is thought to result from intravascular shear stress produced by portal hyperperfusion[24], a reduction in arterial blood flow occurring as a reflexive response to hepatic arterial buffering may also be a contributing factor[25]. Hepatic arterial buffer response (HABR) is best described as the compensatory changes in hepatic arterial blood flow which occur secondary to changes in portal venous flow[26]. While HABR is preserved in a newly implanted liver that is properly sized for the recipient[27], patients with severe SFSS experience an increase in portal blood flow that is associated with significant vasospasm of the hepatic artery. Such vasospasms may lead to functional de-arterialization of the graft, and subsequent infarction of the parenchyma[28].
Several surgical and pharmacological methods have been suggested for modifying HABR. These methods can be classified into those that minimize portal venous blood flow and those that augment arterial blood flow.
Surgical methods for minimizing portal blood flow
When a small for size graft is transplanted, graft inflow modulation has been suggested as an important tool for preventing portal hyperperfusion of the transplanted liver. Modulation of graft inflow can be achieved by splenic artery ligation, splenectomy, or portocaval shunting[21,29]. Although substantial evidence suggests that any of these methods will improve a patient’s outcome, there is no general agreement on when to employ such methods intraoperatively. However, the following stepwise approach for achieving graft inflow modulation has been suggested[30]. The first step is to obtain measurements of both portal venous flow (PVF) and the hepatic vein pressure gradient (gradient between portal venous pressure and hepatic vein pressure). PVF can be measured either directly by using a transonic flowmeter with an ultrasonic probe encircling the main portal vein, or indirectly by Doppler ultrasound. When using Doppler ultrasound, measurements of PVF are obtained by multiplying the cross-sectional area of the portal vein by the portal vein blood velocity. If the value for PVF is between 360-500 mL/min/100 g LW and/or the graft weight/recipient weight ratio is < 0.8%, the splenic artery should be ligated. However, if the value for PVF is very high (> 500 mL/min/100 g LW), splenic arterial ligation will not likely be effective, and insertion of a hemiportocaval shunt should be considered. Some medical centers have advocated the use of splenectomy when the value for PVP is > 15 mmHg[31]. The option to perform splenectomy has expanded the criteria used for graft selection to include a left lobe graft and graft weight/recipient weight ratio of 0.7%.
Pharmacological methods for minimizing portal blood flow
If the portal hyperperfusion theory truly explains the pathogenesis of SFSS, a pharmacological intervention that reduces portal pressure but preserves HABR should be of clinical value. Mehrabi et al[32] reported that infusions of epinephrine and norepinephrine significantly decreased total hepatic blood flow in transplanted livers in pigs[32]. Those investigations concluded that reduced hepatic perfusion seen during infusion of both vasopressors was caused by reductions in both PVF and hepatic arterial flow. These results suggest that catecholamines may not be ideal agents for minimizing portal pressure, as they compromise hepatic perfusion in a dose-dependent manner[32].
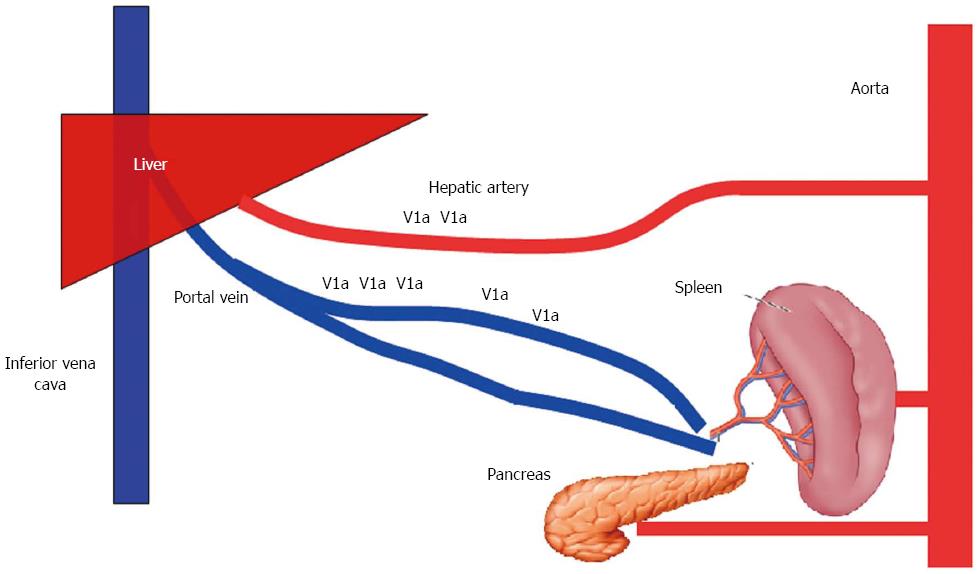
Vasopressin and terlipressin are two other splanchnic vasoconstrictors that reduce PVP while maintaining HABR. Their selective effects are due to the fact that V1a receptors are more prevalent in the portal venous side than the arterial side. Thus the effects of vasopressin on the liver are heterogeneous, and more pronounced on the portal vein than the hepatic artery (Figure 6)[33]. Krejci et al[34] examined the effects of vasopressin in an animal model of septic shock, and found that an infusion of vasopressin significantly decreased blood flow through the portal vein, while flow through the hepatic artery remained unchanged. A similar vasoconstrictive effect on portal blood flow was found when administering terlipressin to liver cirrhosis patients; however, the results showed an increase in hepatic blood flow concomitant with a reduction in hepatic arterial resistance[35]. Although their usefulness for managing SFSS has not been studied, the effects of vasopressors on portal pressure when performing a liver transplantation have been previously examined[20,36]. Wagener[36] infused vasopressin into 16 patients undergoing liver transplantation and reported that vasopressin significantly decreased portal vein pressure and the flow of native liver blood, without reducing cardiac output. In our study, terlipressin infusion produced significant reductions in PVP. However, neither our study nor the previous study was able to examine the effect of vasopressor agents on hepatic blood flow, as all patients in the studies had their hepatic artery ligated prior to infusion of a vasopressor drug. Although the rationale for minimizing portal vein flow during LDLT is well accepted, the safety of applying this technique soon after liver transplantation has yet to be determined. Several studies have recently investigated the effect of terlipressin on hepatic hemodynamics in a small for size liver animal model. In a preliminary study, Ren et al[37] created a small for size rat model by resecting > 90% of the liver parenchyma, and then randomly assigned the resected rats to two separate groups. One group received terlipressin (0.05 mg/kg) and the other group received saline. Those investigators found that elevated portal pressure in the resected rats could be ameliorated by administration of terlipressin during the first week following liver resection. Moreover, terlipressin improved the 10-d survival rate from 30% to 80% in animals that received a small-for-size liver. More recently, Farmer et al[38] demonstrated similar findings in a murine model. In that study, portal venous pressure in mice was significantly increased following an 80% resection of liver parenchyma. Terlipressin injection produced a significant reduction in portal pressure, and a concomitant improvement in liver regeneration. A plausible mechanism for this improved outcome is that terlipressin reduced endothelial cell damage, as documented by electron microscopy[38].
Figure 6 Distribution of vasopressin receptors in the splanchnic area.
V1a receptors are more prevalent on the portal venous side than the arterial side.
Despite the potential beneficial effects of both vasopressin and terlipressin in patients with liver cirrhosis, extreme caution should be taken when using these drugs to treat patients with a history of ischemic heart disease. Although data comparing the coronary effects of terlipressin and vasopressin are scarce, terlipressin appears to be safer than vasopressin and produces a smaller cardiovascular effect[39].
Pharmacological techniques to augment arterial blood flow
Several pharmacologic agents have been suggested for use in augmenting hepatic arterial blood flow in small-for-size livers. A previous study showed that infusion of adenosine into the hepatic artery restored hepatic artery flow and improved survival in an animal model[40]. Additionally, infusion of prostaglandin E1 either alone or in combination with somatostatin reduced tissue injury in small-for-size grafts[41,42].
MAINTENANCE OF PERIOPERATIVE RENAL FUNCTION
Acute kidney injury (AKI) is a frequent and serious complication that may occur following liver transplantation. The reported incidence of AKI varies widely (8%-78%) depending on its definition, and this complication is associated with an eight-fold increase in patient mortality[43,44]. When based on risk, injury, failure, loss, and end-stage kidney disease classification, the prevalence of AKI following LDLT is approximately 60%[45]. The Acute Kidney Injury Network (AKIN) has recently classified AKI into three different stages based on changes in creatinine levels and/or reduced urine output. When based on these new AKIN criteria, the overall prevalence of AKI following liver transplantation is 38%[32,46]. Most cases of postoperative AKI are either related to preoperative hepatorenal syndrome (HRS) or result from intraoperative renal hypoperfusion.
Pre-transplant HRS
HRS develops as a consequence of a severely reduced effective circulating blood volume resulting from both extreme splanchnic arterial vasodilatation and reduced cardiac output[47]. In early phase portal hypertension, normal or near normal renal perfusion is maintained as a result of vasodilatory systems antagonizing the renal effects of vasoconstrictor systems[48]. However, as liver disease progresses in severity, a critical level of vascular underfilling is finally reached. When this occurs, renal vasodilatory systems can no longer counteract the effects produced by fully activated endogenous vasoconstrictors and/or intrarenal vasoconstrictors; resulting in uncontrolled renal vasoconstriction[48]. This hypothesis is supported by studies in which co-administration of splanchnic vasoconstrictors and volume expanders produced improvements in arterial pressure, renal plasma flow, and glomerular filtration rates[49]. An alternative theory proposes that in patients with HRS, renal vasoconstriction is unrelated to systemic hemodynamics, but instead is due to either deficient synthesis of a vasodilatory factor or a hepatorenal reflex that produces renal vasoconstriction[50].
While liver transplantation is the ideal treatment for HRS, the majority of transplant centers have a long waiting list, and most patients die before transplantation. As a result, there is an urgent need to identify effective alternative therapies that can increase the likelihood that a patient with HRS will survive until transplantation can be performed. This need is reinforced by a study which reported that patients who received successful medical treatment for HRS prior to liver transplantation had post-transplantation outcomes and survival rates comparable to those of patients who underwent transplantation without first being treated for HRS[51]. Once HRS has been diagnosed, treatment with terlipressin starting 24-48 h later usually results in a good clinical outcome[52]. Patients who respond to terlipressin show improved renal function as defined by either a reduction in their serum creatinine level to < 133 μmol/L at the end of treatment (complete response) or a > 50% reduction in their serum creatinine level compared to their pre-treatment level, but with an end-of-treatment value ≥ 133 μmol/L (partial response)[53]. Moreover, terlipressin improves survival in patients with combined HRS and advanced cirrhosis. The effects of terlipressin on survival are strongly related to its ability to improve renal function[47]. Survival rates among patients who respond to terlipressin treatment are better than those among non-responders. High baseline values for total serum bilirubin and serum creatinine, and a minimal response to treatment as reflected by mean arterial pressure, are predictors of a poor response to terlipressin[54]. A wealth of information is available regarding the benefits of terlipressin in treating HRS, and this agent can be used for long-term therapy in specific types of patients who have an ongoing recurrence of HRS, despite treatment. While retrospective studies clearly indicate that liver transplantation can significantly increase survival times in patients with HRS independent of their prior therapy, improving renal function prior to liver transplantation remains an important objective when treating patients with HRS, as this measure makes post-transplant management of this difficult group of patients much easier[55].
Intraoperative risk factors for AKI
Small-for-size graft: Several studies have shown a significant relationship between small-for-size grafts and the incidence of AKI following LDLT[56,57]. However, the mechanism by which a small-for-size graft induces AKI remains unclear. One theory is that the presence of persistent portal hyperperfusion and a hyperdynamic state in patients with a small-for-size graft impairs the balance between vasodilatory and vasoconstrictive factors, and thereby causes renal injury. Recently, we retrospectively analyzed the medical records of 303 patients who underwent LDLT, and identified a small-for-size graft as an independent predictor for AKI after LDLT [odds ratio (OR), 2.65; 95% confidence interval (CI), 1.1-6.3][46].
Impaired renal perfusion: Most cases of post-operative AKI are related to renal hypoperfusion due to hypovolemia, hypotension, cardiac dysfunction or caval clamping for > 70 min[58]. Such findings support modulation of splanchnic circulation as an appealing option for maintaining renal perfusion and preventing AKI.
Modulation of splanchnic circulation and renal protection
Splanchnic vasoconstrictors such as terlipressin have two mechanisms by which they might provide some protection against AKI in patients undergoing liver transplantation. First, they reduce portal pressure and hyperdynamic state; this is especially important in patients with a small-for-size graft. We recently reported a significantly lower incidence of AKI among patients given terlipressin when compared to patients in a control group. This result occurred despite the fact that more patients in the terlipressin group received a small-for-size graft[52]. Second, because terlipressin selectively stimulates V1 receptors, it induces arteriolar vasoconstriction in the splanchnic area, and shifts blood from splanchnic to systemic circulation[19,59]. Thus, terlipressin enhances renal perfusion by increasing both effective blood volume and mean arterial pressure. Fayed et al[60] recently reported the effect of intraoperative terlipressin administration on kidney function in patients with chronic liver disease who had undergone LDLT. In their study, 80 recipients were randomly selected to receive either saline alone or terlipressin at an initial dose of 3 μg/kg/h, which was later reduced to 1.5 μg/kg/h after reperfusion, and then continued for three postoperative days. Their results showed that renal function in the group receiving saline alone was significantly worse than in the group receiving terlipressin. Moreover, those same investigators had previously reported that perioperative use of terlipressin helped establish and maintain satisfactory early post-transplant kidney function in patients that underwent LDLT[20].
CONCLUSION
Modulation of splanchnic circulation provides certain benefits when employed during the perioperative management of liver transplant recipients. These benefits include the opportunity to maintain adequate central blood volume, minimize high portal flow when transplanting a small-for-size liver, and ensure the maintenance of proper renal perfusion. Although vasopressin agents appear promising for use as splanchnic modulators, evidence is needed to identify the best splanchnic vasoconstrictors is still lacking.