Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.7135
Peer-review started: March 18, 2016
First decision: May 12, 2016
Revised: May 26, 2016
Accepted: June 13, 2016
Article in press: June 13, 2016
Published online: August 21, 2016
Processing time: 151 Days and 19.4 Hours
AIM: To establish a new animal model for the research of human rotavirus (HRV) infection, its pathogenesis and immunity and evaluation of potential vaccines.
METHODS: 5-d, 30-d and 60-d-old Chinese mini-pigs, Guizhou and Bamma, were inoculated with a single oral dose of attenuated strain Wa, G1, G3 of HRV, and PBS (control), respectively, and fecal samples of pigs from 0 to 7 d post infection (DPI) were collected individually. Enzyme linked immunosorbent assay was used to detect HRV antigen in feces. The HRV was tested by real-time PCR (RT-PCR). The sections of the intestinal tissue were stained with hematoxylin and eosin to observe the morphologic variation by microscopy. Immunofluorescence was used to determine the HRV in intestinal tissue. HRV particles in cells of the ileum were observed by electron micrography.
RESULTS: When inoculated with HRV, mini-pigs younger than 30 d developed diarrhea in an age-dependent manner and shed HRV antigen of the same inoculum, as demonstrated by RT-PCR. Histopathological changes were observed in HRV inoculated mini-pigs including small intestinal cell tumefaction and necrosis. HRV that was distributed in the small intestine was restricted to the top part of the villi on the internal wall of the ileum, which was observed by immunofluorescence and transmission electron microscopy. Virus particles were observed in Golgi like follicles in HRV-infected neonatal mini-pigs. Guizhou mini-pigs were more sensitive to HRV than Bamma with respect to RV antigen shedding and clinical diarrhea.
CONCLUSION: These results indicate that we have established a mini-pig model of HRV induced diarrhea. Our findings are useful for the understanding of the pathogenic mechanisms of HRV infection.
Core tip: Rotavirus (RV) is the leading cause of serious dehydrating diarrhea in infants and young children worldwide. Animal models for RV infection research have provided key insights into the RV infection, its pathogenesis and immunity, and have offered opportunities for design and evaluation of potential vaccines. Our study indicated that human RV (HRV) could effectively replicate in the intestinal villi of the ileum of Chinese mini-pigs and lead to histopathological alterations and diarrhea. This research offers a new animal model for studying the pathological changes and immunogenicity of HRV infection and a useful tool in the design and evaluation of RV vaccines.
- Citation: Li JT, Wei J, Guo HX, Han JB, Ye N, He HY, Yu TT, Wu YZ. Development of a human rotavirus induced diarrhea model in Chinese mini-pigs. World J Gastroenterol 2016; 22(31): 7135-7145
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/7135.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.7135
Rotavirus (RV) is well known as the major cause of severe gastroenteritis in infants under five years of age, responsible for an estimated 480000 to 640000 deaths per year worldwide[1]. Currently, there is no specific medicine for treating RV diarrhea; thus, a safe and effective vaccine would be the best way to control RV[2]. To date, all approved RV vaccines delivered through oral imitation to mimic natural infection have been in attenuated form. Although natural RV infection has shown substantial protection against RV infection, potential virulence recovery of attenuated RV remains a safety concern. Moreover, the immune response mechanism to RV infection is elusive, which hampers the development of RV vaccines. Therefore, an ideal animal model of human RV (HRV) infection is urgently needed to improve the understanding of the immune response to RV infection in humans. Previously tested animal models include mouse, rabbit, rat, lamb, calf and gnotobiotic pig[3-8]. However, our understanding of the immune protection mechanism against HRV infection has been limited to these models. Due to host restriction, small animals such as rodents and rabbits are prone to refractory HRV infection, limiting their potential for studying the pathogenesis and immunity of HRV infection. On the other hand, while larger animals, such as Large White cross-bred pigs could be infected with HRV, genetic consistency and hereditary stability may affect the HRV infection[9-24].
Chinese mini-pigs have been used in many medical experiments as animal models[25]. In this study, two breeds of Chinese mini-pigs, Guizhou and Bamma, were selected to develop an HRV induced diarrhea model because of the following advantages: (1) higher genetic consistency and hereditary stability compared to conventional pigs, with the group similarity coefficient close to 0.928 after inbreeding for nearly 20 generations; (2) early sexual maturation with high reproduction rate; and (3) small size (the neonate Guizhou mini-pig weighs 0.3-0.5 kg and the one-month-old weighs 1.0-1.5 kg), comparable to rabbits[26,27]. We examined whether HRV can replicate efficiently in Chinese mini-pigs due to the different gene background. Furthermore, we were interested in elucidating whether different G serotypes of human group A RV can replicate, spread, and induce disease. Our study findings indicated that HRV could effectively replicate in the intestinal villi of the ileum of Chinese mini-pigs and lead to histopathological alterations and diarrhea. This research offers a new animal model for studying the pathological change and immunogenicity of HRV infection and a useful tool in the design and/or evaluation of RV vaccines.
Guizhou and Bamma mini-pigs from the Experimental Animal Center of the Third Military Medical University (Chongqing, China) were placed in germ-free isolator units immediately after birth. Prior to inoculation with HRV, all mini-pigs were seronegative for RV antibodies for a dilution ratio of 1:10, as detected by enzyme linked immunosorbent assay (ELISA).
Wa strain of HRV was originally obtained from Dr. Elschner and MA104 cells were used to grow the virus in the presence of 0.5 μg/mL trypsin. The dose and median infection dose (PID50) of Wa strain HRV were determined as previously described[13]. The PID50 of the Wa HRV stock inoculum for inoculation in mini-pigs was almost 106 focus forming units (FFUs).
A total of 42 stool samples collected from children with acute RV induced diarrhea in two consecutive winter seasons from October 2003 to April 2005 were used in this study. Viral RNA was extracted and used as templates for real-time PCR (RT-PCR). G and P typing was realized by nested amplification of various gene sequences of the proteins of VP7 and VP4, which respectively consist of six G- and five P-type-specific primers (multiplex PCR)[28]. Two of the samples typed as serotype G1 or G3 were selected for use as animal inocula. After multiple centrifugations to remove the fecal debris from the selected samples, the supernatant was used to inoculate mini-pigs orally for 3-5 d. The intestinal contents were collected and three passages were subsequently conducted. The intestinal content obtained from the 4th mini-pig passage was used as the virulent Wa HRV stock inoculum. The titers of infectious virus from the virulent Wa HRV stock inoculum were detected by fluorescent-focus assay (FFA) and confirmed by FFU[29]. The stock inoculum was identified and analyzed by dilution of serially diluted (10-fold, in duplicate) single layer cells of MA104. Infectivity titers were indicated via FFU. On the basis of the toxicity, 1:10 was the minimum dilution for fecal samples identified for infectivity titers. If the fluorescent foci with the dilution ratio of 1:10 were not visualized by fluorescence microscopy, the sample was labeled as negative.
In this study phase, 5-, 30- and 60-d-old mini-pigs were inoculated with a single oral dose of attenuated strain Wa, G1, G3 of HRV, and PBS (control), respectively. Prior to oral inoculation, mini-pigs received 5 mL NaHCO3 (100 mmol/L) to reduce gastric acidity and, after 5 to 10 min, 5 mL of virus were slowly instilled into the mouth at the back of the throat using a needleless syringe.
The fecal samples from the studied pigs were collected from 0 to 7 d post infection (DPI) individually. In order to avoid cross-contamination, cages and bedding were changed every day during the sample collection period. Presence of diarrhea was identified in the individual fecal samples by gently pressing the abdomen of neonatal pigs every day. As described earlier, cold (4 °C) PBS containing gentamicin (2 mg/mL, Sangon, Shanghai, China), streptomycin (200 U/mL), and penicillin (200 U/mL) was used to process fecal samples as 10% solution for the subsequent test[30]. Based on the assessment of color, consistency, and the amount of feces, diarrhea was scored from 1 to 4[31]. A score > 2 was considered indicative of diarrhea. Three 1 cm long sections, consisting of the duodenum (which was proximal to the stomach), ileum (located at the distal part of the intestine), and jejunum (situated in the middle section of the intestine between the duodenum and ileum) of each pig, were obtained and homogenized.
As described previously, the expression of HRV antigen in samples of fecal or intestinal contents was detected using aforementioned mono-antibodies by ELISA[32]. A reaction was considered positive if: (1) the difference between the optical density (OD) values of the well to which the sample containing virus was added and the well lacking antigen was > 0.1; and (2) the difference between the OD values of the well to which the sample containing virus and the negative control well was equal to or exceeded two standard deviations.
To further ensure that pigs only shed the impregnated virus, fecal suspensions obtained from the pigs that were virus-infected as well as positive for RV (as established by ELISA) were examined by RT-PCR. Nucleic acids were extracted from virus that was recovered from fecal materials, and the results were determined by RT-PCR. The PCR serotype-specific primers were as previously described[33]. All experiments were performed in triplicate at the minimum, yielding reproducible results.
To evaluate the distribution of neutral and acid mucopolysaccharides and the histopathology, 10% zinc-formalin was used to fix sections obtained from the small intestine 24 h prior to transferring to 70% graded ethanol series for dehydration. Next, intestinal tissue samples were embedded in paraffin wax before being sectioned at the 4 μm thickness, as described previously[27,32].
Histopathological findings in sections obtained from the small intestine of newborn pigs sacrificed at 48 h post-inoculation (hpi) were evaluated. The sections were stained with hematoxylin and eosin[34]. The stained villi were observed under a light microscope in order to identify any changes in vacuolization, presence of enterocyte injury or inflammation.
Frozen sections from pigs sacrificed at 24, 48, and 72 hpi were allowed to air dry completely before being fixed for 10 min with 95% alcohol. PBS containing 0.05% Tween 20 (PBS-T) was used to wash the slides twice before placing them in distilled water and blocking them with 2% normal bovine serum (Jingmei, China) for 0.5 h at room temperature (RT). After decanting the excess bovine serum, and at room temperature, the slides were incubated with either sheep anti-VP6 or anti-VP7 serum (Dako Diagnostics, Inc.) at a dilution of 1:100 for 2 h at RT. Next, slides were washed twice with PBS-T and incubated for 2 h at RT with donkey anti-sheep Ig antibody conjugated with fluorescein isothiocyanate, which was mixed with PBS at a dilution of 1:200 (Sigma Chemical Co., St. Louis, MO, United States). After incubation, slides were flushed gently with PBS-T and sealed with moderate aqueous mounting medium (Dako Diagnostics, Inc.) and covered with coverslips. The Leica TCS-SP5 microscope was used to detect fluorescence (Leica, Inc., Germany).
Additional samples of mid-duodenum, mid-jejunum, and mid-ileum collected from newborn pigs sacrificed at 24, 48 and 72 hpi were fixed with buffer containing 2.5% glutaraldehyde and 0.1 mol/L cacodylate (pH 7.4) before adding buffer containing 1% osmium tetraoxide. Plastic was used to embed the fixed samples, to ensure that they could be sectioned in thin slices and analyzed by transmission electron microscopy (TEM). Sections (400 Å) were stained with uranyl acetate and were observed under a HITACHIH600 TEM.
SPSS Version 11 for MS windows (SPSS, Inc., Chicago, IL, United States) was used to perform statistical analyses. In brief, one-way analysis of variance (ANOVA) was used for the comparison of distinct data for the virus shedding (in mean days), diarrhea rate and severity scores of disease for groups of different ages. In addition, the gradients of the cumulative weight data obtained each day were analyzed by linear regression and were compared for equality of means by using the t-test. Pearson’s correlation coefficient was used to calculate correlation coefficients. Difference was deemed statistically significant at P < 0.05.
We inoculated 5-d-old mini-pigs with G1 HRV field strain, G3 HRV field strain, G1 HRV attenuated Wa strain, or PBS. Mini-pigs were randomly divided into four groups. All mini-pigs received oral inoculations of G1 HRV field strain and G3 HRV field strain and developed obvious clinical signs of infection within the 7-d observation period. In brief, mini-pigs infected with human RV produced feces that were profuse, watery and often floccular; thus, they became anorexic within 12-72 h of the inoculation. In contrast, none of the mini-pigs that were treated with oral inoculations of G1 HRV attenuated Wa strain or PBS developed clinical signs of infection.
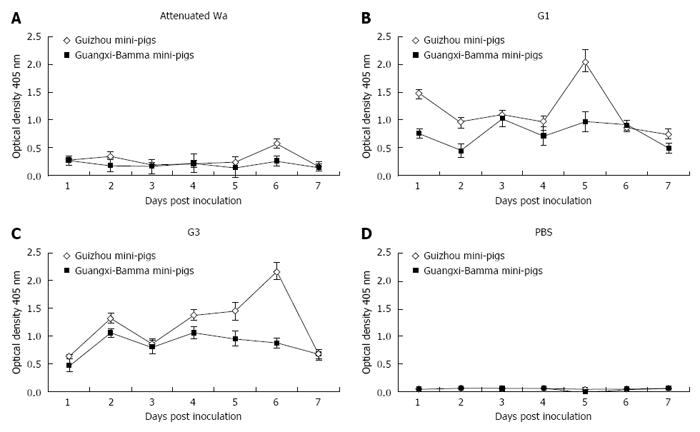
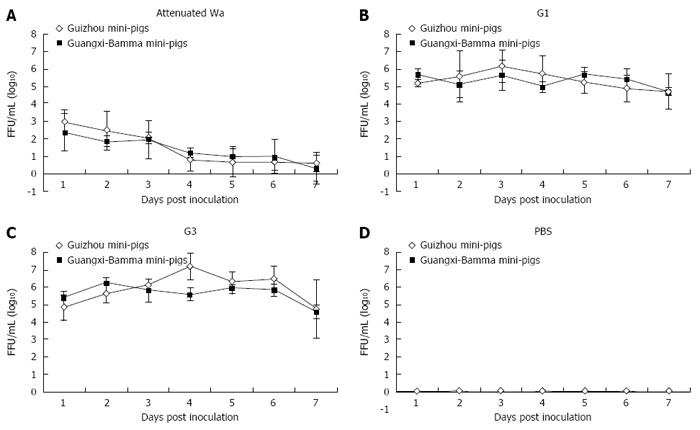
Antigen of HRV (Figure 1A) or infectious virus (Figure 2A) was not detected by ELISA or FFA in 5-d-old mini-pigs that were inoculated with G1 HRV attenuated Wa strain over the 7-d study period. The pigs that were inoculated with G1 HRV attenuated Wa strain shed virus antigen, starting from as early as 1 DPI (Figure 1A), while in those inoculated with G1 HRV field strain (Figure 1B) and G3 HRV field strain (Figure 1C) these effects were noted during a period of 1 to 3 d. The virus antigen quantities shed by the mini-pigs were correlated with each of the infectious viruses (r = 0.852, P = 0.019; Pearson’s correlation coefficient). Neonatal pigs that were impregnated with G1 HRV attenuated Wa strain shed virus antigen for 2 d, while they shed infectious virus for 1 d (Figures 1A and 2A). The quantity of the infectious virus shed by pigs impregnated with G3 HRV field strain was higher compared to those inoculated by G1 HRV field strain, for both Guizhou pigs and Bamma pigs (Figure 1B and C, Figure 2B and C, respectively). Neonatal pigs inoculated with G1 HRV field strain and G3 HRV field strain shed virus antigen or inoculated virus during the entire 7-d period (Figure 1B and C, Figure 2B and C, respectively). The control group, mini-pigs impregnated with PBS only shed neither antigen nor infective virus (Figures 1D and Figure 2D, respectively).
We found that the excreted virus and human virus with which pigs were inoculated were the same, rather than porcine RV, by detecting the RNA extracted from the virus collected from selected fecal samples by RT-PCR. The virus from newborn mini-pigs inoculated with G1 HRV field strain and G3 HRV field strain was the same to the respective virus inoculum. No evidence of virus was found in samples from neonatal mini-pigs inoculated with G1 HRV attenuated Wa strain, most likely because of the low quantity of virus excreted by these pigs.
All 5-d-old virus-inoculated Guizhou pigs that were not PBS-inoculated, as well as Bamma pigs, developed diarrhea (Table 1). However, because of efficient horizontal transmission, the severity of diarrhea developed by PBS-inoculated littermates of G1 HRV field strain and G3 HRV field strain inoculated group and their respective virus-inoculated littermates was similar, while the PBS-inoculated littermates of G1 HRV attenuated Wa strain inoculated group developed mild diarrhea (score of 4 in the Guizhou group and 6 in the Bamma group, respectively). The mean severity of disease was based on the scores obtained by dividing the total score by the total number of samples every day.
| Virus shedding1 | Diarrhea3 | ||||||||
| Inoculum RVserotype (G) | Animalspecies | Animalnumber | Percentage shedding | Mean onset (d) | Mean duration (d) | Peak titer shed (FFUs/mL) | Percentage with diarrhea | Mean onset (d) | Mean cumulative faecal score |
| Wa (attenuate) | Guizhou | 3 | 33%2 | 2 | 2 | 1 × 103 | 0 | 0 | 4 |
| Bamma | 3 | 33%2 | 2 | 1 | 5 × 102 | 0 | 0 | 6 | |
| G1 | Guizhou | 3 | 100% | 1 | 6 | 2 × 106 | 100% (3/3) | 1.2 | 14 |
| Bamma | 3 | 100% | 1 | 6 | 1 × 106 | 100% (3/3) | 1 | 12 | |
| G3 | Guizhou | 3 | 100% | 1 | 7 | 2 × 107 | 100% (3/3) | 1.5 | 16 |
| Bamma | 3 | 100% | 1 | 7 | 5 × 106 | 100% (3/3) | 1 | 12 | |
| PBS | Guizhou | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bamma | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
In order to confirm whether human RV infection caused histopathological lesions in the intestine of the mini-pigs that were inoculated with only 0.5 mL PBS or 0.5 × 107 FFUs of G1 HRV field strain and G3 HRV field strain, these individuals were euthanatized from 0 to 168 hpi. Postmortem examination revealed no obvious changes in the intestine of the control mini-pigs that were euthanatized at each time point. In G1 HRV field strain and G3 HRV field strain infected mini-pigs, fluid accumulation in the small intestine was first viewed at 12 hpi, and again from 96 to 168 hpi, and was accompanied by distention that extended throughout the entire gut, including the small and large intestine.
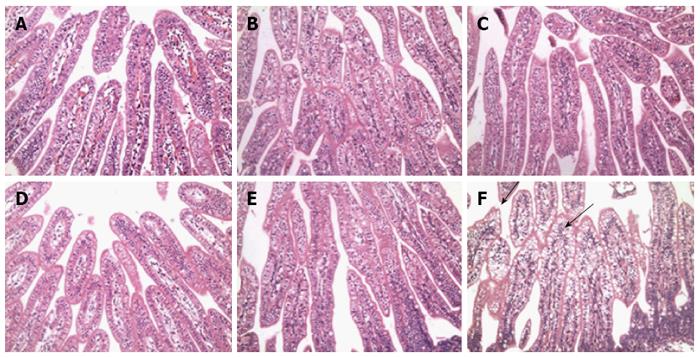
No histological lesions were observed in all of the 5-d-old control mini-pigs at any time point (Figure 3A-C). While obvious signs of lymphocyte inflammation could be noted, villus height and width in the G1 HRV field strain and G3 HRV field strain inoculated 5-d-old mini-pigs changed. In addition, obvious histopathological lesions in the ileum were observed, including large vacuoles in the enterocyte lining, which were mainly distributed on the surface of the villi (Figure 3D-F). Vacuolization at the tops of the villi in the ileum was rarely observed, and was identified only in some of the control mini-pigs inoculated with PBS. Vacuolization in the ileum reached the maximum from 48 to 72 hpi and declined at 96 hpi. Histopathological changes of vacuoles declined at 168 hpi, in terms of both size and number.
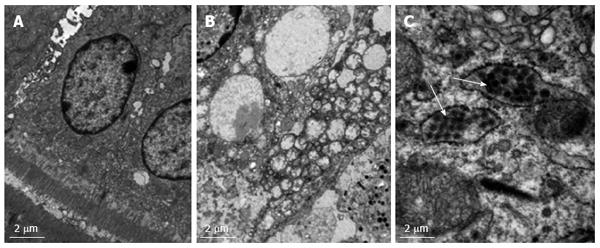
Using TEM analysis, we determined that supra nuclear cytoplasmic vacuoles were present in the ileum of RV-inoculated mini-pigs (Figure 4A and B). The vacuoles in the virus-exposed mini-pigs, however, were significantly distensible (Figure 4B). The markedly enlarged enterocytes indicated that intracellular organelles were pushed aside, and nuclei were squeezed. There were small, ill-defined fragments in the vacuoles, and cytoplasmic debris was noted in the background of general amorphous or finely granular structure. Therefore, normal transport vesicles appeared to be expanding. Viral particles in the intestine or near the vacuoles of the RV-inoculated mini-pigs at 24 hpi were detected by TEM (Figure 4C).
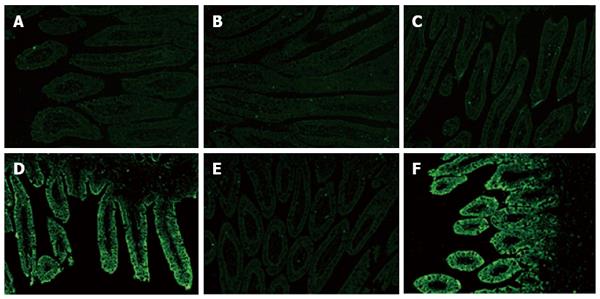
To confirm the distribution of structural protein VP6 in RV, frozen sections of intestinal tissue (including the duodenum, jejunum and ileum) of HRV-impregnated mini-pigs were stained by immunofluorescence (Figure 5). No RV antigen was tested in any section of the small intestine collected from 5-d-old control mini-pigs (Figure 5A-C). VP6 protein of RV was tested in epithelial cells which were located at the top part of the villi in samples of the jejunum and ileum, but not in the duodenum of HRV-infected 5-d-old mini-pigs at 24 hpi (Figure 5D-F). At any time point, RV antigen was not detected in the lower part of the villi of the whole small intestine collected from HRV-inoculated 5-d-old mini-pigs, and the same finding was noted for crypts. The distribution of RV antigen at 24, 48 and 72 hpi was almost identical. Little or no RV antigen was found over 168 hpi in any region of the intestine.
To observe the age difference in mini-pigs inoculated with human Group A RV, we examined the mini-pigs at 30 d, 60 d and 6 mo of age. The time points were chosen because mini-pigs were weaned at age of 2 mo and sexually mature at 6 mo of age. Six mini-pigs (n = 6) were inoculated with 0.5 × 107 FFUs of HRV. Similar to previous reports pertaining to rats, mice and conventional pigs, RV disease was age restricted to neonatal and 30 d old mini-pigs[13,32,35,36]. None of the 60-d-old or 6-mo-old mini-pigs inoculated with RV developed disease (Table 2). All the RV-inoculated mini-pigs started to shed virus antigen at 1 to 2 DPI and continued to do so for 1 or 2 d.
| Age (d) | Number | Inoculum | Diarrhea1 | Viral antigen shedding2 | ||||
| Percentage (%) | Average (days happened) | Persistent (d) | Percentage (%) | Average (d) | Persistent (d) | |||
| 3-5 | 3 | G1 HRV | 100 (3/3) | 1 | 3-5 | 100 (3/3) | 1 | 5-7 |
| 3 | G3 HRV | 100 (3/3) | 1 | 3-6 | 100 (3/3) | 1 | 5-7 | |
| 2 | PBS | 0 | 0 | 0 | 0 | 0 | 0 | |
| 30-35 | 3 | G1 HRV | 100 (3/3) | 3 | 3-5 | 100 (3/3) | 1 | 4-7 |
| 2 | PBS | 0 | 0 | 0 | 0 | 0 | 0 | |
| 56-60 | 3 | G1 HRV | 0 | 0 | 0 | 0 | 2 | 2-3 |
| 2 | PBS | 0 | 0 | 0 | 0 | 0 | 0 | |
Compared to the neonatal mini-pigs or 30-d-old mini-pigs inoculated with the corresponding RV strains, the timing and duration of virus antigen shedding in mini-pigs aged 60 d and 6 mo significantly decreased (P < 0.001). These findings indicate that RV infection in mini-pigs is also age limited (data not shown), in line with the observations pertaining to rabbits, mice and rats. To confirm that virus antigen test was not simply the result of residual virus inoculums, we evaluated the fecal samples shed by the mini-pigs inoculated with virus to establish the infectious virus quantity. As the quantity of the virus antigen shed was negligible, the infectivity with rotavirus was not detectable.
In this study, two breeds of Chinese mini-pigs, namely, Guizhou and Bamma, were used to develop Group A HRV induced diarrhea models. Their smaller size and susceptibility to infection with HRV allowed us to conveniently study HRV induced diarrhea disease. To our knowledge, this is the first study to demonstrate that Chinese mini-pigs can be infected with HRV and subsequently develop clinically typical diarrhea. Mini-pigs inoculated with either G1 or G3 HRV shed RV and had clinical diarrhea for an average of 3 d. Pigs inoculated with G3 HRV shed RV at slightly higher rates than those inoculated with G1 HRV. Moreover, compared to Bamma pigs, Guizhou pigs seem more sensitive to HRV, with respect to shedding RV and developing clinical diarrhea.
The animal models for RV infection have offered key insights for understanding of RV infection, its pathology and immunity, and testing of prospective vaccines in children[12-21,23,24,27,29,32,36-56]. To date, five models - including two small (mouse and rabbit) and three large laboratory (sheep, pig and cow) animal species - had been used to research the pathogenesis of both human and animal RV infection. Large animal models have been utilized to study the pathogenesis of RV infection because they are sensitive not only to homologous but also to heterologous RV, and develop severe disease symptoms. The limitations of the large animal models include high cost, limited availability, the need of specialized equipment and staff, and difficulty in handling multiple animals, which restrict their use in large-scale studies. Conversely, models of small animals have some merit, such as cost-effectiveness, short gestation and multiple births, the feasibility of including a large number of animals in experimental studies, the practicability of segregating sufficient number of infected animals, and other merits. However, using adult mouse and rabbit models of RV infection for vaccine testing is affected by several restrictions, in particular: (1) HRV strains in either animal fail to replicate efficiently[26]; (2) clinical disease was not observed in extant studies[27]; and (3) only isogeny virus strains that were isolated from the same species replicate effectively and transmit horizontally to the control animals that had not been inoculated. Therefore, development of an ideal small animal model combines the efficient intestinal replication of HRV strains with small size and convenience for large-scale studies.
Both Guizhou mini-pigs and Guangxi mini-pigs examined in this study have been used widely as animal models in medical research[25]. We investigated whether G1 HRV field strain and G3 HRV field strain could efficiently replicate in mini-pigs. To our knowledge, HRV in mini-pigs has not been reported to date. The present study was designed to develop a mini-pig model of human G1 or G3 serotype RV infection that is useful for defining basic parameters of active immunity, immunogenicity, and protective efficacy of vaccines[57,58].
In this study, mini-pigs inoculated with HRV (including serotype 1 and serotype 3) developed clinical symptoms and pathological features of homologous virulent RV infections in children, lambs, calves, rodents and rabbits. Our results have successfully established virological and clinical parameters of RV infection in mini-pigs. More specifically, we have demonstrated that (1) viral replication, transmission, and disease of attenuated and virulent RV of human origin can persist from birth to 30 d, as detected by viral antigen or infectious virus shed by the virus-incubated pigs; (2) mini-pigs that were inoculated with human RV can develop diarrhea before reaching 30-d maturity, indicating that the disease has age-related restrictions; (3) pathological changes that do appear in newborn mini-pigs include small intestinal cell tumefaction, necrosis and lymph node inflammation[30]; (4) the distribution of RV antigen is restricted to the top part of the villi in the ileum; and (5) RV infection of mini-pigs (including neonatal and those that are 30 d old) resulted in weight reduction during the early stages of infection.
On average, the mini-pigs included in the study developed diarrhea within 3 d from inoculation, with the earliest onset noted at 1 d after impregnation. The onset of diarrhea coincides with the early stages of viral shedding despite different RV serotypes (either G1 or G3) and different mini-pig breeds (either Guizhou or Bamma). Viral antigen in small intestinal tissue was tested from 24 to 72 hpi, showing that diarrhea is connected with the presence of replicating virus in the intestinal tissue. Fluorescence intensity of structural RV protein VP6 in the ileum was higher than in the jejunum and less pronounced fluorescence staining was detected in the duodenum in mini-pigs inoculated with RV.
In this study, for the first time, we have identified human RV particles in the ileum of mini-pigs by electron microscopy. No previous study has reported HRV particles in this region of the small intestine. As only porcine RV particles have been previously reported in the pig intestine[31], our novel findings indicate that mini-pigs are as sensitive to human RV as conventional pigs and other animals.
It has been three decades since RV has been determined as the most common cause of severe dehydrating diarrhea[59]. However, children’s morbidity and mortality remain high. Yet, making progress in developing successful vaccine strategies is hindered by our limited understanding of pathogenic mechanism of RV. Knowledge of the molecular and cellular basis of host responsiveness to RV infection remains crucial, especially in the context of malnutrition, which exacerbates severity of the disease and will most likely affect the efficacy of RV vaccines. Our results suggest that mini-pigs provide important advantages over normal pigs, mice and rabbits as animal models in the study of pathogenic mechanisms of RV infections, particularly in studies designed to evaluate RV vaccines and protection against infection and disease.
In conclusion, two breeds of Chinese mini-pigs, namely, Guizhou and Bamma, were used to develop Group A HRV induced diarrhea models. Their small size and susceptibility to infection with HRV allowed us to conveniently study HRV induced diarrhea. To our knowledge, this is the first study to demonstrate that Chinese mini-pigs can be infected with HRV and subsequently develop clinically typical diarrhea. Mini-pigs inoculated with either G1 or G3 HRV shed RV and had clinical diarrhea for an average of 3 d. Pigs inoculated with G3 HRV shed RV at slightly higher levels compared to those inoculated with G1 HRV. Moreover, Guizhou pigs seem more sensitive to HRV than Bamma pigs with respect to shedding RV and developing clinical diarrhea. Our results suggest that mini-pigs provide important advantages over normal pigs, mice and rabbits as animal models in investigations of pathogenic mechanisms of RV infections, particularly in studies designed to evaluate RV vaccines and protection against infection and disease.
We are thankful to Dr. Elschner for his kind gifts of rotavirus Wa and MA104 cells that were used in this study. We are also grateful to Ms. Zhang Rong for her technical help in immunofluorescence. We also wish to thank Mr. Lu-Shen Wu of Harvard University and Professor Yun Zhang of the Foreign Language Department of the Third Military Medical University for critical reading and insightful comments, which greatly improved this manuscript.
Rotavirus (RV) can cause severe gastroenteritis in infants in the world, and there is no specific medicine for treating RV diarrhea. Therefore, a safe and efficacious vaccine is very necessary. The unclearness of immune response mechanism of RV infection limits the development of vaccines. An ideal animal model of human RV (HRV) infection is urgently needed to improve the understanding of the immune response to RV infection in humans.
It was reported that Chinese mini-pigs, Guizhou and Bamma, have many advantages as animal models in medical experiments.
This is the first to use Chinese mini-pigs to establish an infection model of RV.
This research offers a new animal model for studying the pathological changes and immunogenicity of HRV infection and a useful tool in the design and evaluation of RV vaccines.
Mini-pigs inoculated with either G1 or G3 HRV shed RV and had clinical diarrhea for an average of 3 d. Pigs inoculated with G3 HRV shed RV at slightly higher levels compared to those inoculated with G1 HRV and Guizhou pigs seem more sensitive to HRV than Bamma pigs with respect to shedding RV and developing clinical diarrhea.
The manuscript has reported an original research performed in two kinds of Chinese mini-pig. Both can be infected efficiently with HRV. Obvious histopathological changes and rotavirus particles can be observed in the intestinal villi of the ileum by TEM. The small size of Chinese mini-pigs gave convenience for understanding pathogenic mechanisms of human rotavirus. The idea is new and the findings provided potential use for testing of vaccine efficacy for preclinical test. The manuscript is in general well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tagbo BN S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 945] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 2. | Ebrahim GJ. Rotaviruses and rotavirus vaccines. J Trop Pediatr. 2008;54:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Mondal A, Chakravarti S, Majee SB, Bannalikar AS. Detection of picobirnavirus and rotavirus in diarrhoeic faecal samples of cattle and buffalo calves in Mumbai metropolis, Western India. Vet Ital. 2013;49:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Schoondermark-van de Ven E, Van Ranst M, de Bruin W, van den Hurk P, Zeller M, Matthijnssens J, Heylen E. Rabbit colony infected with a bovine-like G6P[11] rotavirus strain. Vet Microbiol. 2013;166:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Tonietti Pde O, da Hora AS, Silva FD, Ferrari KL, Brandão PE, Richtzenhain LJ, Gregori F. Simultaneous detection of group a rotavirus in Swine and rat on a pig farm in Brazil. ScientificWorldJournal. 2013;2013:648406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Honeyman MC, Laine D, Zhan Y, Londrigan S, Kirkwood C, Harrison LC. Rotavirus infection induces transient pancreatic involution and hyperglycemia in weanling mice. PLoS One. 2014;9:e106560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kandasamy S, Chattha KS, Vlasova AN, Rajashekara G, Saif LJ. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes. 2014;5:639-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Li T, Lin H, Zhang Y, Li M, Wang D, Che Y, Zhu Y, Li S, Zhang J, Ge S. Improved characteristics and protective efficacy in an animal model of E. coli-derived recombinant double-layered rotavirus virus-like particles. Vaccine. 2014;32:1921-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Saif LJ, Bohl EH, Theil KW, Cross RF, House JA. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980;12:105-111. [PubMed] |
| 10. | Tzipori SR, Makin TJ, Smith ML. The clinical response of gnotobiotic calves, pigs and lambs to inoculation with human, calf, pig and foal rotavirus isolates. Aust J Exp Biol Med Sci. 1980;58:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Tzipori S, Unicomb L, Bishop R, Montenaro J, Vaelioja LM. Studies on attenuation of rotavirus. A comparison in piglets between virulent virus and its attenuated derivative. Arch Virol. 1989;109:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Schaller JP, Saif LJ, Cordle CT, Candler E, Winship TR, Smith KL. Prevention of human rotavirus-induced diarrhea in gnotobiotic piglets using bovine antibody. J Infect Dis. 1992;165:623-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl. 1996;12:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075-3083. [PubMed] |
| 15. | Tô TL, Ward LA, Yuan L, Saif LJ. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79:2661-2672. [PubMed] |
| 16. | Chang KO, Kim YJ, Saif LJ. Comparisons of nucleotide and deduced amino acid sequences of NSP4 genes of virulent and attenuated pairs of group A and C rotaviruses. Virus Genes. 1999;18:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Yuan L, Geyer A, Saif LJ. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103:188-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Yuan L, Iosef C, Azevedo MS, Kim Y, Qian Y, Geyer A, Nguyen TV, Chang KO, Saif LJ. Protective immunity and antibody-secreting cell responses elicited by combined oral attenuated Wa human rotavirus and intranasal Wa 2/6-VLPs with mutant Escherichia coli heat-labile toxin in gnotobiotic pigs. J Virol. 2001;75:9229-9238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Iosef C, Chang KO, Azevedo MS, Saif LJ. Systemic and intestinal antibody responses to NSP4 enterotoxin of Wa human rotavirus in a gnotobiotic pig model of human rotavirus disease. J Med Virol. 2002;68:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Iosef C, Van Nguyen T, Jeong Ki, Bengtsson K, Morein B, Kim Y, Chang KO, Azevedo MS, Yuan L, Nielsen P, Saif LJ. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine. 2002;20:1741-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | González AM, Nguyen TV, Azevedo MS, Jeong K, Agarib F, Iosef C, Chang K, Lovgren-Bengtsson K, Morein B, Saif LJ. Antibody responses to human rotavirus (HRV) in gnotobiotic pigs following a new prime/boost vaccine strategy using oral attenuated HRV priming and intranasal VP2/6 rotavirus-like particle (VLP) boosting with ISCOM. Clin Exp Immunol. 2004;135:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Yuan L, Azevedo MS, Gonzalez AM, Jeong KI, Van Nguyen T, Lewis P, Iosef C, Herrmann JE, Saif LJ. Mucosal and systemic antibody responses and protection induced by a prime/boost rotavirus-DNA vaccine in a gnotobiotic pig model. Vaccine. 2005;23:3925-3936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122:175-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Wang X, Chen H, Cao HH. Phylogenesis analysis of 10 chinese indigenous pig populations. Yi Chuan. 2005;27:715-718. [PubMed] |
| 26. | Bell LM, Clark HF, O’Brien EA, Kornstein MJ, Plotkin SA, Offit PA. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc Soc Exp Biol Med. 1987;184:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Ciarlet M, Estes MK, Barone C, Ramig RF, Conner ME. Analysis of host range restriction determinants in the rabbit model: comparison of homologous and heterologous rotavirus infections. J Virol. 1998;72:2341-2351. [PubMed] |
| 28. | Argüelles MH, Villegas GA, Castello A, Abrami A, Ghiringhelli PD, Semorile L, Glikmann G. VP7 and VP4 genotyping of human group A rotavirus in Buenos Aires, Argentina. J Clin Microbiol. 2000;38:252-259. [PubMed] |
| 29. | Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Crawford SE, Patel DG, Cheng E, Berkova Z, Hyser JM, Ciarlet M, Finegold MJ, Conner ME, Estes MK. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J Virol. 2006;80:4820-4832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Pearson GR, McNulty MS. Pathological changes in the small intestine of neonatal pigs infected with a pig reovirus-like agent (rotavirus). J Comp Pathol. 1977;87:363-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ciarlet M, Conner ME, Finegold MJ, Estes MK. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J Virol. 2002;76:41-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, Fang ZY. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276-282. [PubMed] |
| 34. | Franco MA, Feng N, Greenberg HB. Molecular determinants of immunity and pathogenicity of rotavirus infection in the mouse model. J Infect Dis. 1996;174 Suppl 1:S47-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Kalter SS, Heberling RL, Rodriguez AR, Lester TL. Infection of baboons (“Papio cynocephalus”) with rotavirus (SA11). Dev Biol Stand. 1983;53:257-261. [PubMed] |
| 36. | Burns JW, Krishnaney AA, Vo PT, Rouse RV, Anderson LJ, Greenberg HB. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Wyatt RG, James WD, Bohl EH, Theil KW, Saif LJ, Kalica AR, Greenberg HB, Kapikian AZ, Chanock RM. Human rotavirus type 2: cultivation in vitro. Science. 1980;207:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 186] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Gaul SK, Simpson TF, Woode GN, Fulton RW. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982;16:495-503. [PubMed] |
| 39. | Offit PA, Clark HF, Plotkin SA. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983;42:293-300. [PubMed] |
| 40. | Bohl EH, Theil KW, Saif LJ. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984;19:105-111. [PubMed] |
| 41. | Steel RB, Torres-Medina A. Effects of environmental and dietary factors on human rotavirus infection in gnotobiotic piglets. Infect Immun. 1984;43:906-911. [PubMed] |
| 42. | Torres A, Ji-Huang L. Diarrheal response of gnotobiotic pigs after fetal infection and neonatal challenge with homologous and heterologous human rotavirus strains. J Virol. 1986;60:1107-1112. [PubMed] |
| 43. | Ebina T, Ohta M, Kanamaru Y, Yamamoto-Osumi Y, Baba K. Passive immunizations of suckling mice and infants with bovine colostrum containing antibodies to human rotavirus. J Med Virol. 1992;38:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, Ogra PL. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch Virol Suppl. 1996;12:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Saif L, Yuan L, Ward L, To T. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv Exp Med Biol. 1997;412:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Yuan L, Kang SY, Ward LA, To TL, Saif LJ. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330-338. [PubMed] |
| 48. | Parreño V, Hodgins DC, de Arriba L, Kang SY, Yuan L, Ward LA, Tô TL, Saif LJ. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol. 1999;80:1417-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Yuan L, Geyer A, Hodgins DC, Fan Z, Qian Y, Chang KO, Crawford SE, Parreño V, Ward LA, Estes MK. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J Virol. 2000;74:8843-8853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Chang KO, Vandal OH, Yuan L, Hodgins DC, Saif LJ. Antibody-secreting cell responses to rotavirus proteins in gnotobiotic pigs inoculated with attenuated or virulent human rotavirus. J Clin Microbiol. 2001;39:2807-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Nguyen TV, Iosef C, Jeong K, Kim Y, Chang KO, Lovgren-Bengtsson K, Morein B, Azevedo MS, Lewis P, Nielsen P. Protection and antibody responses to oral priming by attenuated human rotavirus followed by oral boosting with 2/6-rotavirus-like particles with immunostimulating complexes in gnotobiotic pigs. Vaccine. 2003;21:4059-4070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, Gochnauer M, Zhang W, Azevedo A, Saif LJ. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79:5428-5436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Iosef C, Lovgren-Bengtsson K, Morein B, Lewis P, Saif LJ. High titers of circulating maternal antibodies suppress effector and memory B-cell responses induced by an attenuated rotavirus priming and rotavirus-like particle-immunostimulating complex boosting vaccine regimen. Clin Vaccine Immunol. 2006;13:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26:3655-3661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008;121:222-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Murphy TV, Smith PJ, Gargiullo PM, Schwartz B. The first rotavirus vaccine and intussusception: epidemiological studies and policy decisions. J Infect Dis. 2003;187:1309-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 59. | Van Damme P, Van der Wielen M, Ansaldi F, Desgrandchamps D, Domingo JD, Sanchez FG, Gray J, Haditsch M, Johansen K, Lorgelly P. Rotavirus vaccines: considerations for successful implementation in Europe. Lancet Infect Dis. 2006;6:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |