INTRODUCTION
Esophageal cancer is an aggressive malignant disease, as well as a growing health concern, with regard to mortality and prognosis. Esophageal squamous cell carcinoma (ESCC) is the prevailing histological type of esophageal cancer worldwide but has a great degree of regional variability, with a high incidence in eastern countries. Ninety percent of cases of esophageal cancer are squamous cell carcinomas in the so-called “esophageal cancer belt”, which covers the area from Northern Iran through the Central Asian Republics to North-Central China[1]. Northern and central China have an incidence of ESCC as high as 100 cases per 100000 persons per year; ESCC is 3 to 4 times more common in men than in women, and the peak incidence occurs in the seventh decade of life[2].
Risks factors for ESCC include alcoholism, smoking, diet, human papillomavirus (HPV) infection, esophageal diseases (e.g., achalasia) and a personal history of head and neck cancer[3-5]. ESCC typically appears in the lower two-thirds of the esophagus, with only 10%-15% of cases occurring in the upper one-third of the esophagus[6]. Poor outcomes in patients with ESCC are related to diagnosis at an advanced stage and the tendency to spread at an early stage[7]. To improve the prognosis of ESCC, accurate staging is necessary for guiding treatment protocols.
In this paper, we review the common imaging modalities used in staging preoperative ESCC that are reported in the literature.
DIAGNOSTIC CRITERIA
Generally, patients with squamous intraepithelial lesions and half of patients with superficial ESCC show no symptoms at all when diagnosed[8]. Symptoms are present only when tumors are large enough to interfere with esophageal function or to have spread through the esophageal wall. Usually, intermittent dysphagia is the first symptom. As the frequency and severity of dysphagia increase, weight loss follows. Patients with advanced disease often suffer from odynophagia, chest pain radiating to the back and anemia and may even develop a tracheoesophageal fistula.
The diagnostic work-up for ESCC should involve various aspects, including symptoms, history, physical examination findings, imaging results and pathological findings. Once a diagnosis is made, accurate staging must be performed before treatment to guarantee that the most appropriate treatment schemes are applied, the results of which can be sufficiently assessed[2,9].
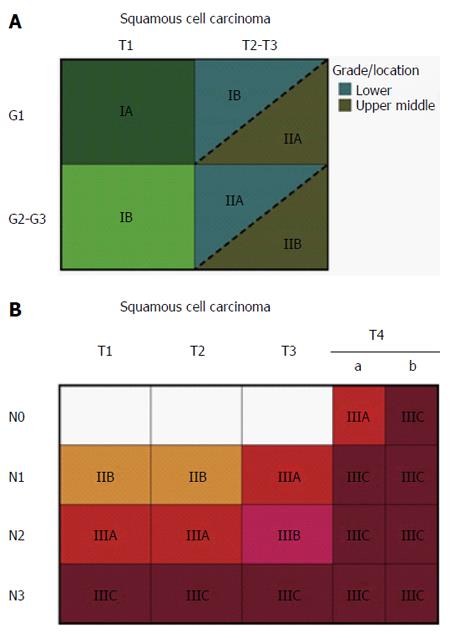
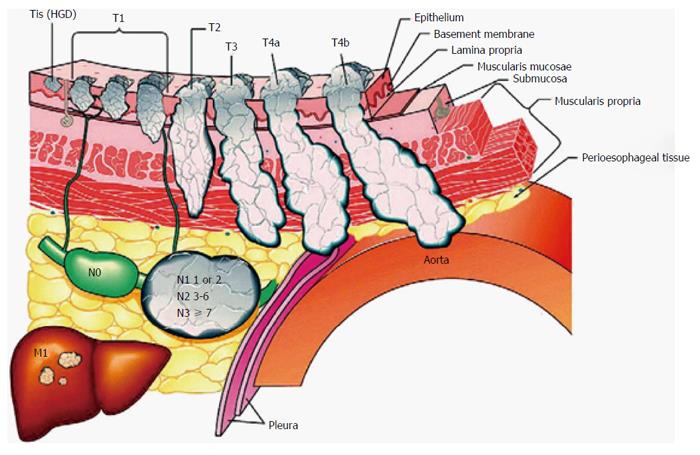
At present, the widely accepted staging criteria are those of the TNM system developed by the American Joint Committee on Cancer (AJCC). The TNM staging system takes into consideration the depth of tumor invasion (T-staging), the nodal status (N-staging) and metastatic spread (M-staging). This system has been updated over time, and the current version is shown in Figure 1[10]. It is worth noting that in the latest version, T4 lesions are classified as resectable (T4a) or unresectable (T4b), and the division of N-staging is based on the lymph nodes involved. Tis lesions (i.e., intraepithelial neoplasias) have been redefined as high-grade dysplasia, and M-staging is classified as no distant metastases (M0) or distant metastases (M1; Figure 2[10]).
Figure 1 Stage groupings for M0 esophageal squamous cell carcinoma[10].
A: Stage groupings for T1N0M0 and T2-3N0M0 esophageal squamous cell carcinoma (ESCC) by histologic grade (G) and cancer location; B: Stage groupings for all other M0 ESCCs. M1 stage is grouped as IV, regardless of tumor depth, nodal status or tumor location.
Figure 2 Features used to stage esophageal squamous cell carcinoma according to the latest version of the TNM classification system[10].
*Tis = intraepithelial neoplasia. T1: cancer invades lamina propria, muscularis mucosa, or submucosa; T2: cancer invades muscularis propria; T3: cancer invades adventitia; T4a: resectable cancer invades adjacent structures, such as pleura, pericardium, or diaphragm; and T4b; unresectable cancer invades other adjacent structures, such as aorta, vertebral body, or trachea. N is classified as N0: no regional lymph node metastasis; N1: regional lymph node metastases involving 1 to 2 nodes; N2: regional lymph node metastases involving 3 to 6 nodes; and N3: regional lymph node metastases involving 7 or more nodes. M is classified as M0: no distant metastasis; and M1: distant metastasis. HGD: High-grade dysplasia.
The common imaging techniques for staging ESCC that are currently in use are endoscopy, especially endoscopic ultrasound (EUS) and its innovations, computed tomography (CT), positron emission tomography (PET) and magnetic resonance imaging (MRI).
ENDOSCOPY
On white light endoscopy, squamous dysplasias may be flat, erythematous, nodular, plaque-like or erosive appearing. The majority of lesions are abnormal under endoscopic examination[11].
Endoscopic ultrasound
In the TNM system, the T-stage is determined by the depth of tumor invasion. EUS is a highly regarded technique for diagnosing and staging ESCC before treatment because it is able to distinguish the layers of the esophageal wall with endoscopic sonographic transducers (7.5-12 MHz). The accuracy of T-staging varies based on the stage. A meta-analysis of 49 studies showed that EUS had better accuracy in advanced disease than in early stage disease, with a pooled sensitivity of 92% in T4 and 82% in T1[12]. Further studies showed that EUS had an excellent performance in differentiating sub-stages of early disease. Thosani et al[13] conducted a meta-analysis of 19 studies and determined that EUS had a pooled sensitivity and specificity of 85% and 87%, respectively, for T1a, and 86% and 86%, respectively, for T1b. Recently, submucosal saline injection combined with EUS was reported to have better performance in staging T1b than EUS alone (86.7% vs 60%)[14]. It was reported that the echogenicity differences in tumor lesions and the submucosa were enhanced after the submucosa was filled with saline, which made it easier to identify lesions and the surrounding normal tissue. However, few studies have focused on sub-staging, especially for an advanced stage, to differentiate a resectable stage (T4a) from an unresectable stage (T4b).
With regard to N-staging, EUS is reported to have an accuracy of up to 84% in node-positive patients, but its accuracy falls to 69% when node-negative patients are taken into consideration[15]. In clinical practice, EUS has a limitation in detecting node status because some adjacent lymph nodes may be beyond its area of detection. In a meta-analysis of 49 studies, Puli et al[12] compared EUS alone and EUS-guided fine needle aspiration (EUS-FNA) and found that EUS-FNA (pooled sensitivity and specificity of 96.7% and 95.5%, respectively) was superior to EUS alone (pooled sensitivity and specificity of 84.7% and 84.6%, respectively).
Narrow band imaging
Narrow band imaging (NBI) uses spectral characteristics of endoscopic light to enhance the visibility of vasculature in the mucosa[16-18]. An optical filter with a narrow band spectrum is applied to correspond to the peak absorption spectrum of hemoglobin to enhance the visualization of mucosal and submucosal microvascular patterns. A panel of nine experts from Asian-Pacific countries agreed that NBI could replace chromoendoscopy, which distinguishes normal esophageal mucosa by applying Lugol’s iodine stain during routine examination, because it is easy to use and imparts considerable information[19]. On NBI, early lesions appear brown and well demarcated, and it is reported that microvascular patterns correspond to the depth of tumor invasion, which is classified into five types and several subtypes[20]. Thus, NBI is being increasingly used to stage superficial ESCC.
Several clinical studies have been conducted to assess the effectiveness of NBI for detecting superficial lesions, but the results have led to varying conclusions. In a randomized controlled multicenter trial, NBI was able to detect superficial lesions more frequently than white light imaging in the esophagus (97% vs 55%, P < 0.01)[21]. In contrast, Sharma et al[22] indicated that the performances of NBI and white light imaging were the same. To obtain more detail, magnification endoscopy in conjunction with NBI has been employed. Goda et al[23] investigated 72 patients with 101 superficial ESCCs and found that magnification endoscopy with NBI has the potential to reduce the overestimation risk of high-resolution endoscopy. However, NBI does have an advantage in detecting superficial lesions, and it is believed that with protocol adjustment, NBI is promising for the staging of preoperative ESCC.
CT
Esophageal wall thickness is a basic finding when assessing the esophagus by CT, and a lack of symmetry with respect to wall thickness is another classic but not specific CT finding in ESCC. Li et al[24] reported that CT had a sensitivity of 77.4% and a specificity of 74.8% for distinguishing T1/2 from T3 in ESCC with whole esophageal circumferential tumor involvement. Recently, a prospective, nonrandomized study showed that CT had higher accuracy than EUS in the precise assessment of superficial ESCC limited to the epithelium or lamina propria mucosa (94.6% vs 80.6%, P < 0.05)[25].
However, CT has a limited ability to accurately distinguish T1, T2 and T3 diseases. The combination of CT and EUS has an overall staging accuracy of approximately 85%[26]. The general protocol after the diagnosis of ESCC is to perform an initial CT because EUS shows no advantage in detecting distant metastases. If no distant metastases are present, then EUS is performed.
Therefore, the most useful aspect of CT in detecting tumor depth is in evaluating advanced stages (T4), when adjacent structures are invaded. Several studies have reported the effectiveness of multidetector computed tomography (MDCT), a great modality for performing multiplanar reformation imaging to confirm a true lesion from three or more plane images in the case of mediastinal invasion. A retrospective study involving 21 patients with ESCC reported that the accuracies for MDCT to differentiate T3 and T4 disease were 77.3% and 81.8%, respectively, and that the sensitivity and specificity were generally high (over 75% for all)[27]. In addition, Ba-Ssalamah et al[28] believe that with the specific assessment criteria of MDCT, two different readers can achieve a sensitivity as high as 95% and a high positive predictive value of 96%.
CT assessment of nodal status depends on the size of the lymph nodes. Most studies consider the common size criterion of 1 cm to be enlarged. It has been reported that CT has a sensitivity of 30%-60% and a specificity of 60%-80% for identifying enlarged lymph nodes[29]. Tio et al[30] reported that the accuracy of CT in staging nodal status was 46% to 58%. However, an obvious limitation of CT in N-staging involves metastatic nodes without obvious enlargement in size, as well as the fact that enlarged nodes may contain no metastasis. Thus, regular application of EUS-FNA and CT for patients with ESCC may show advantages in N-staging compared with CT alone.
PET
18F-fluorodeoxyglucose PET (FDG-PET), a non-invasive modality for management, has been increasingly used to stage ESCC, but it is not useful for detecting tumor depth (T-staging) and its accuracy in assessing nodal status varies widely. Little et al[31] investigated 58 patients with superficial esophageal cancer and found that FDG-PET was not indicated for staging superficial lesions because it could not differentiate Tis (5/11, 45%) from T1 (26/47, 55%, P = 0.03). FDG-PET is believed to be the most important modality for detecting unsuspected metastatic disease[32] (M-staging) because of its ability to distinguish physiologically normal tissues from malignant tumors due to enhanced glucose transport in tumors. Early use of PET in the staging of patients with ESCC may facilitate treatment strategies and identify unsuspected metastases in up to 20% of patients. Metastatic spread from ESCC can occur in unusual and unexpected locations, and whole body PET allows the detection of these metastases[33,34]. In a meta-analysis, PET had a moderate sensitivity of 67% and a high specificity of 97% in diagnosing distant metastatic disease[35].
Several clinical studies indicate that FDG-PET has a high sensitivity in identifying lesions in the entire body. Meltzer et al[36] conducted an independent, blinded retrospective evaluation of FDG-PET findings obtained in 47 patients referred for initial preoperative staging of ESCC. They found that PET had a great specificity in detecting nodal status (approximately 90%) and a sensitivity of 70% (n = 10) in detecting distant metastases when it was applied in the high-sensitivity mode. Furthermore, in comparison with CT, PET correctly identified one more site of metastasis that was missed by CT. A multicenter study involving 349 patients with ESCC reported that PET had false-positive results in only 5 (1.43%) patients in describing the extent of the disease[37].
MRI
In addition to PET scans, MRI with the functional feature of diffusion-weighted imaging (DWI) is another advancing imaging technology, which has current and future potential to overcome the limitations of conventional staging methods in patients with ESCC. MRI is often considered technically challenging because of artifacts due to organ motion and blood flow[38]. However, the usefulness of MRI in ESCC may expand as technical improvements allow proper visualization of the esophagus. van Rossum et al[39] believe that an anticipated MRI protocol consisting of T2-weighted and DWI sequences will be effective in future clinical practice by guiding treatment options for patients with ESCC.
In early studies of MRI, the results of T-staging were disappointing, especially for early stage[40]. However, as technology has improved, combining T2-weighted and DWI scans has yielded detection rates for MRI in T-staging of ESCC of 33% for T1, 58% for T2, 96% for T3 and 100% for T4[41]. Recent studies reported that high-resolution MRI for T-staging was able to achieve an accuracy as high as that for standard EUS (81% vs 81%-92%)[42,43]. With regard to lymph node staging, a prospective study with 24 patients reported that T2-weighted MRI in combination with DWI had a sensitivity of 77.8% and a specificity of 55.6%[41]. Moreover, a more recent prospective study that performed PET and whole-body MRI in 49 patients found that whole-body MRI and PET had similar accuracies in T–staging (98% vs 94%) and N-staging (67% vs 59%), and even for metastatic disease[44].
CONCLUSION AND FUTURE DIRECTIONS
As the incidence of ESCC increases worldwide, substantial work is required to improve diagnostic and staging accuracy. Imaging plays a pivotal role in the management of ESCC. At present, there is no single staging modality that is superior to another in preoperative TNM staging for ESCC. Local staging is usually best accomplished using a combination of EUS and CT, while distant metastatic disease is better assessed by PET. In the near future, MRI has the potential to improve staging, thereby compensating for the limitations of current imaging modalities. However, staging techniques still need to be refined to better assess prognosis, as well as to achieve the best possible outcomes by individualizing treatment. Technological advances in EUS and its innovations are being investigated and will ideally improve the outcomes of patients with ESCC.