Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6145
Peer-review started: March 29, 2016
First decision: May 12, 2016
Revised: May 18, 2016
Accepted: June 13, 2016
Article in press: June 13, 2016
Published online: July 21, 2016
Processing time: 109 Days and 14.4 Hours
Living-donor liver transplantation has provided a solution to the severe lack of cadaver grafts for the replacement of liver afflicted with end-stage cirrhosis, fulminant disease, or inborn errors of metabolism. Vascular complications remain the most serious complications and a common cause for graft failure after hepatic transplantation. Doppler ultrasound remains the primary radiological imaging modality for the diagnosis of such complications. This article presents a brief review of intra- and post-operative living donor liver transplantation anatomy and a synopsis of the role of ultrasonography and color Doppler in evaluating the graft vascular haemodynamics both during surgery and post-operatively in accurately defining the early vascular complications. Intra-operative ultrasonography of the liver graft provides the surgeon with useful real-time diagnostic and staging information that may result in an alteration in the planned surgical approach and corrections of surgical complications during the procedure of vascular anastomoses. The relevant intra-operative anatomy and the spectrum of normal and abnormal findings are described. Ultrasonography and color Doppler also provides the clinicians and surgeons early post-operative potential developmental complications that may occur during hospital stay. Early detection and thus early problem solving can make the difference between graft survival and failure.
Core tip: In this article we focus on the role of intra- and post-operative Doppler ultrasonography in the early detection of potential vascular complications in living-donor liver transplantation, in addition to monitoring the surgical and interventional vascular therapeutic procedures.
- Citation: Abdelaziz O, Attia H. Doppler ultrasonography in living donor liver transplantation recipients: Intra- and post-operative vascular complications. World J Gastroenterol 2016; 22(27): 6145-6172
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6145
Living donor liver transplantation (LDLT) is an established therapeutic modality for adults and paediatrics with end-stage liver diseases and congenital hepato-biliary diseases, especially in countries where deceased donors for liver transplantation (LT) are not available and where the waiting lists for orthotopic liver transplantation are too long for critically ill patients with end-stage liver disease.
Vascular complications (VCs) remain the most serious complications and a common cause for graft failure after LT[1]. The overall incidence of VCs is higher in LDLT than in deceased donor liver transplantation (DDLT) due to the complex nature of the vascular anastomoses. The reported incidence of vascular complications after LT in adults varies widely among transplant centres, ranging between 8%-15%. However, this rate can be as high as 20%, especially in cases such as split liver transplantation or LDLT[2-5]. Higher incidence has been reported in paediatric patients because of their smaller vessels in addition to the short vascular pedicles that are available for reconstruction[6].
Intraoperative ultrasound (IOUS), a non-invasive test that allows real-time and quantitative evaluation of the graft vasculature, has been considered an integral component of the recipient surgery. Its use in early detection and, thus, in the immediate surgical correction of intra-operative (IO) VCs ensures adequate graft perfusion after revascularization. The sonographer should be able to provide the surgeon with sufficient data regarding the morphology and integrity of the different surgical anastomoses. Furthermore, with the aid of different Doppler flow measurements, the hemodynamic changes can be described and interpreted. The decision to perform vascular repair of the anastomoses is multidisciplinary and depends on a variety of factors rather than solely the Doppler ultrasound (DU) findings.
DU is the modality of choice for PO recipient surveillance as it is non-invasive, portable and provides rapid, comprehensive and accurate evaluation of the entire hepatic vasculature. Knowledge of the immediate and early physiological graft hemodynamics after graft perfusion and early identification of VCs are essential for improving graft and patient survival[7].
In this article, we review the role of intra- and post-operative DU in the evaluation of the recipient surgery during LDLT, with descriptions of the surgical background, the IOUS technique, normal DU appearance of different vascular anastomoses, the spectrum of normal and abnormal graft haemodynamics and potential VCs.
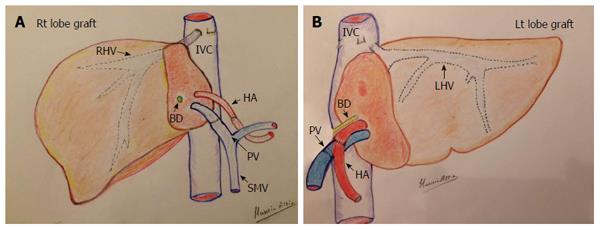
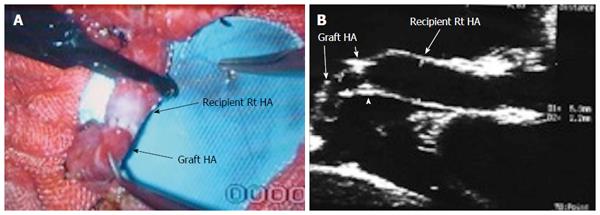
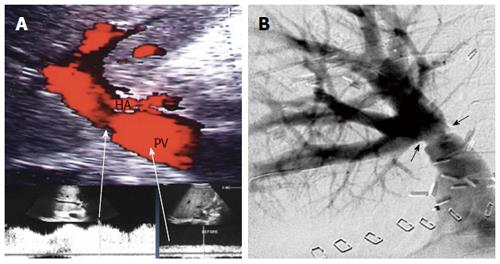
In an adult recipient, a right lobe graft that is drained by the right hepatic vein (RHV) is obtained by transecting the liver on the right side of the middle hepatic vein (MHV). The resection typically includes the entire right lobe, RHV, right portal vein, right hepatic artery and right bile duct. The middle hepatic artery (segment IV artery) and MHV are preserved for survival of the medial segment of the donor liver and are critical for avoiding venous complications in the donor[8,9] (Figure 1A).
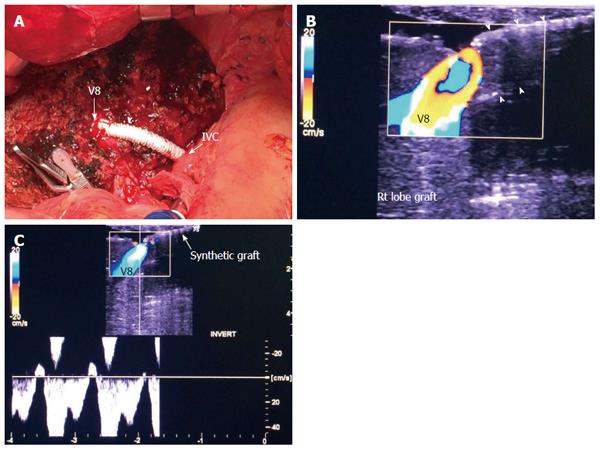
Implantation of the graft starts with hepatic vein (HV) reconstruction, followed by portal vein (PV) and then hepatic artery (HA) reconstruction. The RHV is either reconstructed in an end-to-end fashion to the stump of the RHV in the recipient or anastomosed in an end-to-side fashion directly to the inferior vena cava (IVC). Reconstruction of large-calibre MHV tributaries, such as HV draining segment V (V5) and segment VIII (V8), is often essential to avoid congestion of the segment drained by this tributary[10]. Various reconstruction techniques have been reported using autologous vein grafts such as the greater saphenous vein, Left PV (LPV) or the para-umbilical vein. Moreover, some techniques have been reported using cryo-preserved veins or arteries[11]. Additionally, synthetic grafts are widely used in many centres for venous reconstruction. The large-calibre inferior RHV might also require reconstruction, usually in an end-to-side fashion to the IVC[12,13].
Modalities for PV reconstruction are chosen according to the diameter, size mismatch, wall status, and length of the recipient PV. Reconstruction is performed by end-to-end anastomosis between the graft right PV and recipient main PV. When PV reconstruction is impossible, for example, due to pre-operative PV thrombosis (PVT), a venous jump or interposition conduit must be obtained[14].
HA reconstruction carries its own challenges due to the small vessel diameter, the deeply seated vessels, the short stump, and the moving field. Furthermore, tachycardia and tachypnoea can add to the difficulties[15]. The selection of the recipient artery is critical for successful anastomosis. The artery is chosen according to the patency, size match, length, direction, and very importantly, the upfront blood flow[16]. One common problem is the discrepancy in size between the graft and recipient arteries. Different techniques have been described for an anastomosis in such cases, including oblique cut, fish mouth method, funnelization and end-to-side techniques[17]. In cases of grafts with double arteries, a single opening may be created or two separate arterial anastomoses may be performed[18,19]. A single anastomosis may also be also performed using the larger artery, provided the other artery presents good backflow[15].
For most paediatric recipients, the donor liver graft is the left lobe or lateral segment (Figure 1B). The graft left HA and left PV are anastomosed end-to-end to the appropriate HA and PV of the recipient. The left HV is anastomosed in an end-to-end fashion with the LHV stump of the recipient[16].
IOUS has become an integral part of LT surgery that provides the surgeon with real-time information regarding the integrity of vascular anastomoses and graft hemodynamics. Reconstructive procedures are monitored by DU guidance until optimum flow is established. This technique reduces post-transplant vascular complications that might necessitate re-transplantation[20].
Before performing IOUS, the sonographer should revise the pre-operative imaging, including the DU data and the multi-detector computed tomography angiography of both the recipient and the donor to verify the normal and variant vascular branching anatomy and the liver haemodynamics prior to transplantation. The surgical technique is discussed with the surgeons regarding the type of anastomoses, size mismatch, usage of interposition grafts and the presence of any technical problems during the surgical reconstruction.
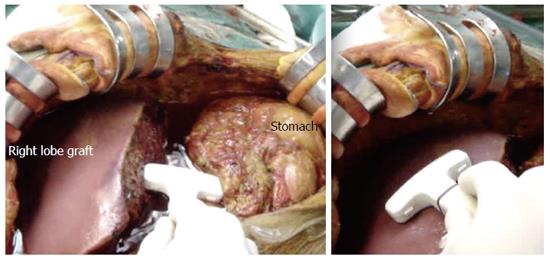
IOUS is performed before starting the biliary anastomosis. Scanning may be delayed for few minutes after graft perfusion to allow the HA to recover from spasticity. Linear array, 7-8 MHz transducers are most frequently used[21]. T- or V-shaped transducers are preferred to facilitate scanning the liver surface and the vascular pedicle. Some machines are equipped with remote control units that facilitate the scanning technique. The upper abdominal cavity is filled with warm saline as an acoustic medium for the US beam (Figure 2). The operating table can be tilted to the left side to avoid fluid spillage. During examination of the extrahepatic vessels (PV and HA), the whole vessel should be well immersed in the fluid, at a certain distance from the transducer. Assistance may be required from the surgeon to unfold vascular kinks and to move vascular clamps away from the US field. During the examination of the intrahepatic vessels (post- anastomotic and segmental branches), the transducer can be applied directly to the surface or the cut surface of the graft, very gently to avoid injuring the potentially bleeding cut surface.
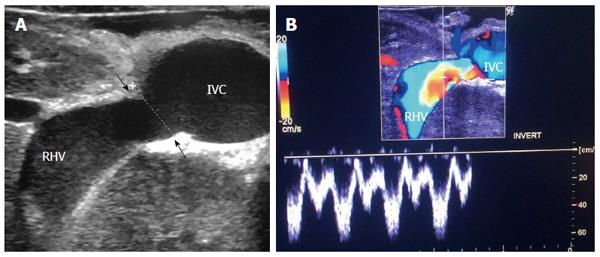
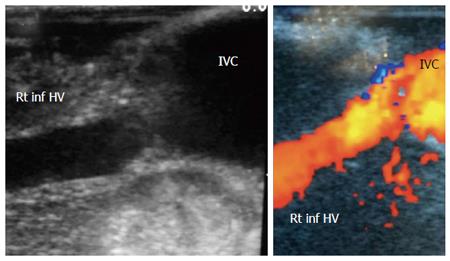
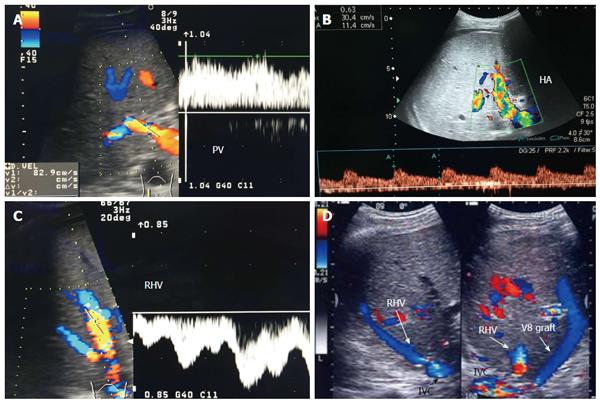
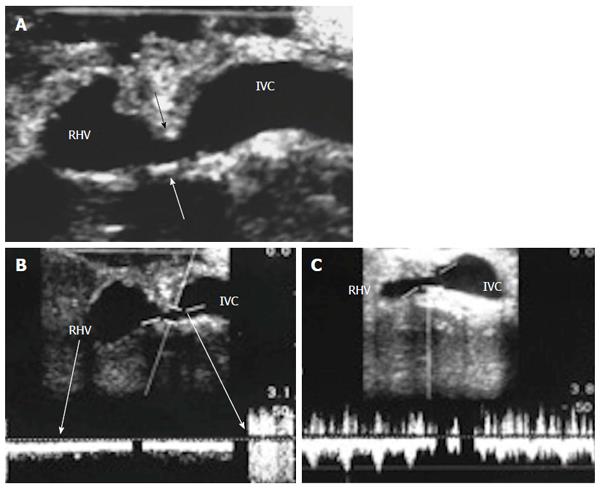
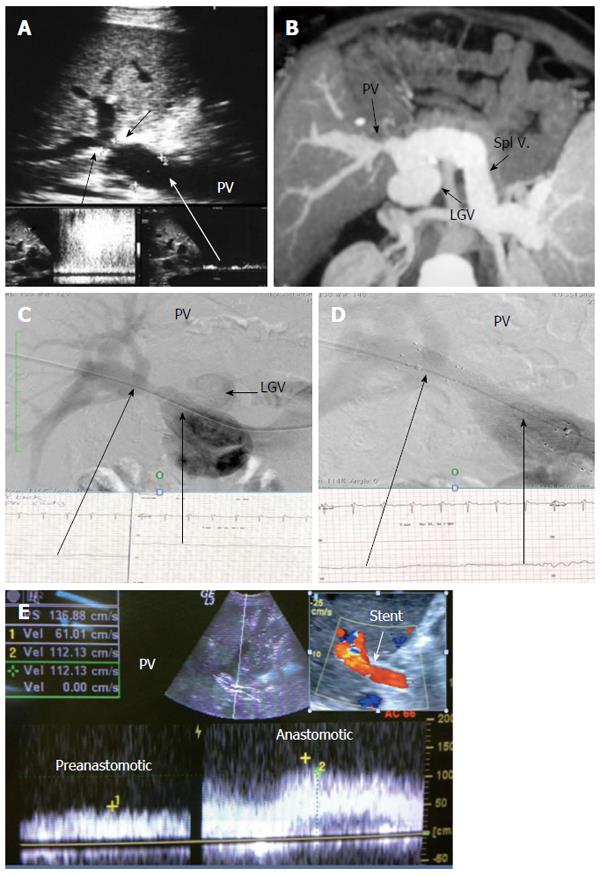
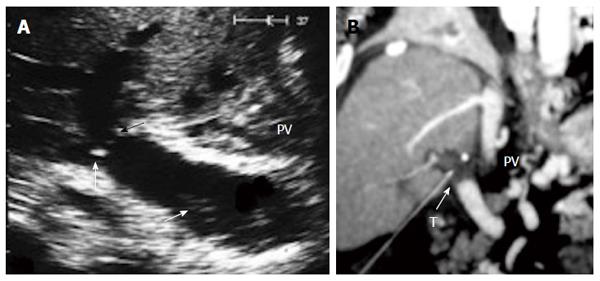
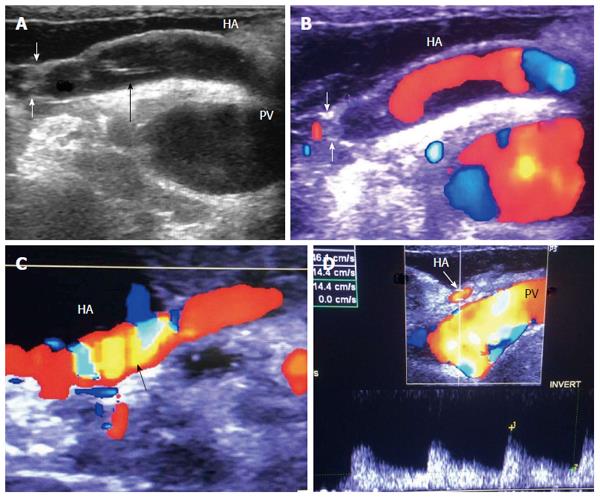
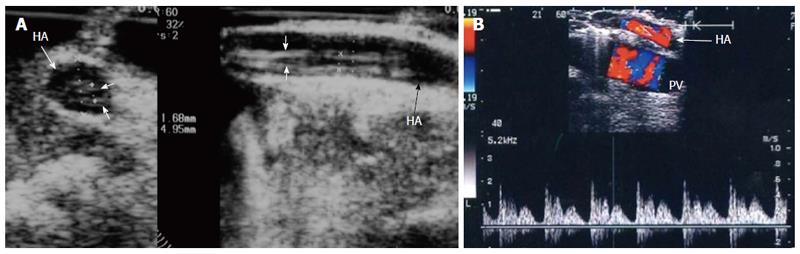
The probe can be tilted in the axial plane, cranially guided by the IVC, with gradual angulation towards the graft cut surface. In the b-mode, the vein is unfolded to be in line with the anastomosis. The anastomosis is usually elliptical in shape and in this axial oblique plane, only the antro-posterior diameter of the anastomosis can be visualized. The cranio-caudal diameter cannot be demonstrated using this technique; it is necessary to scan the anastomosis cranially and caudally until the widest point in the anastomosis is visualized (Figure 3). The anastomotic/intra-hepatic HV velocity ratio can then be calculated. Reconstructed segment V or segment VIII grafts and an accessory right hepatic vein anastomosed to the IVC can be scanned in the same manner (Figure 4). The use of synthetic grafts usually hinders the US beam; in such a condition, assessment of the intrahepatic flow is usually sufficient to ensure patency of the graft (Figure 5).
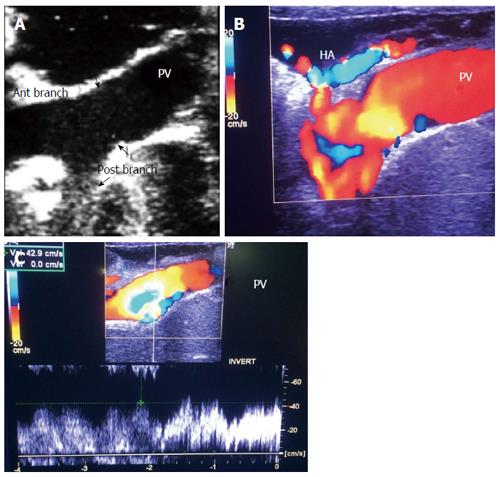
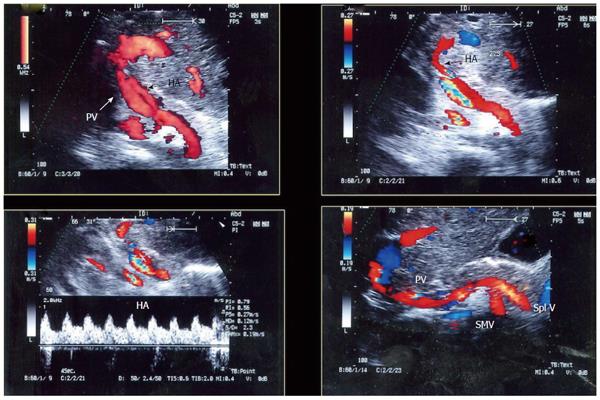
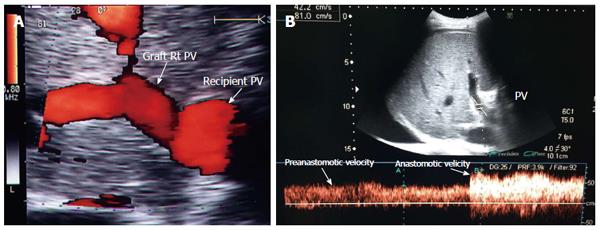
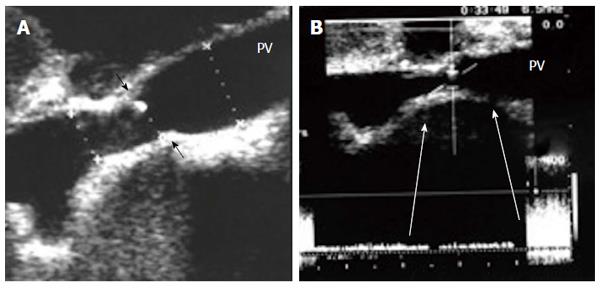
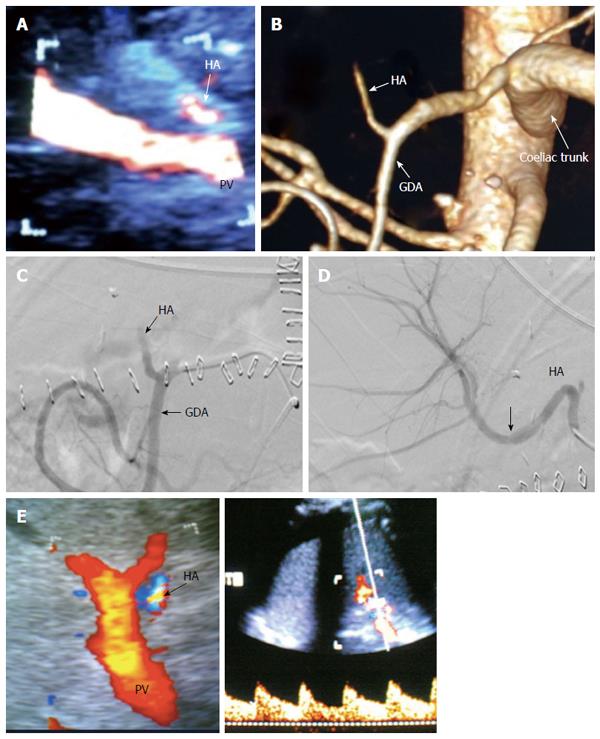
The PV is examined at the graft hilum (Figure 6); the probe is angulated to demonstrate the entire length of the recipient PV down to the confluence of the splenic and superior mesenteric veins for detection of thrombi. The size mismatch between the recipient and donor veins and the diameter of the anastomosis are measured, after which the anastomotic/pre-anastomotic velocity ratio can be calculated. Finally, examination of the intrahepatic segmental branches is important to ensure adequate graft perfusion.
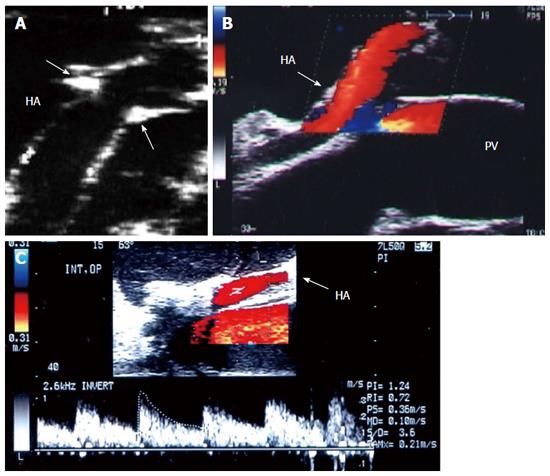
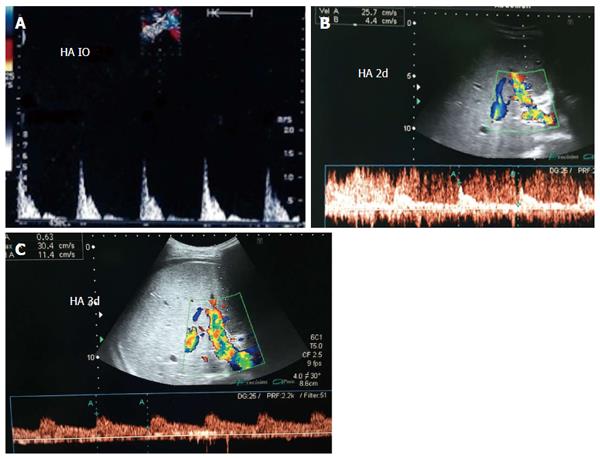
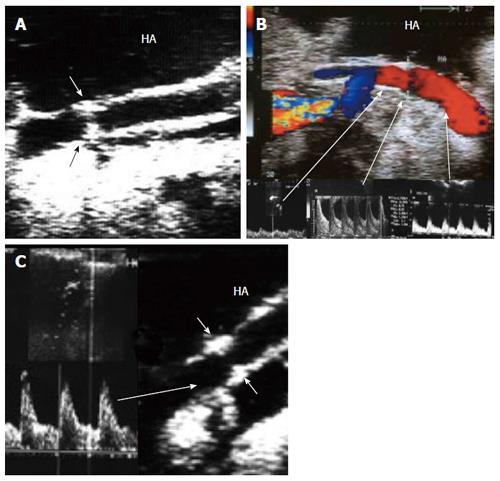
The HA can be unfolded with the aid of the surgeon, and its patency and the presence of any thrombi, dissecting flaps or anastomotic strictures can be evaluated. The site of anastomosis can be visualized as two echogenic dots on the wall (Figure 7). Measurements of peak systolic velocity (PSV), end diastolic velocity (EDV) and resistivity index (RI) are taken after proper angle correction in the pre-anastomotic, anastomotic and post-anastomotic segments. The velocity ratio (anastomotic/pre-anastomotic) can then be calculated. Examination of the intrahepatic arterial waveform is of utmost importance as a proper intrahepatic arterial flow with a good arterial spectral waveform often signifies a proper anastomosis.
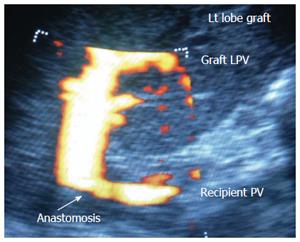
In paediatric patients, the scanning technique is different; the PV and HA anastomoses lie beneath the left lobe graft in the neutral position. The oblique entry of the left PV and left HA into the graft (C-shaped) creates technical difficulties in unfolding the vessel to evaluate the anastomoses (Figure 8). The surgeon can retract the liver gently upwards to allow scanning however, this retraction places the vessel and anastomoses under tension that may alter the DU measurements. One option is to perform the scanning from the surface of the graft while in the neutral position. This technique may require low- frequency transducers, and probing should be performed gently to avoid injury to the graft surface during probe angulations.
DU is often employed in the initial recipient work-up post-LDLT. DU is the imaging technique of choice to assess early and late surgical complications as it provides rapid, comprehensive and accurate evaluation of the entire hepatic vasculature and graft parenchymal abnormalities (Figure 9).
The timing and frequency of postoperative US screening ranges from a single examination on the postoperative day or every 12 h to daily or alternate-day examinations for 14 d, or daily until discharge[22], which is our practice.
DU is performed at the patient’s bedside with the patient in the supine or semi-sitting position using low-frequency (4-5 MHz) curvilinear transducers. Sterilized gel is preferred to avoid wound contamination. In addition to DU evaluation of the graft perfusion, ultrasound of the abdomen is performed for the evaluation of the hepatic parenchyma, peri-hepatic fluid collection and ascites[7].
Scanning of the vascular anastomoses is usually performed in the anterior axillary intercostal window. The HA and PV are examined extrahepatically, the DU parameters across the anastomoses are recorded, and their segmental intrahepatic branches are then examined to evaluate the graft perfusion. The anterior segmental branches are usually chosen as they are nearer to the probe and are easily assessed. The main HV and accessory veins are evaluated intrahepatically. If definite diagnosis of a VC is established by DU, interventional radiological treatment or surgery can be performed. In equivocal cases, cross-sectional imaging by MDCT angiography is performed prior to intervention.
The HV in a healthy graft displays a similar spectral waveform as a healthy native liver, typically a triphasic, hepato-fugal waveform corresponding to the cardiac cycle, with two antegrade major peaks followed by a small retrograde component in the early ventricular systolic phase (Figures 3B and 9C). Changes in the HV spectrum can be noted with phases of respiration, fluid status, cardiac dysfunction or abnormal anastomosis[23]. Elevation of the HV velocity at the anastomotic site (< 3-fold) compared to the intrahepatic velocity can be accepted in case the phasic pattern is preserved and the flow velocities are satisfactory (Figure 10).
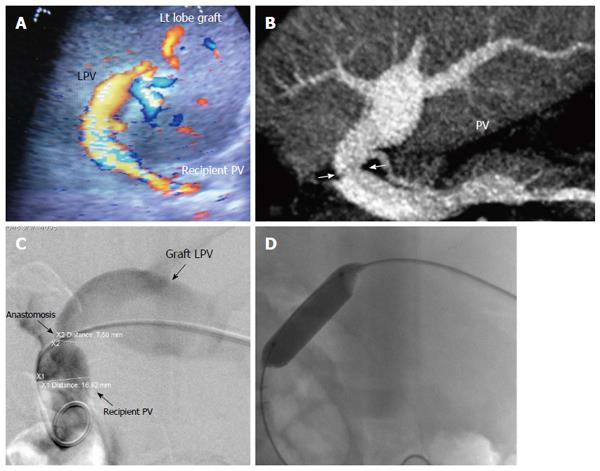
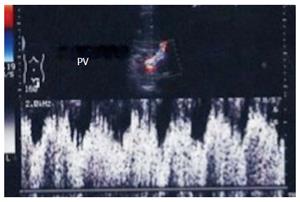
The PV has a wavy venous spectral waveform with respiratory variant pattern changes and has been described in earlier reports as being non-pulsatile or continuous (parabolic). With the recent interest in analysis of the portal vein waveform, a more precise description has emerged concerning the undulant fluctuation of flow; hence, different authors have used the term pulsatility[24]. Average blood flow velocity in the main PV is approximately (12-30 cm/s) in healthy adults[25]. However, in LDLT the normal range of PV velocities has not been agreed upon but is usually more than 30 cm/s as the donor PV component calibre, the right main branch, is usually smaller than recipient’s main PV component in right lobe grafts. Additionally, the recipient large portal flow volume is directed to a partial (split liberal) instead of a whole native liver size. These 2 factors contribute to the higher velocity values measured immediately after portal anastomoses (Figure 11). In left lobe grafts the recipient PV is usually smaller than the graft PV and the elevation in the anastomotic and post anastomotic velocities are not usually expected (Figure 12).
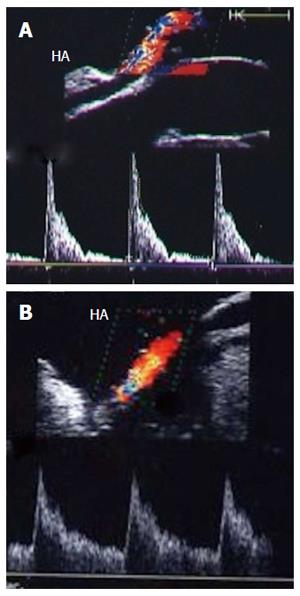
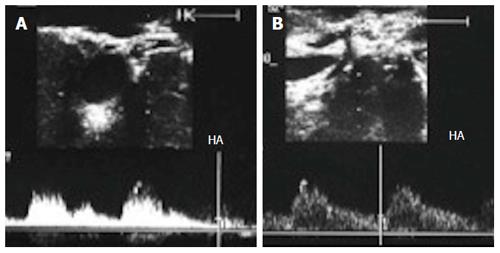
In healthy individuals, the spectral Doppler of the HA presents a biphasic waveform pattern; both systolic and diastolic waves are observed above the baseline, and no waves are detected below it (Figures 7 and 9B). The RI (PSV- EDV/ PSV), which allows a semi-quantitative estimation of the resistance to arterial flow into the liver, is the most commonly used Doppler parameter in hepatic artery evaluation. Under physiologic conditions, the HA flow shows low-resistance flow with antegrade flow throughout diastole because of the broad arterio-portal and arterio-sinus connections in the hepatic micro-vascular system. Its normal value in both healthy individuals and those with transplants, ranges from 0.55 to 0.8[26,27].
In cirrhotic patients with portal hypertension, the hyper-dynamic splanchnic circulation may results in hypertrophy of the HA with increased dimension[28-30]. This explains the frequent finding of HA size mismatch between the recipient and graft HA especially in right lobe transplants (Figure 13). Variations in the HA flow may occur normally due to anastomotic caliber discrepancies, central arterial pressure differences and the frequent occurrence of transient arterial spasm.
Early detection of haemodynamic abnormalities after LT requires knowledge of the normal or physiological changes that accompany the graft perfusion immediately and in the early PO period. The haemodynamics of the transplanted graft differ from those of a native liver as they depend on the inflow, represented by the PV and HA, and outflow through a single HV[15]. Analysis of DU findings may be confusing intra-operatively and in the early PO period due to complex vascular anastomoses, donor-recipient vascular size mismatch and reduced graft volume.
For patients with cirrhosis, immediately after LT, the mechanical component of portal hypertension is relieved by the healthy graft but without immediate restoration of the systemic or the splanchnic circulation to normal levels[31]. The splanchnic circulation shows rapid and potentially reversible changes in the portal and arterial perfusion that may not be clinically significant[32-34]. However, the changes in the portal and arterial parameters by DU are still under debate.
After orthotopic liver transplantation (OLT), a high perfusion state develops that is predominantly portal, with increases in the PV flow and velocities and the hepatic arterial resistance. In LDLT, the haemodynamic changes are much more pronounced than those occurring in DDLT, with a higher perfusion state and increases in the portal venous flow and velocities[35-37]. The increased PV velocity has been attributed to the persistence of the hyperkinetic haemodynamic splanchnic circulation in patients with cirrhosis and portal hypertension, reduction in the liver vasculature and small PV anastomotic stoma that could induce elevations in PV resistance and pressure, effects of the loss of sympathetic hepatic innervation or elevated cardiac output[31,33,34,36,38,39]. A wide range of PV velocities (15-400 cm/s) have been reported in the immediate post-OLT period in patients without vascular complications[40].
High-resistance arterial flow is frequently detected in patients with a normal hepatic artery. This flow returns to normal in few days and has not been associated with deterioration in the clinical course or decreased graft and patient survival[15,41,42]. The elevated RI has been attributed to the regulatory mechanisms and the hepatic arterial buffer response that induces HA vasoconstriction in response to portal hyper-perfusion and produces a high resistive index with poor arterial perfusion[43,44]; it has been demonstrated by temporary clamping of the portal vein that resulted in improvement of the hepatic arterial flow[15]. Other theories related the early and transient elevation of the HA RI after DDLT to the older donor graft, prolonged periods of cold ischaemia and preservation injury, graft steatosis and chronic cholestatic disease[43,45,46].
Different techniques and innovations have been proposed for reconstruction of the main graft HV and large accessory veins and tributaries. Ensuring the integrity of those anastomoses by DU is crucial because there is no collateral route for blood to exit the liver; failure to establish proper venous drainage may result in congestion and life-threatening graft dysfunction[10,47,48]. HV complications after LT are relatively uncommon, with reported incidence ranging from 1%-6%; this incidence is 2 times higher in LDLT[4,49-52].
HV outflow obstruction is a general term referring to obstruction of the HV that may occur intra-operatively due to surgical error or graft torsion and post-operatively due to compression/twisting of the anastomosis resulting from graft regeneration or intimal hyperplasia and fibrosis at the anastomosis. Clinical signs are usually nonspecific and include congestion of the liver parenchyma with abnormal laboratory values, hepatomegaly, ascites, and pleural effusions[53].
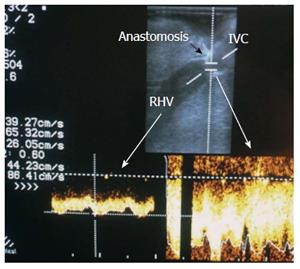
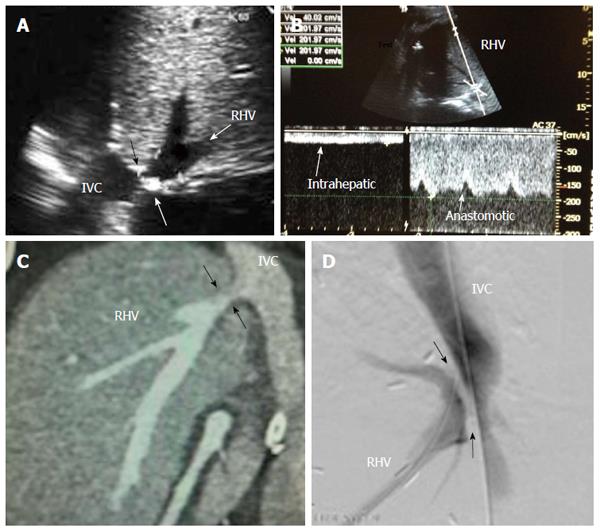
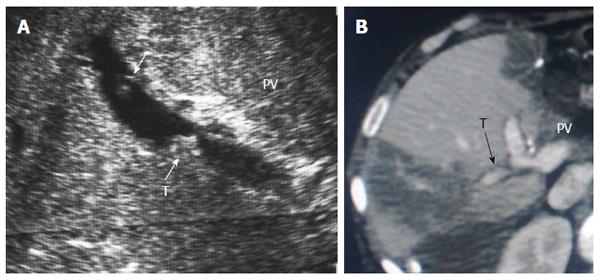
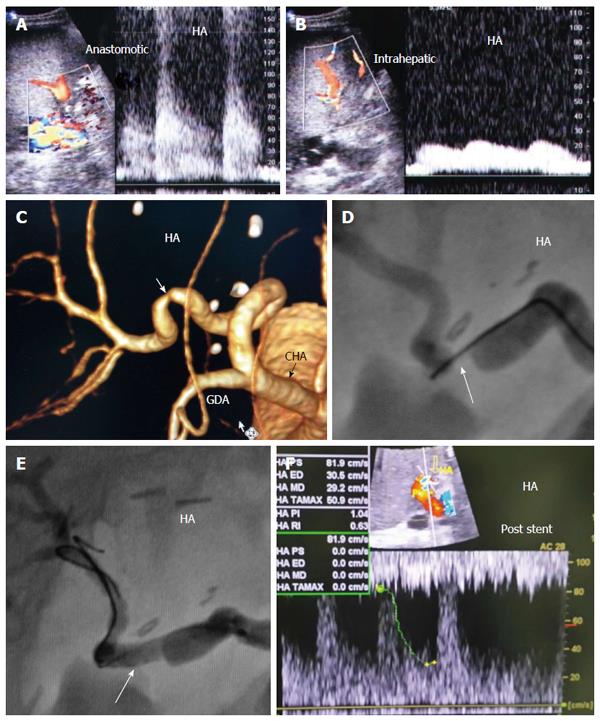
Diagnostic criteria of HV stenosis (HVS) using DU include the morphological appearance of the anastomosis by b-mode US, alteration of the Doppler waveform, velocity measurements and secondary compromise in the portal flow (Figures 14 and 15). HVS appears on b-mode US as an abrupt shouldering (waist) of the anastomosis or gradual tapering (beak-like)[54]. Loss of the triphasic pattern of the HV and absence of the retrograde flow are believed to reflect increased stiffness of the liver parenchyma around the HVs and had a 98.4% negative predicted value for venous obstruction[55]. In contrast, a persistent triphasic wave pattern on Doppler ultrasound images can exclude the possibility of substantial stenosis[53].
However, monophasic waveforms alone are not specific for HVS and associated dampening of the flow velocities usually accompany the loss of various components of the triphasic pattern[54,56]. Low peak HV velocity (as low as 10-11 cm/s) has been reported in previous studies[57,58]. In addition, dilatation of the hepatic vein and secondary compromise of the portal flow with extreme decrease in PV velocity < 14 cm/s have been reported[58]. We believe that measuring the velocity gradient (anastomotic/intrahepatic) is essential for grading the degree of venous outflow compromise and we consider anastomotic jets > 3-fold compared to the intrahepatic velocities to be significant (Figure 14B). In equivocal cases, measuring the pressure gradient intra-operatively or during PO radiological interventional procedures can help distinguish haemodynamically significant lesions from pseudo-stenosis. A pressure gradient between the HV and the IVC > 10 mmHg was considered substantial HVS[53]. In addition IO graft discoloration and increased stiffness and PO clinical signs would aid in establishing the diagnosis.
Because revision of the HV anastomosis carries its own challenges and might require clamping of the vascular pedicle, it is important to rule out torsion before rushing to the diagnosis of surgical anastomotic stricture based on the DU findings. Repositioning the graft in either direction and repeating the DU can be performed several times until a proper flow and waveform are established; hepatopexy can then be performed in the optimum position.
For treatment of PO HVS, percutaneous trans-jugular or trans-hepatic balloon angioplasty and stent placement are the modalities of choice, with adequate clinical and technical success rates[51,52,59,60].
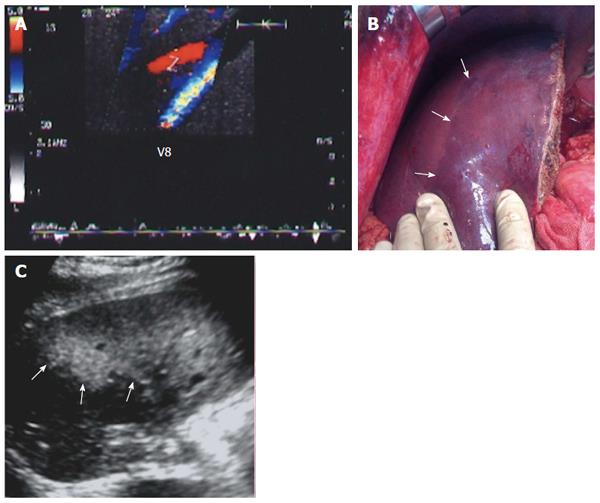
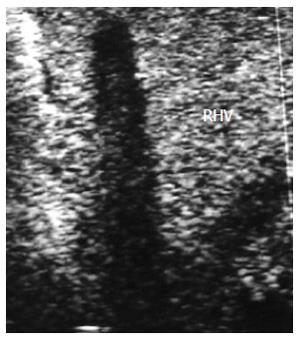
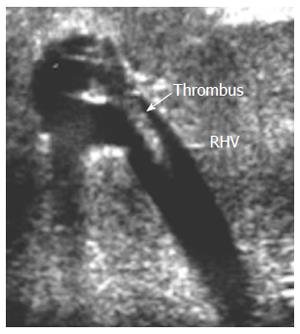
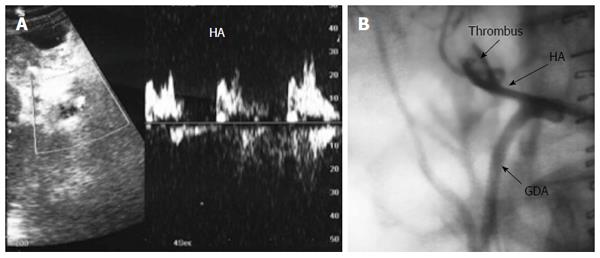
HV thrombosis is a rare complication in LT that has been reported in a few PO cases with clinical manifestations of HV outflow obstruction and is considered a sequel of untreated HVS[61-63]. Acute thrombosis within the segmental ligated HVs (V5 and V8) appears on DU as hypo-echoic with absent flow on color Doppler mapping and sometimes shows a very damped (to-and-fro flow) within the vein. On serial PO examination, the echogenicity of the thrombus gradually increases and is associated with echogenic geographically congested areas along the vein distribution, that usually resolve within 1-2 wk PO. If the ligated branch is large, large areas of congestion results that might cause graft dysfunction (Figure 16). Whereas thrombosis in the main HV is a very rare and dreadful complication that necessitates immediate intervention, we experienced one case of fatal IO massive thrombosis of the main RHV and one patient with a small adherent thrombus that was managed conservatively (Figures 17 and 18).
PV complications following LT are relatively uncommon, occurring in 1%-3% in most reports (with an incidence reaching 13%), and are associated with high morbidity and graft loss[1,64-69]. They are more common with split liver and LDLT[65,70]. Risk factors include significant size mismatch between the recipient and donor PV, graft position causing venous redundancy and kinking, prior surgery on the portal or splanchnic venous system, pre-transplant PV thrombosis (PVT) requiring thrombectomy, small diameter of the PV, use of venous conduits for reconstruction, and decreased PV flow due to the presence of porto-systemic (P-S) shunts[2,69,71-73]. In paediatric LT and as a result of previous Kasai surgery and recurrent cholangitis, the PV becomes narrow and hypoplastic. The impaired quality of PV flow increases the risk of complications, with an incidence ranging between 3.6% and 14%[74]. PV complications include high or low portal flow, PV stenosis (PVS) and PVT.
High portal flow: As previously described, IO and early PO benign elevation of the portal flow velocities and flow volume are expected after LT; however, there are no cut-off DU parameters that would define the need for surgical intervention. Therefore, it is important to correlate the Doppler measurements with the graft volume and the portal venous pressure (PVP) in such situation. When a high portal flow is accompanied by a suboptimal graft recipient weight ratio, hyper-perfusion injury and small for size syndrome may result where the portal flow should be modulated in such cases[75,76]. In addition, a PVP > 20 mmHg in the early period showed close associations with morbidity and poor graft function[77]. Splenic artery ligation and portocaval shunts have been described to reduce the portal flow[35,75].
Low portal flow: Reduced portal flow after graft perfusion may predispose to graft dysfunction and PVT. Low portal flow may occur due to the presence of P-S shunts that could be large enough to cause steal phenomena or the creation of porto-caval shunts for portal flow diversion in small for size grafts; the condition may also occur secondary to HV outflow obstruction. A PV flow velocity of less than 10 cm/s has been considered unacceptable and requires intervention. Repositioning the graft to relieve graft torsion and kinking of the PV may allow adequate blood flow to the graft, as can collateral shunt ligation, anastomosis revision, inferior mesenteric vein cannulation and endovascular stent placement[74,78,79]. Doppler US can provide accurate guidance when monitoring portal flow during these manipulations[79]. Post-operatively, heparinized saline infused via the IMV catheter to increase the PV flow and to lower the risk of thrombosis have been described[80].
Portal vein stenosis: With advancements in surgical innovation, PVS in LDLT is relatively uncommon, with an incidence of < 3%[66]. IO and early PO PVS usually result from technical errors or early PO anastomotic edema, whereas late PO PVS is usually secondary to fibrosis or intimal hyperplasia[81]. The majority of patients with PVS are asymptomatic and the diagnosis of PO stenosis is an incidental finding detected on routine DU screening and can be referred to as pseudo-stenosis (Figure 19). PVS usually develops slowly after transplantation and is suspected on the basis of the presence of manifestations of portal hypertension, such as gastrointestinal varices, ascites and splenomegaly[78,82-86]. Liver function tests and graft failure are not consistently reliable signs for PVS diagnosis[66].
It is necessary to differentiate between PVS and PV size mismatch by DU. PV calibre differences appear on b-mode US as tapering of the anastomosis without encroachment of the suture line on the lumen. By pulsed Doppler, elevations of the anastomotic velocities (usually < 3-fold) compared to the pre-anastomotic velocities are apparent (Figure 11). In PVS, encroachment of the suture line on the lumen can cause focal color aliasing with > 3-fold elevation of the anastomotic velocities by Color Doppler flow (Figures 20, 21 and 22). Haemodynamically significant stenosis may produce post-stenotic aneurysmal dilation as well[78] (Figure 23). A PV stenotic ratio has been proposed to define the degree of stenosis using the formula: PV stenotic ratio (%) = pre-stenotic calibre-anastomotic calibre/pre-stenotic calibre. Anastomotic calibre < 5 mm and stenotic ratio > 50% have been defined as the critical points for PVS cases that require interventional management for good long-term graft survival[87]. In equivocal cases, MDCT can confirm the morphological degree of the stricture and other signs of portal hypertension but will not provide quantitative information about the degree of stricture. A PVP gradient across the anastomosis can be performed in the recipient surgery or during the PO interventional angioplasty procedures, pressure gradients > 3-5 mmHg are considered significant and necessitate intervention[81,88] (Figure 21).
In patients with clinical manifestations and radiological confirmation of significant stenosis, therapeutic intervention is mandatory. Interventional angioplasty and stent placement have become widely recognized as the first choice for treatment[80,81,89] (Figures 21 and 22).
Portal vein thrombosis: PVT is a severe complication that may occur during either the recipient surgery or PO. Early identification by DU allows early intervention to avoid prolonged warm ischaemia, which may result in graft failure. The incidence of PVT in OLT ranges from 0.3%-2.6%[1,4,69], and a higher incidence up to 4% has been reported in LDLT due to technical difficulties in PV reconstructions, mainly related to shorter vessel pedicles and limited vessel grafts. The condition occurs more frequently in the early PO period, defined as within 3 mo after transplantation[71]. The clinical presentation depends on the timing of thrombosis. If the condition occurs early, severe acute liver insufficiency or graft failure predominates; if the condition occurs late, portocaval collateral circulation usually exists and the patient may present with manifestations of portal hypertension[69,73,90].
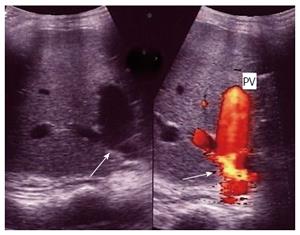
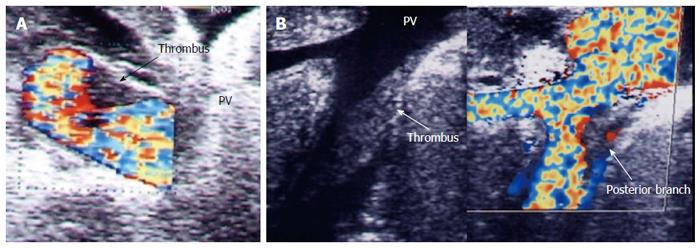
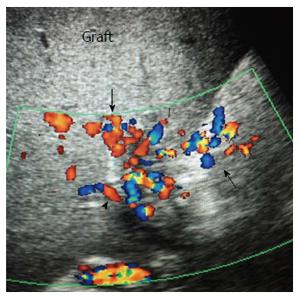
IO total PV thrombosis is rare whereas, detection of residual adherent thrombi in the recipient PV after thrombectomy is common for pre-existing PVT. Old thrombi appear as echogenic filling defects that adhere to the wall (Figure 24). Thrombi that are small in size and do not cause flow obstruction may be conserved; large thrombi that impede the flow necessitate further thrombectomy. On b-mode US, acute PVT appears as a hypo-echoic or slightly echogenic shadow within the PV with absent flow by color Doppler (Figures 25 and 26). Proper color Doppler evaluation of portal flow is crucial so that a slow portal flow is not mistaken as thrombosis[7,78]. Secondary hepatic infarction may be observed on US as an irregular, wedge-shaped low attenuation areas of heterogeneous echo pattern and devoid of vascular activity that are mainly in the periphery of the liver. Those areas appear as perfusion defects on CT examination (Figure 26B). In late onset or gradual PVT, portal cavernoma may be observed at the graft hilum with attenuated or non-visualized main graft PV (Figure 27).
In early PVT, within the first 3 d, surgical revision of the anastomosis is mandatory, together with systemic anticoagulation[2]. In more delayed cases, non-surgical treatment, such as percutaneous thrombolysis and stent placement, has shown good results and acceptable safety[2,91-94].
Arterial complications are the most common VCs after LT that can lead to ischaemia and graft loss with high mortality and morbidity rates[3,95,96]. Anatomical variations of the HA, diameter and length, kinking, quality of the recipient artery and mismatch between donor and recipient arteries should be carefully considered during DU interpretation and can be managed accordingly[78,97]. HA complications include vasospasm, HA thrombosis (HAT), HA stenosis (HAS), HA dissection, arterial steal phenomena and pseudo-aneurysm formation.
Hepatic artery vasospasm: Vasospasm is a state of arterial constriction; the affected segment or entire vessel becomes rigid and its lumen narrows or even becomes occluded[98,99]. It is a common complication during the recipient surgery secondary to excessive manipulations and suturing. The results of animal and clinical studies suggested that the plasma norepinephrine level is elevated because of surgical stress, occlusion of hepatic inflow and removal of the donor liver, which might have an appreciable effect on donor arterial spasm[100-102]. The potential impact of vasospasm in transplants is still undetermined, it is possible that vasospasm may cause reduction of HA flow and may induce the formation of thrombosis[99,103]. Richards et al[104] pointed out that vasospasm in re-vascularized tissue could impair tissue perfusion even though the microsurgical anastomoses remain patent.
There is lack of standard diagnostic criteria of HA vasospasm by Doppler US; it may appear as a short attenuated segment of the artery without focal anastomotic narrowing and usually results in increased RI, sometimes with absent or reversed diastolic flow, and can be differentiated from other causes of increased RI, previously described, that it responds to IO local application or PO administration of vasodilators (Figure 28). The increased RI can be explained according to Poiseuille’s equation, R = 8λη/πρ4, the resistance (R) depends on the viscous properties of the blood (η) and on the dimensions of the vessels. Whenever the hepatic artery is contracted, its diameter will correspondingly reduce, and, as a result, the resistance of the hepatic arterial flow will increase[103,105]. The diagnosis of HA vasospasm can be also supported by the follow-up PO DU results, and can be used to monitor the dynamic changes of the involved arteries before and after vasodilatation[103] (Figure 29).
Hepatic artery thrombosis: HAT is the most frequent VC following LT occurring with an incidence of 1%-12%, and is a graft-threatening condition that necessitates rapid revascularization to avoid graft loss[1,65,95,106]. Potential mechanical risk factors include vasospasm, dissection, kinks, significant mismatch, anastomotic stricture, arterial steal, bench reconstruction, arterial conduit use and multiple anastomoses[106]. Nonsurgical risk factors include hyper-coagulable disorders, cytomegalovirus, ABO incompatibility, arterial reperfusion time, prolonged operation times and acute rejection[107,108]. Early HAT within the first few weeks PO usually presents with fulminant graft failure and sepsis whereas delayed thrombosis occurring after the first month has a more insidious clinical course with delayed biliary complications[106].
Acute HAT can be diagnosed by b-mode US as a hypo-echoic or an-echoic shadow inside the artery and complete absence of color flow and arterial wave by pulsed Doppler (Figures 30 and 31). However, some earlier haemodynamic changes may occur and can anticipate total occlusion; these changes are referred to as signs of impending thrombosis[41]. Some authors have observed that absent or reversed diastolic flow appeared just before the HA flow vanished in patients with HAT[41,42,103] (Figure 32), indicating that under certain circumstances, HAT may share a common DUS appearance with vasospasm[103]. However, other authors found this sign to be poorly associated with HAT because the incidence of HAT was low among patients who had this highly resistant arterial flow[109]. In our experience, dampening of the PSV can differentiate between impending thrombosis and vasospasm; in vasospasm, the PSV is usually high, with good systolic upstroke, whereas in impending thrombosis, the systolic upstroke is usually damped and irregular (Figure 32). Chen et al[103] differentiated between impending thrombosis and vasospasm by the absence of diastolic flow in the main HA and in its parenchymal branches in vasospasm, whereas in impending thrombosis, the absent diastolic flow occurs around the anastomotic site only. HAT requires retransplantation in a majority of cases, whereas surgical revision and endovascular intervention are the primary options if DDLT is not available[2,3,95,110].
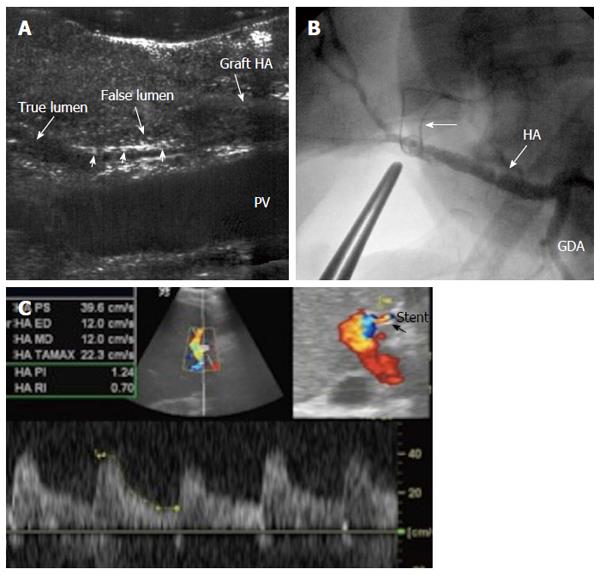
Hepatic artery dissection: Arterial dissection involves the separation of the intimal lining of an artery from the media and, less frequently, the separation of the media from the adventitia. Dissection is usually accompanied by haemorrhage into the arterial wall, which creates a blind pouch or a parallel sub-intimal second channel or false lumen[111]. HA dissection is a predisposing factor to arterial stenosis and occlusion and usually occurs secondary to surgical trauma and clamp injury. It can occur in either the recipient or the donor side of the artery.
IO dissection of the recipient artery is not uncommon, especially in hypertrophied, diseased arteries in elderly patients. The dissection is usually self-limiting because the direction of blood flow seals the tear and can be managed either by anastomotic revision or using another recipient artery[112]. However, dissection of the graft artery is more dramatic as the direction of flow increases the dissection and can extend to the intrahepatic branches, eventually occluding the artery and requiring repair (Figure 33).
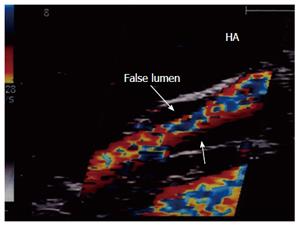
The most common US finding of HA dissection is a double lumen that is separated by an echogenic intimal flap or two parallel intraluminal flaps in cases of circumferential intimal dissection (Figure 34A). An intramural haematoma can also be demonstrated as an eccentric echogenicity that surrounds a relatively narrowed arterial lumen. Color Doppler may demonstrate the true and false lumina. Surgical anastomotic revision of a dissected HA is mandatory in the absence of arterial flow, in the presence of low flow velocity or if the flow is seen in both the true and false lumina with double systolic peaks, signifying ongoing dissection (Figure 34B).
When DU shows a single stream from the main lumen with a sealed or thrombosed false lumen, the anastomosis may not be revised[95] (Figure 35).
Arterial steal phenomena: Arterial steal syndromes have been described as a cause of arterial hypo-perfusion after LT. These syndromes are characterized by low arterial flow towards the graft, caused by shift of flow into either the splenic artery or the gastro-duodenal artery, and are usually diagnosed angiographically[95,113]. The PO DU findings describing these phenomena are nonspecific, such as loss of HA flow signal, decreased HA PSV, elevated or reduced RI, and total absence of diastolic flow[114-116]. Data regarding IO arterial steal are lacking, though in our experience, a low arterial flow coming from the HA proper may be due to coeliac artery stenosis, arcuate ligament syndrome or arterial steal. Experimental clamping of the gastroduodenal artery and the splenic artery may be performed to diagnose arterial steal (Figure 36).
Hepatic artery stenosis: HAS can cause complications similar to HAT, but the condition is less grave with a more insidious course, occurring in 2% to 13% of transplants[2,50,117]. IO recognition of HAS is extremely important, especially in significant cases, because by itself, it causes graft ischaemia. Untreated stenosis can progress to the even more devastating HAT, early graft failure or acute bile duct necrosis and biliary sepsis[118,119]. Post-operatively, patients’ with mild HAS may be asymptomatic or may present with abnormal liver enzymes or biliary strictures.
On DU, HAS can be diagnosed by direct visualization of the anastomotic narrowing by b-mode, anastomotic velocity jet with turbulence and aliasing and distal dampening of the flow (Figures 37 and 38). The PSV at the stenotic segment is usually elevated to greater than 200 cm/s[57,120,121]. Vascular kinks due to vessel redundancy can lead to marked elevation of the PSV, where correction of the angle of interrogation can help differentiate from true stenosis. Anastomotic edema may also cause temporarily increased HA velocity mimicking HAS in the immediate PO scans[40].
Secondary changes seen downstream of the stenosis are very useful in establishing the diagnosis, they are very reliable if there is technical difficulty in direct visualization of the anastomosis due to kinks or small arteries. Distal to the stenosis, the resistance of the arterial tree decreases, resulting in increased diastolic flow and a corresponding decrease in RI to less than 0.55. The rapid hepatic arterial systolic upstroke gets delayed with an increased systolic acceleration time ≥ 0.08 second (tardus parvas waveform), and the sensitivity and specificity of the secondary changes range from 73%-83% and 60%-73%, respectively[121-124]. In the PO period, the tardus parvas waveform has a false-positive diagnosis due to conditions other than HAS, including severe aorto-coeliac atherosclerotic disease, arterio-venous or arterial-biliary fistula formation, and hepatic venous or portal venous thrombosis[125,126].
In our practice we do not rely on the absolute value of the PSV at the anastomosis as it is influenced by many factors, including the systemic pressure, source of the recipient artery, size mismatch, kinks, and spasm. A high- anastomotic jet (> 3-fold) compared with the pre-anastomotic segment with high pitched sound and an intrahepatic tardus parvas waveform are considered signs of HAS (Figure 38B). Management of HAS includes either surgical revision or percutaneous endovascular balloon dilatation and stent placement[127,128].
HA pseudo aneurysms and peripheral arterioportal fistula: HA pseudo aneurysm is rare complication after LT with a reported incidence of 0.27%-3%. It results in leakage of the blood outside the arterial wall into surrounding tissue. Risk factors include technical problem in the arterial anastomosis, peritoneal infections and biliary leak[129-131]. Patients may be asymptomatic or present with abdominal pain, fever, gastrointestinal bleeding or bleeding through the abdominal drain. On sonography HAP appears as a peri-portal or cystic structure with a disorganized arterial flow pattern or a characteristic bidirectional flow[132]. Management is either by surgical repair or interventional radiology[129,133].
Intra-hepatic arterio-portal fistula usually occurs following surgical biopsy or percutaneous liver biopsy or local infection and is often detected incidentally. They appear on DU as an intra-hepatic cystic structure with pulsatile portal vein branch communicating with a fistula to the arterial tree at the biopsy site[78] (Figure 39).
Doppler ultrasonography is the primary imaging modality and the most important diagnostic tool for the evaluation of the graft vascular perfusion in LDLT, both during surgery and post-operatively. It is sensitive to blood flow dynamics and can provide the clinicians and surgeons intra-operative and post-operative potential complications that may occur. A multidisciplinary approach, early detection and thus early problem solving can make the difference between graft survival and failure.
We would like to thank the liver transplantation team of Cairo University and Dar Al-Fouad Hospitals, Cairo, Egypt. For the past 15 years, they adopted a tireless stance and continued relentlessly for hours on end to improve the patient care and survival.
Manuscript source: Invited manuscript
P- Reviewer: Ferraioli G, Sheth RA S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896-903; discussion 903-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Piardi T, Lhuaire M, Bruno O, Memeo R, Pessaux P, Kianmanesh R, Sommacale D. Vascular complications following liver transplantation: A literature review of advances in 2015. World J Hepatol. 2016;8:36-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (7)] |
| 3. | Hejazi Kenari SK, Zimmerman A, Eslami M, F Saidi R. Current state of art management for vascular complications after liver transplantation. Middle East J Dig Dis. 2014;6:121-130. [PubMed] |
| 4. | Khalaf H. Vascular complications after deceased and living donor liver transplantation: a single-center experience. Transplant Proc. 2010;42:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 5. | Sindhi R, Rosendale J, Mundy D, Taranto S, Baliga P, Reuben A, Rajagopalan PR, Hebra A, Tagge E, Othersen HB. Impact of segmental grafts on pediatric liver transplantation--a review of the United Network for Organ Sharing Scientific Registry data (1990-1996). J Pediatr Surg. 1999;34:107-110; discussion 110-111. [PubMed] |
| 6. | Shirouzu Y, Kasahara M, Morioka D, Sakamoto S, Taira K, Uryuhara K, Ogawa K, Takada Y, Egawa H, Tanaka K. Vascular reconstruction and complications in living donor liver transplantation in infants weighing less than 6 kilograms: the Kyoto experience. Liver Transpl. 2006;12:1224-1232. [PubMed] |
| 7. | Sanyal R, Zarzour JG, Ganeshan DM, Bhargava P, Lall CG, Little MD. Postoperative doppler evaluation of liver transplants. Indian J Radiol Imaging. 2014;24:360-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 8. | Broelsch CE, Malagó M, Testa G, Valentin Gamazo C. Living donor liver transplantation in adults: outcome in Europe. Liver Transpl. 2000;6:S64-S65. [PubMed] |
| 9. | Ghobrial RM, Hsieh CB, Lerner S, Winters S, Nissen N, Dawson S, Amersi F, Chen P, Farmer D, Yersiz H. Technical challenges of hepatic venous outflow reconstruction in right lobe adult living donor liver transplantation. Liver Transpl. 2001;7:551-555. [PubMed] |
| 10. | Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, Koh K, Han S, Choi K, Hwang K. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812-814. [PubMed] |
| 11. | Soejima Y, Ueda N, Fukuhara T, Yoshizumi T, Ikegami T, Yamashita Y, Sugimachi K, Taketomi A, Maehara Y. One-step venous reconstruction for a right lobe graft with multiple venous orifices in living donor liver transplantation. Liver Transpl. 2008;14:706-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ikegami T, Shirabe K, Yoshiya S, Soejima Y, Yoshizumi T, Uchiyama H, Toshima T, Motomura T, Maehara Y. One-step reconstruction of the right inferior hepatic veins using auto-venous grafts in living-donor liver transplantation. Surg Today. 2013;43:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hwang S, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Jung DH, Park GC, Namgoong JM, Jung SW. Reconstruction of inferior right hepatic veins in living donor liver transplantation using right liver grafts. Liver Transpl. 2012;18:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Sugawara Y, Makuuchi M, Tamura S, Matsui Y, Kaneko J, Hasegawa K, Imamura H, Kokudo N, Motomura N, Takamoto S. Portal vein reconstruction in adult living donor liver transplantation using cryopreserved vein grafts. Liver Transpl. 2006;12:1233-1236. [PubMed] |
| 15. | Amin AA, Kamel R, Hatata Y, Attia H, Marawan I, Hosney A, El-Malt O, Tanaka K. Crucial issues of hepatic artery reconstruction in living donor liver transplantation: our experience with 133 cases at Dar El-Fouad Hospital, Egypt. J Reconstr Microsurg. 2009;25:307-312. [PubMed] |
| 16. | Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, Sawada H, Shirahase I, Kim HJ, Yamaoka Y. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82-91. [PubMed] |
| 17. | Inomoto T, Nishizawa F, Sasaki H, Terajima H, Shirakata Y, Miyamoto S, Nagata I, Fujimoto M, Moriyasu F, Tanaka K. Experiences of 120 microsurgical reconstructions of hepatic artery in living related liver transplantation. Surgery. 1996;119:20-26. [PubMed] |
| 18. | Alper M, Gundogan H, Tokat C, Ozek C. Microsurgical reconstruction of hepatic artery during living donor liver transplantation. Microsurgery. 2005;25:378-383; discussion 383-384. [PubMed] |
| 19. | Wei WI, Lam LK, Ng RW, Liu CL, Lo CM, Fan ST, Wong J. Microvascular reconstruction of the hepatic artery in live donor liver transplantation: experience across a decade. Arch Surg. 2004;139:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Cheng YF, Huang TL, Chen CL, Lee TY, Chen TY, Chen YS, Liu PP, Chiang YC, Eng HL, Wang CC. Intraoperative Doppler ultrasound in liver transplantation. Clin Transplant. 1998;12:292-299. [PubMed] |
| 21. | Machi J, Oishi AJ, Furumoto NL, Oishi RH. Intraoperative ultrasound. Surg Clin North Am. 2004;84:1085-1101, vi-i. [PubMed] |
| 22. | Bowen A, Hungate RG, Kaye RD, Reyes J, Towbin RB. Imaging in liver transplantation. Radiol Clin North Am. 1996;34:757-778. [PubMed] |
| 23. | Wu CC. Ultrasonographic evaluation of portal hypertension and liver cirrhosis. J Med Ultrasound. 2008;16:188-193. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Barakat M. Portal vein pulsatility and spectral width changes in patients with portal hypertension: relation to the severity of liver disease. Br J Radiol. 2002;75:417-421. [PubMed] |
| 25. | Gallix BP, Taourel P, Dauzat M, Bruel JM, Lafortune M. Flow pulsatility in the portal venous system: a study of Doppler sonography in healthy adults. AJR Am J Roentgenol. 1997;169:141-144. [PubMed] |
| 26. | Lafortune M, Patriquin H. The hepatic artery studies using Doppler sonography. Ultrasound Q. 1999;15:9-26. |
| 27. | McCuskey RS. Hepatic microvascular system. The Liver: Biology and Pathobiology. New York, NY: Raven Press 1994; 1089-1098. |
| 28. | Vilgrain V. Ultrasound of diffuse liver disease and portal hypertension. Eur Radiol. 2001;11:1563-1577. [PubMed] |
| 29. | Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667-673. [PubMed] |
| 30. | Han SH, Rice S, Cohen SM, Reynolds TB, Fong TL. Duplex Doppler ultrasound of the hepatic artery in patients with acute alcoholic hepatitis. J Clin Gastroenterol. 2002;34:573-577. [PubMed] |
| 31. | Hadengue A, Lebrec D, Moreau R, Sogni P, Durand F, Gaudin C, Bernuau J, Belghiti J, Gayet B, Erlinger S. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993;17:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Han H, Liu R, Wang WP, Ding H, Wen JX, Lin XY. Postoperative haemodynamic changes in transplanted liver: Long-term follow-up with ultrasonography. J Int Med Res. 2014;42:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Piscaglia F, Zironi G, Gaiani S, Mazziotti A, Cavallari A, Gramantieri L, Valgimigli M, Bolondi L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: a long-term prospective study. Hepatology. 1999;30:58-64. [PubMed] |
| 34. | Bolognesi M, Sacerdoti D, Bombonato G, Merkel C, Sartori G, Merenda R, Nava V, Angeli P, Feltracco P, Gatta A. Change in portal flow after liver transplantation: effect on hepatic arterial resistance indices and role of spleen size. Hepatology. 2002;35:601-608. [PubMed] |
| 35. | Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429-436. [PubMed] |
| 36. | Jiang SM, Zhang QS, Zhou GW, Huang SF, Lu HM, Peng CH. Differences in portal hemodynamics between whole liver transplantation and living donor liver transplantation. Liver Transpl. 2010;16:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Sainz-Barriga M, Reyntjens K, Costa MG, Scudeller L, Rogiers X, Wouters P, de Hemptinne B, Troisi RI. Prospective evaluation of intraoperative hemodynamics in liver transplantation with whole, partial and DCD grafts. Am J Transplant. 2010;10:1850-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Henderson JM, Millikan WJ, Hooks M, Noe B, Kutner MH, Warren WD. Increased galactose clearance after liver transplantation: a measure of increased blood flow through the denervated liver? Hepatology. 1989;10:288-291. [PubMed] |
| 39. | Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697-1703. [PubMed] |
| 40. | Stell D, Downey D, Marotta P, Solano E, Khakhar A, Quan D, Ghent C, McAlister V, Wall W. Prospective evaluation of the role of quantitative Doppler ultrasound surveillance in liver transplantation. Liver Transpl. 2004;10:1183-1188. [PubMed] |
| 41. | Nolten A, Sproat IA. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198:553-559. [PubMed] |
| 42. | Gilabert R, Bargallo X, Forns X, Bru C, Rimola A, Salmeron JM, Garcia-Valdecasas JC, Grande L, Visa J, Rodes J. Value of duplex-doppler ultrasound findings in liver transplant recipients with poor graft function. Transplantation. 1996;61:832-835. [PubMed] |
| 43. | Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046-6057. [PubMed] |
| 44. | Quintini C, Hirose K, Hashimoto K, Diago T, Aucejo F, Eghtesad B, Vogt D, Pierce G, Baker M, Kelly D. “Splenic artery steal syndrome” is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl. 2008;14:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | García-Criado A, Gilabert R, Salmerón JM, Nicolau C, Vilana R, Bianchi L, Buñesch L, García-Valdecasas JC, Rimola A, Brú C. Significance of and contributing factors for a high resistive index on Doppler sonography of the hepatic artery immediately after surgery: prognostic implications for liver transplant recipients. AJR Am J Roentgenol. 2003;181:831-838. [PubMed] |
| 46. | Sanyal R, Lall CG, Lamba R, Verma S, Shah SN, Tirkes T, Berry WA, Sandrasegaran K. Orthotopic liver transplantation: reversible Doppler US findings in the immediate postoperative period. Radiographics. 2012;32:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 47. | Shapiro RS, Fishbein T, Schwartz M, Miller CM. Use of intraoperative Doppler ultrasound to diagnose hepatic venous obstruction in a right lobe living donor liver transplant. Liver Transpl. 2001;7:547-550. [PubMed] |
| 48. | Uchida K, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Ota M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Three-dimensional computed tomography scan analysis of hepatic vasculatures in the donor liver for living donor liver transplantation. Liver Transpl. 2010;16:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Glockner JF, Forauer AR, Solomon H, Varma CR, Perman WH. Three-dimensional gadolinium-enhanced MR angiography of vascular complications after liver transplantation. AJR Am J Roentgenol. 2000;174:1447-1453. [PubMed] |
| 50. | Wozney P, Zajko AB, Bron KM, Point S, Starzl TE. Vascular complications after liver transplantation: a 5-year experience. AJR Am J Roentgenol. 1986;147:657-663. [PubMed] |
| 51. | Miraglia R, Maruzzelli L, Caruso S, Milazzo M, Marrone G, Mamone G, Carollo V, Gruttadauria S, Luca A, Gridelli B. Interventional radiology procedures in adult patients who underwent liver transplantation. World J Gastroenterol. 2009;15:684-693. [PubMed] |
| 52. | Darcy MD. Management of venous outflow complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:240-245. [PubMed] |
| 53. | Ko EY, Kim TK, Kim PN, Kim AY, Ha HK, Lee MG. Hepatic vein stenosis after living donor liver transplantation: evaluation with Doppler US. Radiology. 2003;229:806-810. [PubMed] |
| 54. | Hwang S, Ha TY, Ahn CS, Moon DB, Song GW, Kim KH, Jung DH, Park GC, Sung KB, Ko GY. Hemodynamics-compliant reconstruction of the right hepatic vein for adult living donor liver transplantation with a right liver graft. Liver Transpl. 2012;18:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Choi JY, Lee JY, Lee JM, Kim SH, Lee MW, Han JK, Choi BI. Routine intraoperative Doppler sonography in the evaluation of complications after living-related donor liver transplantation. J Clin Ultrasound. 2007;35:483-490. [PubMed] |
| 56. | Bolondi L, Li Bassi S, Gaiani S, Zironi G, Benzi G, Santi V, Barbara L. Liver cirrhosis: changes of Doppler waveform of hepatic veins. Radiology. 1991;178:513-516. [PubMed] |
| 57. | Chong WK. Ultrasound evaluation of liver transplants. Abdom Imaging. 2004;29:180-188. [PubMed] |
| 58. | Someda H, Moriyasu F, Fujimoto M, Hamato N, Nabeshima M, Nishikawa K, Okuma M, Tanaka K, Ozawa K. Vascular complications in living related liver transplantation detected with intraoperative and postoperative Doppler US. J Hepatol. 1995;22:623-632. [PubMed] |
| 59. | Ko GY, Sung KB, Yoon HK, Kim JH, Song HY, Seo TS, Lee SG. Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantation. J Vasc Interv Radiol. 2002;13:591-599. [PubMed] |
| 60. | Kubo T, Shibata T, Itoh K, Maetani Y, Isoda H, Hiraoka M, Egawa H, Tanaka K, Togashi K. Outcome of percutaneous transhepatic venoplasty for hepatic venous outflow obstruction after living donor liver transplantation. Radiology. 2006;239:285-290. [PubMed] |
| 61. | Aydogdu S, Tumgor G, Parildar M, Arikan C, Aydin U, Yuzer Y, Kilic M. Acute hepatic vein thrombosis after liver transplantation in a child with biliary atresia and absent inferior vena cava. Transplant Proc. 2006;38:1459-1460. [PubMed] |
| 62. | Akun E, Yaprak O, Killi R, Balci NC, Tokat Y, Yuzer Y. Vascular complications in hepatic transplantation: single-center experience in 14 years. Transplant Proc. 2012;44:1368-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Kilic M, Aydinli B, Aydin U, Alper M, Zeytunlu M. A new surgical technique for hepatic vein reconstruction in pediatric live donor liver transplantation. Pediatr Transplant. 2008;12:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Pérez-Saborido B, Pacheco-Sánchez D, Barrera-Rebollo A, Asensio-Díaz E, Pinto-Fuentes P, Sarmentero-Prieto JC, Rodríguez-Vielba P, Martínez-Díaz R, Gonzalo-Martín M, Rodríguez M. Incidence, management, and results of vascular complications after liver transplantation. Transplant Proc. 2011;43:749-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Steinbrück K, Enne M, Fernandes R, Martinho JM, Balbi E, Agoglia L, Roma J, Pacheco-Moreira LF. Vascular complications after living donor liver transplantation: a Brazilian, single-center experience. Transplant Proc. 2011;43:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK. Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:233-239. [PubMed] |
| 67. | Pawlak J, Grodzicki M, Leowska E, Małkowski P, Michałowicz B, Nyckowski P, Rowiński O, Pacho R, Zieniewicz K, Andrzejewska M. Vascular complications after liver transplantation. Transplant Proc. 2003;35:2313-2315. [PubMed] |
| 68. | Parrilla P, Sánchez-Bueno F, Figueras J, Jaurrieta E, Mir J, Margarit C, Lázaro J, Herrera L, Gómez-Fleitas M, Varo E. Analysis of the complications of the piggy-back technique in 1,112 liver transplants. Transplantation. 1999;67:1214-1217. [PubMed] |
| 69. | Sánchez-Bueno F, Hernández Q, Ramírez P, Robles R, Acosta F, Rodríguez JM, Parrilla P. Vascular complications in a series of 300 orthotopic liver transplants. Transplant Proc. 1999;31:2409-2410. [PubMed] |
| 70. | Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, Kelly S, Brady L, Leef JA, Millis JM. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236:658-666. [PubMed] |
| 71. | Kyoden Y, Tamura S, Sugawara Y, Matsui Y, Togashi J, Kaneko J, Kokudo N, Makuuchi M. Portal vein complications after adult-to-adult living donor liver transplantation. Transpl Int. 2008;21:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Stieber AC, Zetti G, Todo S, Tzakis AG, Fung JJ, Marino I, Casavilla A, Selby RR, Starzl TE. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg. 1991;213:199-206. [PubMed] |
| 73. | Lerut J, Tzakis AG, Bron K, Gordon RD, Iwatsuki S, Esquivel CO, Makowka L, Todo S, Starzl TE. Complications of venous reconstruction in human orthotopic liver transplantation. Ann Surg. 1987;205:404-414. [PubMed] |
| 74. | Lin TL, Chiang LW, Chen CL, Wang SH, Lin CC, Liu YW, Yong CC, Lin TS, Li WF, Jawan B. Intra-operative management of low portal vein flow in pediatric living donor liver transplantation. Transpl Int. 2012;25:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Boillot O, Delafosse B, Méchet I, Boucaud C, Pouyet M. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002;359:406-407. [PubMed] |
| 76. | Heaton N. Small-for-size liver syndrome after auxiliary and split liver transplantation: donor selection. Liver Transpl. 2003;9:S26-S28. [PubMed] |
| 77. | Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313-1317. [PubMed] |
| 78. | Attia H, Kamel R. Post operative ultrasonography. In Tanaka K, Inomata Y, Kaihara S. Evolution of living-donor liver transplant. Provenza 388, 08025. Barcelona , Spain: Prous Science 2008; 185-208. |
| 79. | Cheng YF, Ou HY, Yu CY, Tsang LL, Huang TL, Chen TY, Hsu HW, Concerjero AM, Wang CC, Wang SH. Interventional radiology in living donor liver transplant. World J Gastroenterol. 2014;20:6221-6225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Cheng YF, Ou HY, Tsang LL, Yu CY, Huang TL, Chen TY, Concejero A, Wang CC, Wang SH, Lin TS. Vascular stents in the management of portal venous complications in living donor liver transplantation. Am J Transplant. 2010;10:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Wei BJ, Zhai RY, Wang JF, Dai DK, Yu P. Percutaneous portal venoplasty and stenting for anastomotic stenosis after liver transplantation. World J Gastroenterol. 2009;15:1880-1885. [PubMed] |
| 82. | Schneider N, Scanga A, Stokes L, Perri R. Portal vein stenosis: a rare yet clinically important cause of delayed-onset ascites after adult deceased donor liver transplantation: two case reports. Transplant Proc. 2011;43:3829-3834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Carnevale FC, de Tarso Machado A, Moreira AM, Dos Santos AC, da Motta-Leal-Filho JM, Suzuki L, Cerri GG, Tannuri U. Long-term results of the percutaneous transhepatic venoplasty of portal vein stenoses after pediatric liver transplantation. Pediatr Transplant. 2011;15:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Carnevale FC, Borges MV, Moreira AM, Cerri GG, Maksoud JG. Endovascular treatment of acute portal vein thrombosis after liver transplantation in a child. Cardiovasc Intervent Radiol. 2006;29:457-461. [PubMed] |
| 85. | Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM. Angioplasty treatment of portal vein stenosis in children with segmental liver transplants: mid-term results. AJR Am J Roentgenol. 1997;169:551-554. [PubMed] |
| 86. | Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM, Piper JB, Whitington PF. Portal vein stenosis in children with segmental liver transplants: treatment with percutaneous transhepatic venoplasty. AJR Am J Roentgenol. 1995;165:161-165. [PubMed] |
| 87. | Huang TL, Cheng YF, Chen TY, Tsang LL, Ou HY, Yu CY, Wang CC, Wang SH, Lin CL, Cheung HK. Doppler ultrasound evaluation of postoperative portal vein stenosis in adult living donor liver transplantation. Transplant Proc. 2010;42:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Shibata T, Itoh K, Kubo T, Maetani Y, Shibata T, Togashi K, Tanaka K. Percutaneous transhepatic balloon dilation of portal venous stenosis in patients with living donor liver transplantation. Radiology. 2005;235:1078-1083. [PubMed] |
| 89. | Azzam AZ, Tanaka K. Management of vascular complications after living donor liver transplantation. Hepatogastroenterology. 2012;59:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 90. | Langnas AN, Marujo W, Stratta RJ, Wood RP, Shaw BW. Vascular complications after orthotopic liver transplantation. Am J Surg. 1991;161:76-82; discussion 82-83. [PubMed] |
| 91. | Cherukuri R, Haskal ZJ, Naji A, Shaked A. Percutaneous thrombolysis and stent placement for the treatment of portal vein thrombosis after liver transplantation: long-term follow-up. Transplantation. 1998;65:1124-1126. [PubMed] |
| 92. | Haskal ZJ, Naji A. Treatment of portal vein thrombosis after liver transplantation with percutaneous thrombolysis and stent placement. J Vasc Interv Radiol. 1993;4:789-792. [PubMed] |
| 93. | Bhattacharjya T, Olliff SP, Bhattacharjya S, Mirza DF, McMaster P. Percutaneous portal vein thrombolysis and endovascular stent for management of posttransplant portal venous conduit thrombosis. Transplantation. 2000;69:2195-2198. [PubMed] |
| 94. | Baccarani U, Gasparini D, Risaliti A, Vianello V, Adani GL, Sainz M, Sponza M, Bresadola F. Percutaneous mechanical fragmentation and stent placement for the treatment of early posttransplantation portal vein thrombosis. Transplantation. 2001;72:1572-1582. [PubMed] |
| 95. | Abdelaziz O, Hosny K, Amin A, Emadeldin S, Uemoto S, Mostafa M. Endovascular management of early hepatic artery thrombosis after living donor liver transplantation. Transpl Int. 2012;25:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Singhal A, Stokes K, Sebastian A, Wright HI, Kohli V. Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl Int. 2010;23:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Settmacher U, Stange B, Haase R, Heise M, Steinmüller T, Bechstein WO, Neuhaus P. Arterial complications after liver transplantation. Transpl Int. 2000;13:372-378. [PubMed] |
| 98. | Uchida Y, Nakamura F, Tomaru T, Sonoki H, Sumino S, Sugimoto T. Angiographic and angioscopic observations of the arterial luminal changes induced by vasospasm. Am Heart J. 1987;114:1096-1101. [PubMed] |
| 99. | Sakamoto Y, Harihara Y, Nakatsuka T, Kawarasaki H, Takayama T, Kubota K, Kimura W, Kita Y, Tanaka H, Ito M. Rescue of liver grafts from hepatic artery occlusion in living-related liver transplantation. Br J Surg. 1999;86:886-889. [PubMed] |
| 100. | Acosta F, Diaz J, Sansano T, Palenciano CG, Reche M, Beltran R, Roques V, Robles R, Bueno FS, Ramirez P. Evolution of the plasma concentration of norepinephrine in cirrhotic patients during liver transplantation. Transplant Proc. 2000;32:2659-2660. [PubMed] |
| 101. | Irita K, Okamoto H, Sakaguchi Y, Takahashi S. A possible increase in plasma norepinephrine by removal of the liver. Acta Anaesthesiol Scand. 1998;42:1164-1167. [PubMed] |
| 102. | Kogure K, Suzuki M. Effects of hepatic inflow occlusion on changes in plasma potassium, histamine, and norepinephrine in rats. Circ Shock. 1992;36:290-298. [PubMed] |
| 103. | Chen W, Facciuto ME, Rocca JP, Marvin MR, Sheiner PA, Rachlin S, Rodriguez MI. Doppler ultrasonographic findings on hepatic arterial vasospasm early after liver transplantation. J Ultrasound Med. 2006;25:631-638. [PubMed] |
| 104. | Richards RR, Seaber AV, Urbaniak JR. Chemically induced vasospasm: the effect of ischemia, vessel occlusion, and adrenergic blockade. Plast Reconstr Surg. 1985;75:238-244. [PubMed] |
| 105. | Carter SA. Hemodynamic considerations in peripheral and cerebrovascular disease. Introduction to Vascular Ultrasonography. Philadelphia, PA: WB Saunders Co 2000; 3-17. |
| 106. | Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 107. | Proposito D, Loinaz Segurola C, Garcia Garcìa I, Jimènez C, Gonzalez Pinto I, Gomez Sanz R, De La Cruz J, Moreno Gonzàlez E. [Assessment of risk factors in the incidence of hepatic artery thrombosis in a consecutive series of 687 liver transplantations]. Ann Ital Chir. 2001;72:187-205. [PubMed] |
| 108. | Warner P, Fusai G, Glantzounis GK, Sabin CA, Rolando N, Patch D, Sharma D, Davidson BR, Rolles K, Burroughs AK. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation - univariable and multivariable analysis. Transpl Int. 2011;24:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | Propeck PA, Scanlan KA. Reversed or absent hepatic arterial diastolic flow in liver transplants shown by duplex sonography: a poor predictor of subsequent hepatic artery thrombosis. AJR Am J Roentgenol. 1992;159:1199-1201. [PubMed] |
| 110. | Kim BW, Won JH, Lee BM, Ko BH, Wang HJ, Kim MW. Intraarterial thrombolytic treatment for hepatic artery thrombosis immediately after living donor liver transplantation. Transplant Proc. 2006;38:3128-3131. [PubMed] |
| 111. | Kim YK, Schulman S. Cervical artery dissection: pathology, epidemiology and management. Thromb Res. 2009;123:810-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 112. | Abdelaziz O, Sallam K, Mostafa M, Elansary A, Amin A. Hybrid Microsurgical Reconstruction and Percutaneous Endovascular Stent Placement for Management of Dissected Graft Hepatic Artery during Living Donor Liver Transplantation. J Vasc Interv Radiol. 2015;26:916-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | García-Criado A, Gilabert R, Berzigotti A, Brú C. Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR Am J Roentgenol. 2009;193:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Uflacker R, Selby JB, Chavin K, Rogers J, Baliga P. Transcatheter splenic artery occlusion for treatment of splenic artery steal syndrome after orthotopic liver transplantation. Cardiovasc Intervent Radiol. 2002;25:300-306. [PubMed] |
| 115. | Nishida S, Kadono J, DeFaria W, Levi DM, Moon JI, Tzakis AG, Madariaga JR. Gastroduodenal artery steal syndrome during liver transplantation: intraoperative diagnosis with Doppler ultrasound and management. Transpl Int. 2005;18:350-353. [PubMed] |
| 116. | Uslu N, Aslan H, Tore HG, Moray G, Karakayali H, Boyvat F, Arslan G, Haberal M. Doppler ultrasonography findings of splenic arterial steal syndrome after liver transplant. Exp Clin Transplant. 2012;10:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | da Silva RF, Raphe R, Felício HC, Rocha MF, Duca WJ, Arroyo PC, Palini GL, Vasquez AM, Miquelin DG, Reis LF. Prevalence, treatment, and outcomes of the hepatic artery stenosis after liver transplantation. Transplant Proc. 2008;40:805-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Park YS, Kim KW, Lee SJ, Lee J, Jung DH, Song GW, Ha TY, Moon DB, Kim KH, Ahn CS. Hepatic arterial stenosis assessed with doppler US after liver transplantation: frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff. Radiology. 2011;260:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 119. | Kim SY, Kim KW, Kim MJ, Shin YM, Lee MG, Lee SG. Multidetector row CT of various hepatic artery complications after living donor liver transplantation. Abdom Imaging. 2007;32:635-643. [PubMed] |
| 120. | Maceneaney PM, Malone DE, Skehan SJ, Curry MP, Miller JC, Gibney RG, Traynor O, Mccormick PA. The role of hepatic arterial Doppler ultrasound after liver transplantation: an ‘audit cycle’ evaluation. Clin Radiol. 2000;55:517-524. [PubMed] |
| 121. | Dodd GD, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994;192:657-661. [PubMed] |
| 122. | Platt JF, Yutzy GG, Bude RO, Ellis JH, Rubin JM. Use of Doppler sonography for revealing hepatic artery stenosis in liver transplant recipients. AJR Am J Roentgenol. 1997;168:473-476. [PubMed] |
| 123. | Vit A, De Candia A, Como G, Del Frate C, Marzio A, Bazzocchi M. Doppler evaluation of arterial complications of adult orthotopic liver transplantation. J Clin Ultrasound. 2003;31:339-345. [PubMed] |
| 124. | Sidhu PS, Ellis SM, Karani JB, Ryan SM. Hepatic artery stenosis following liver transplantation: significance of the tardus parvus waveform and the role of microbubble contrast media in the detection of a focal stenosis. Clin Radiol. 2002;57:789-799. [PubMed] |
| 125. | Urbani L, Morelli L, Campatelli A, Montin U, Catalano G, Biancofiore G, Bindi L, Bargellini I, Cioni R, Vignali C. False positive tardus-parvus waveforms after liver transplantation: a case of wide discrepancy between donor and recipient hepatic arteries mimicking anastomotic stenosis. Transplant Proc. 2008;40:3816-3818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Tamsel S, Demirpolat G, Killi R, Aydin U, Kilic M, Zeytunlu M, Parildar M, Oran I, Ucar H. Vascular complications after liver transplantation: evaluation with Doppler US. Abdom Imaging. 2007;32:339-347. [PubMed] |
| 127. | Chen GH, Wang GY, Yang Y, Li H, Lu MQ, Cai CJ, Wang GS, Xu C, Yi SH, Zhang JF. Single-center experience of therapeutic management of hepatic artery stenosis after orthotopic liver transplantation. Report of 20 cases. Eur Surg Res. 2009;42:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Uller W, Knoppke B, Schreyer AG, Heiss P, Schlitt HJ, Melter M, Stroszczynski C, Zorger N, Wohlgemuth WA. Interventional radiological treatment of perihepatic vascular stenosis or occlusion in pediatric patients after liver transplantation. Cardiovasc Intervent Radiol. 2013;36:1562-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Boleslawski E, Bouras AF, Truant S, Liddo G, Herrero A, Badic B, Audet M, Altieri M, Laurent A, Declerck N. Hepatic artery ligation for arterial rupture following liver transplantation: a reasonable option. Am J Transplant. 2013;13:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 130. | Stange B, Settmacher U, Glanemann M, Nuessler NC, Bechstein WO, Neuhaus P. Aneurysms of the hepatic artery after liver transplantation. Transplant Proc. 2000;32:533-534. [PubMed] |
| 131. | Volpin E, Pessaux P, Sauvanet A, Sibert A, Kianmanesh R, Durand F, Belghiti J, Sommacale D. Preservation of the arterial vascularisation after hepatic artery pseudoaneurysm following orthotopic liver transplantation: long-term results. Ann Transplant. 2014;19:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Zajko AB, Tobben PJ, Esquivel CO, Starzl TE. Pseudoaneurysms following orthotopic liver transplantation: clinical and radiologic manifestations. Transplant Proc. 1989;21:2457-2459. [PubMed] |
| 133. | Patel JV, Weston MJ, Kessel DO, Prasad R, Toogood GJ, Robertson I. Hepatic artery pseudoaneurysm after liver transplantation: treatment with percutaneous thrombin injection. Transplantation. 2003;75:1755-1757. [PubMed] |