Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6127
Peer-review started: March 25, 2016
First decision: April 14, 2016
Revised: May 2, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: July 21, 2016
Processing time: 112 Days and 5 Hours
Hepatocellular carcinoma (HCC) is one of the most common cancers in Eastern Asia, and its incidence is increasing globally. Numerous experimental models have been developed to better our understanding of the pathogenic mechanism of HCC and to evaluate novel therapeutic approaches. Molecular imaging is a convenient and up-to-date biomedical tool that enables the visualization, characterization and quantification of biologic processes in a living subject. Molecular imaging based on reporter gene expression, in particular, can elucidate tumor-specific events or processes by acquiring images of a reporter gene’s expression driven by tumor-specific enhancers/promoters. In this review, we discuss the advantages and disadvantages of various experimental HCC mouse models and we present in vivo images of tumor-specific reporter gene expression driven by an alpha-fetoprotein (AFP) enhancer/promoter system in a mouse model of HCC. The current mouse models of HCC development are established by xenograft, carcinogen induction and genetic engineering, representing the spectrum of tumor-inducing factors and tumor locations. The imaging analysis approach of reporter genes driven by AFP enhancer/promoter is presented for these different HCC mouse models. Such molecular imaging can provide longitudinal information about carcinogenesis and tumor progression. We expect that clinical application of AFP-targeted reporter gene expression imaging systems will be useful for the detection of AFP-expressing HCC tumors and screening of increased/decreased AFP levels due to disease or drug treatment.
Core tip: It is essential to establish an appropriate animal model of hepatocellular carcinoma (HCC) for monitoring the disease progression and evaluating therapeutic interventions with anticancer drugs. Reporter gene-based molecular imaging can elucidate tumor-specific events or processes through acquisition of images of reporter gene expression driven by tumor-specific enhancers/promoters. In this paper, we describe the advantages and disadvantages of various animal models of HCC and present images of in vivo reporter gene expression controlled by alpha-fetoprotein enhancer/promoter in the various HCC animal models.
- Citation: Kim KI, Chung HK, Park JH, Lee YJ, Kang JH. Alpha-fetoprotein-targeted reporter gene expression imaging in hepatocellular carcinoma. World J Gastroenterol 2016; 22(27): 6127-6134
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6127
Molecular imaging methods can provide novel insights into biological and physiological processes through visualization of cellular or molecular events in a living organism. The greatest significance of molecular imaging methods lies in their ability to non-invasively and repetitively obtain longitudinal and quantitative biological information in an in vivo setting[1]. Moreover, when compared to analytical methods involving biopsied tissues, the non-invasive nature of molecular imaging is less time-consuming and provides more reliable inferences to the organism.
When applied to evaluate the efficacy and therapeutic mechanism of new drugs, molecular imaging can help to determine biochemical or metabolic changes in vivo by use of Tc-99m labeled annexin V (for apoptosis), Tc-99m labeled vascular endothelial growth factor or Cu-64/In-111/Tc-99m labeled arginylglycylaspartic acid peptide (for angiogenesis), Cu-64 labeled methylthiosemicarbazone or F-18 labeled fluoroazomycinarabinoside (for hypoxia), or F-18 labeled 2-deoxy-D-glucose, F-18 labeled 3’-deoxy-3’-fluorothymidine or F-18 labeled fluoroethyltyrosine (for glucose, nucleotide or amino acid metabolism)[2]. The pharmacokinetic and pharmacodynamic properties of new drugs, including tissue biodistribution at designated time points and the binding affinity of ligands to their target receptors, can also be determined by nuclear medicine imaging using radioisotope-labeled drugs[3]. At present, the applicability of molecular imaging methods to drug development is increasing in scale and scope, with the added benefit of providing further clarification of basic biological phenomena[4].
The currently available molecular imaging methods are classified into the following categories according to their technical modalities: optical imaging (fluorescence and bioluminescence); magnetic resonance imaging (MRI); nuclear medicine imaging, including scintigraphy, positron emission tomography (PET) and single-photon emission computed tomography (commonly known as SPECT); and others, including ultrasound imaging and photoacoustic imaging. Optical imaging has high sensitivity, but its clinical utility is limited due to its low depth of penetration. MRI has high resolution and excellent soft-tissue contrast, but poor sensitivity and the expensive cost of the MRI equipment have proven prohibitive to its widespread application. Nuclear medicine imaging has high sensitivity and unlimited depth penetration, but again cost of the equipment is prohibitive and its limited spatial resolution is another limiting factor. Ultrasound imaging has high spatial/temporal resolution and a much more affordable (relatively low cost) profile, but is limited to vascular compartments and its reliability can be operator-dependent[5,6]. Therefore, several multimodality imaging instruments, such as PET/CT, PET/MR, and optical imaging/computed tomography (CT), have been developed to overcome the distinct disadvantages of each and now play an important role in basic and clinical research.
Molecular imaging with reporter gene expression (also known as molecular genetic imaging) is defined as an imaging method that makes use of reporter gene expression in a target cell or tissue. Various imaging reporter genes have been developed for optical and nuclear medicine imaging, with the most popular being those encoding firefly luciferase, a variety of fluorescent proteins, the herpes simplex virus type 1 thymidine kinase (HSV1-tk) and the sodium iodide symporter (NIS)[5,7]. To localize and track target cells in vivo, a reporter gene driven by a strong constitutive promoter, such as that of cytomegalovirus (commonly referred to as CMV), is first introduced into target cells. The reporter gene-expressing target cell is injected into a living organism and images are then acquired at designated time points following target cell injection. Because the HSV1-tk gene is applied for therapy as well as imaging, this gene expression can be monitored by visualization with an imaging technique[5]. Therefore, combining molecular imaging and gene therapy can allow for real-time evaluation of location and duration of expression of a therapeutic gene, and successful application of this method in clinical practice has been reported[8,9].
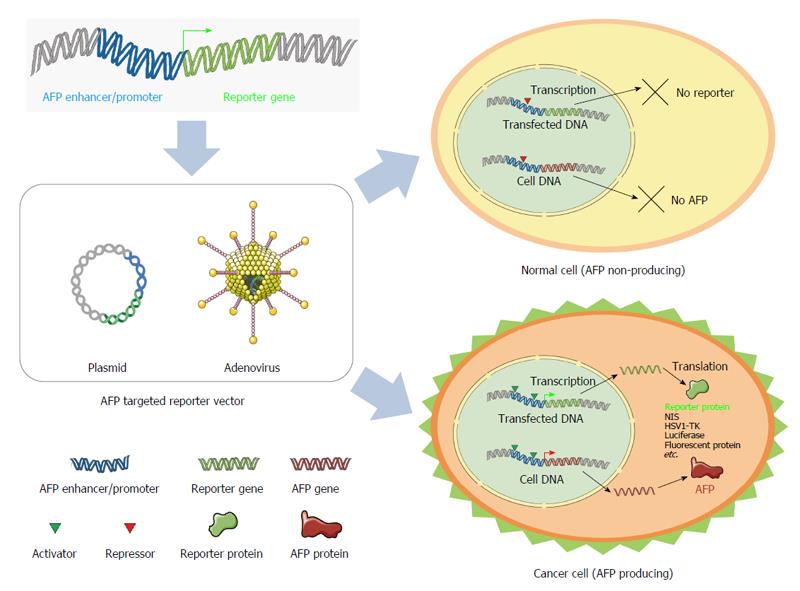
Because tumor-specific enhancers/promoters, such as the alpha-fetoprotein (AFP) enhancer/promoter (Figure 1) and human telomerase reverse transcriptase, are highly active in cancer cells but not in non-cancer cells, they may serve as markers of cancer pathogenesis and progression that can be monitored by imaging of reporter gene expression. Indeed, several studies have already shown the utility of such reporter gene expression imaging using various tumor-specific enhancers/promoters, including survivin, mucin-1, carcinoembryonic antigen, prostate specific antigen and progression elevated gene-3; these studies are summarized in Table 1[10-20].
| Enhancer/promoter | Reporter gene(delivery method) | Imaging modality | Targeted tumor | Ref. |
| Survivin | hNIS (adenovirus) | Gamma camera | Ectopic xenograft | Huang et al[10] |
| PC-3 (prostate cancer) | ||||
| HepG2 (hepatoma) | ||||
| A375 (melanoma) | ||||
| fLuc (adenovirus) | Bioluminescent imaging | Orthotopic xenograft | Ahn et al[11] | |
| McA-RH7777 (rat hepatoma) | ||||
| Mucin-1 | fLuc (adenovirus) | Bioluminescent imaging | Ectopic xenograft (metastasis) | Huyn et al[12] |
| KPL-1 (breast cancer) | ||||
| Hepatocarcinoma-intestine-pancreas (HIP) | NIS (adenovirus) | SPECT-CT | DEN-induced HCC (rat) | Hervé et al[13] |
| Prostate specific antigen (PSA) | fLuc/HSV1-sr39tk (adenovirus) | Bioluminescent imaging/ PET | Ectopic xenograft | Iyer et al[14] |
| LNCaP (prostate cancer) | Jiang et al[15] | |||
| Carcinoembryonic antigen (CEA) | HSV1-tk (adenovirus) | Gamma camera | Ectopic xenograft | Qiao et al[16] |
| MOD (murine breast cancer) | ||||
| hNIS (adenovirus) | Gamma camera | Ectopic xenograft | Spitzweg et al[17] | |
| TT (medullary thyroid cancer) | ||||
| Progression elevated gene (PEG)-3 | fLuc/HSV1-tk (plasmid) | Bioluminescent imaging/ SPECT-CT | Ectopic xenograft (metastasis) | Bhang et al[18] |
| MeWo (melanoma) | ||||
| MDA-MB-231 (breast cancer) | ||||
| Telomerase reverse transcriptase (TERT) | hNIS (plasmid) | SPECT-CT | Ectopic xenograft | Kim et al[19] |
| Hep3B (hepatoma) | ||||
| GFP (lentivirus) | Fluorescence imaging | Ectopic xenograft | Yu et al[20] | |
| HepG2 (hepatoma) | ||||
| SGC-7901 (gastric cancer) | ||||
| SW480 (colon cancer) |
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and is currently ranked fifth in incidence and second in mortality among males[21]. Fetal liver cells normally express AFP, but expression decreases rapidly after birth. In adults, however, AFP expression has been found to resume under abnormal conditions, including liver cancer and cirrhosis. Although the diagnostic accuracy of AFP in small HCC (< 3 cm) is limited, it has been a useful and convenient diagnostic tool for HCC since the 1970s[22]. Thus, many of the studies of HCC-specific imaging have used AFP-targeted reporter gene expression.
It is essential to establish an HCC animal model for monitoring of HCC progression and therapeutic interventions. In this review, we discuss methods for preparation of experimental HCC mouse models and the particular advantages and disadvantages of each. The AFP-specific reporter gene system, in vivo imaging applications for hepatocarcinogenesis and detection of AFP-positive HCC are also presented in the context of the mouse models.
Mouse models of HCC development are established by xenografting, carcinogen induction or genetic engineering, according to the tumor-inducing factor and tumor location of research interest. Table 2 provides an overview of the various HCC mouse modeling methods and their key advantages and disadvantages.
| Category | Inducing factor | Latency | Advantages | Disadvantages | |
| Xenograft | Ectopic | Cancer cell line | Several weeks | Fast and easy modeling | Less clinical relevancy |
| Orthotopic | Easy detection of tumorigenesis | Only reflect the characteristics of the selected cells | |||
| Carcinogen-induced | DEN | 5-10 mo | More clinical relevancy | Lengthy time and high cost to model | |
| Genetically engineered | HBV-derived | 12-24 mo | Uncovering the molecular mechanisms of hepatocarcinogenesis | Difficult to detect tumorigenesis | |
| HCV-derived | 12-24 mo | Closely mimic the pathophysiological features of human HCC | |||
| Oncogene-derived | Several weeks | ||||
Xenograft HCC models involve inoculation of cultured HCC cells into immune-deficient mice, such as the athymic or severe combined immunodeficiency (SCID) strains. Xenograft models can be generated ectopically, by subcutaneous injection of HCC cells into extrahepatic locations such as the flank or thigh, or orthotopically, by direct injection into the liver; the latter approach more accurately reflects the in vivo tumor environment[23]. Xenograft models are considered an easy, rapid and efficient means by which to demonstrate proofs of concept when appropriate cell lines are selected[24]. However, this model has less clinical relevancy than autochthonous HCC models (which are beyond the scope of this review and not discussed herein).
Carcinogens are subdivided into two classes, namely the genotoxic and non-genotoxic (or epigenetic) types. Genotoxic carcinogens irreversibly damage DNA, leading to genetic alterations and interference with normal biological processes; these types of carcinogens comprise chemical or non-chemical agents, such as radiation (ultraviolet or ionizing) and viruses. Non-genotoxic carcinogens do not directly adduct DNA structure, but alter cellular metabolism and promote uncontrolled malignant division. In general, the effect of carcinogens is insidious because they may not be immediately toxic[23,25-27]. Carcinogen-induced HCC mouse models are principally used for evaluation of human hepatocarcinogenesis, and these studies must take into consideration that HCC in both human and mouse can be affected by additional contributing factors - e.g., sex, age and genetic background[28].
Several chemical carcinogens can induce HCC formation to establish the mouse model system; the most commonly used are diethylnitrosamine (DEN)[29], aflatoxin[30], thioacetamide[31], and carbon tetrachloride[32]. Among these, DEN is the preferred agent, since the other chemical carcinogens have human toxicity and yield low tumor incidence and delayed carcinogenesis[23]. The carcinogenic property of DEN arises from its alkylation of cellular DNA[33] and generation of reactive oxygen species[34]. DEN is generally administered to the mice as a single dose between postnatal days 12-15. The single-dose DEN administration to generate HCC must consider the dosage applied as well as the sex and age of the animal[35-37]. Research groups using the single-dose protocol that was originally described by Vesselinovitch and Mihailovich have reported that intraperitoneal injection of DEN results in generation of HCC in 100% of B6C3F1 male mice after 44 wk[38-40]. In addition, the role of androgen receptor (AR) status has been demonstrated as important in DEN-induced murine hepatocarcinogenesis[41].
As a consequence of the known significant differences between mouse and human carcinogenesis, carcinogen-induced HCC mouse models are not appropriate for determining the molecular mechanisms of human hepatocarcinogenesis. In contrast, genetically engineered mouse (GEM) models closely mimic the pathophysiological and molecular features of human HCC[42]. GEM modeling, then, is more feasible for studies to understand the complexities of human diseases, such as HCC, and for assessment of the molecular mechanisms of tumor generation, progression and maintenance in particular[43]. GEM models have already been successfully used in investigations of specific genes and their interactions with other genes[28]. Numerous GEMs have been developed to study liver tumorigenesis in mice, with the most frequently used ones involving overexpression (transgenic mice) or deletion (knockout mice) of a specific gene[44]. Moreover, these GEM models have been developed by various techniques, including pronuclear injection (additive transgenesis), homologous recombination (targeted transgenesis using embryonic stem cell technology), and RNA interference (to generate knockdown mice)[45].
More than 80% of HCCs in humans are attributable to infection with the hepatitis B virus (HBV) and/or the hepatitis C virus[46]. The genome of HBV is characterized by four overlapping open reading frames, which encode surface, core, polymerase and X (HBx) proteins. HBx transgenic mice are more sensitive to a single DEN-injection than their non-transgenic counterparts[47,48]. In general, mutation or overexpression of the Myc gene is associated with tumorigenesis, including that of HCC. A mouse model with overexpression of the human transforming growth factor-α (TGF-α) under control of the methallothionein (MT) 1 promoter develops HCC. Concordantly, it has been reported that Myc- and TGF-α over-expressing transgenic mice are genetically close to human HCC and that the overexpression profile is related with prognosis[49]. In transgenic mice with over-expression of epidermal growth factor (EGF) under the control of the albumin promoter, the overexpression of secreted EGF leads to generation of multiple highly malignant hepatic tumors[50]. In addition, other DNA viruses, such as simian vacuolating virus 40 (SV40), have the potential to cause tumors[51].
AFP is a biomarker of HCC, and much progress has been made in our understanding of the mechanistic underpinnings of AFP expression in HCC. Because the AFP gene becomes re-expressed in HCC, tumorigenesis can be monitored using reporter gene expression imaging. However, few AFP-targeted reporter gene-imaging studies have been published; those studies on reporter gene imaging driven by AFP enhancer/promoter in HCC mouse models are presented in Table 3[52-60].
| Tumor model | Mouse strain | Induction of HCC | Reporter gene | Delivery system | Injection route | Ref. |
| Xenograft | NOD/SCID | HuH-7 cells | hNIS | Adenovirus1 | IV | Kim et al[56] |
| BALB/c nude | TK/fLuc | Adenovirus1 | IT | Park et al[57] | ||
| NOD/SCID | fLuc | Adenovirus1 | IV | Kim et al[58] | ||
| BALB/c nude | hNIS | Plasmid2 | - | Jin et al[52] | ||
| CD-1 nude | HepG2 cells | hNIS | Adenovirus1 | IT | Klutz et al[54] | |
| BALB/c nude | hNIS | Adenovirus1 | IT | Ma et al[55] | ||
| NMRI nude | hNIS | Plasmid2 | - | Willhauck et al[53] | ||
| Carcinogen-induced | C57BL/6 | DEN | fLuc | Adenovirus1 | IV | Kim et al[58] |
| FVB/N | DEN | TK/fLuc | Transgenesis3 | - | Lu et al[59] | |
| C57BL/6 | DEN | fLuc | Transgenesis3 | - | Park et al[60] |
Xenograft tumor models are preferred for use in cancer research because of their ease of tumor establishment. Several studies of AFP-targeted imaging or therapeutic effects in xenograft ectopic HCC mouse models established by the ectopic approach have been reported which carried out assessment via optical or nuclear medicine imaging modalities. A reporter gene under the control of the AFP enhancer/promoter can be delivered into cells using a plasmid vector (cell transfection) or adenoviral vector (systemic or intratumoral administration) system.
Jin et al[52] and Willhauck et al[53] used a plasmid system under the control of the AFP enhancer/promoter to achieve stable NIS gene expression in an HCC cell line. The AFP-targeted NIS gene expression system functioned well in vitro and in vivo, indicating the feasibility of HCC-specific reporter gene expression systems for diagnosis and therapy of AFP-positive HCC. However, this plasmid system proved to be limited in terms of its ability to deliver the reporter gene to the target region in vivo due to use of a plasmid-transfected HCC cell line. In other studies, an adenoviral vector system using the AFP enhancer/promoter was injected intratumorally in a xenograft HCC model established by the ectopic approach[54,55]. Those results indicated the potential of in vivo AFP-targeted imaging and a radiotherapeutic approach in HCC.
Kim et al[56] and Park et al[57] reported in vivo systemic delivery of an adenoviral vector system with a reporter gene (NIS, fLuc or HSV1-tk) driven by the AFP enhancer/promoter. Those studies showed that targeted imaging and therapy through systemic delivery of adenoviral vector was possible. Also, Kim et al[58] introduced AFP-targeted bioluminescent imaging performed using adenovirus in xenograft and carcinogen-induced HCC tumor models. Thus, the adenovirus system is capable of facilitating AFP-targeted imaging and therapy in carcinogen-induced HCC as well as in xenograft tumor models.
Generally, carcinogen-induced HCC animal models can be established upon exposure of genetically susceptible mice to a variety of chemical carcinogens, such as DEN. Induction of HCC using carcinogens is more difficult than by the xenograft approach because of the longer generation time and greater difficulty of verification of AFP expression.
Lu et al[59] reported that a hepatocarcinogenesis reporter (HCR) transgenic mouse model enables monitoring of tumorigenesis by bioluminescent and nuclear medicine imaging. In their HCR mouse model, the HSV1-tk and fLuc genes were concurrently expressed under the control of the AFP enhancer/promoter. The bioluminescent signal was then detected during the early stage of DEN-induced HCC, prior to neoplastic transformation. Detection at later stages revealed high expression of fLuc and HSV1-tk. In another study, Park et al[60] demonstrated non-invasive monitoring of AFP expression and DEN-induced hepatocarcinogenesis using bioluminescent imaging in transgenic mice with the fLuc gene under the control of the AFP enhancer/promoter. These two transgenic mouse models enabled in vivo monitoring of AFP expression throughout the entire disease course and lifetime of the afflicted animal. These studies suggested the usefulness of AFP-targeted reporter gene expression imaging for non-invasive in vivo evaluation of hepatocarcinogenesis.
We have presented here the various experimental HCC animal models, including xenograft, carcinogen-induced and genetically engineered HCC models, and discussed their characteristics. Non-invasive real-time in vivo molecular imaging of reporter gene expression under the control of tumor-specific enhancers/promoters can provide longitudinal information about carcinogenesis and tumor progression. We expect that AFP-targeted reporter gene expression imaging systems will be applied for the detection of AFP-expressing HCC tumors and screening of increased/decreased AFP levels due to disease or drug treatment.
Manuscript source: Invited manuscript
P- Reviewer: Chen CH, Goral V, Zhang Q S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Massoud TF, Gambhir SS. Integrating noninvasive molecular imaging into molecular medicine: an evolving paradigm. Trends Mol Med. 2007;13:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 780] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 3. | Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003;2:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 546] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | de Vries EG, de Jong S, Gietema JA. Molecular Imaging As a Tool for Drug Development and Trial Design. J Clin Oncol. 2015;33:2585-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kang JH, Chung JK. Molecular-genetic imaging based on reporter gene expression. J Nucl Med. 2008;49 Suppl 2:164S-179S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1436] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 7. | Minn I, Menezes ME, Sarkar S, Yarlagadda K, Das SK, Emdad L, Sarkar D, Fisher PB, Pomper MG. Molecular-genetic imaging of cancer. Adv Cancer Res. 2014;124:131-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Jacobs A, Voges J, Reszka R, Lercher M, Gossmann A, Kracht L, Kaestle C, Wagner R, Wienhard K, Heiss WD. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Peñuelas I, Mazzolini G, Boán JF, Sangro B, Martí-Climent J, Ruiz M, Ruiz J, Satyamurthy N, Qian C, Barrio JR. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology. 2005;128:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Huang R, Zhao Z, Ma X, Li S, Gong R, Kuang A. Targeting of tumor radioiodine therapy by expression of the sodium iodide symporter under control of the survivin promoter. Cancer Gene Ther. 2011;18:144-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ahn BC, Ronald JA, Kim YI, Katzenberg R, Singh A, Paulmurugan R, Ray S, Hofmann LV, Gambhir SS. Potent, tumor-specific gene expression in an orthotopic hepatoma rat model using a Survivin-targeted, amplifiable adenoviral vector. Gene Ther. 2011;18:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Huyn ST, Burton JB, Sato M, Carey M, Gambhir SS, Wu L. A potent, imaging adenoviral vector driven by the cancer-selective mucin-1 promoter that targets breast cancer metastasis. Clin Cancer Res. 2009;15:3126-3134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Hervé J, Cunha AS, Liu B, Valogne Y, Longuet M, Boisgard R, Brégerie O, Roux J, Guettier C, Calès P. Internal radiotherapy of liver cancer with rat hepatocarcinoma-intestine-pancreas gene as a liver tumor-specific promoter. Hum Gene Ther. 2008;19:915-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595-14600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Jiang ZK, Sato M, Wei LH, Kao C, Wu L. Androgen-independent molecular imaging vectors to detect castration-resistant and metastatic prostate cancer. Cancer Res. 2011;71:6250-6260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Qiao J, Doubrovin M, Sauter BV, Huang Y, Guo ZS, Balatoni J, Akhurst T, Blasberg RG, Tjuvajev JG, Chen SH. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Spitzweg C, Baker CH, Bergert ER, O’Connor MK, Morris JC. Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum Gene Ther. 2007;18:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Bhang HE, Gabrielson KL, Laterra J, Fisher PB, Pomper MG. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nat Med. 2011;17:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Kim S, Youn H, Song MG, Kang JH, Chung HK, Lee DS, Chung JK. Complementary treatment of siTERT for improving the antitumor effect of TERT-specific I-131 therapy. Cancer Gene Ther. 2012;19:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Yu ST, Yang YB, Liang GP, Li C, Chen L, Shi CM, Tang XD, Li CZ, Li L, Wang GZ. An optimized telomerase-specific lentivirus for optical imaging of tumors. Cancer Res. 2010;70:2585-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21367] [Article Influence: 2136.7] [Reference Citation Analysis (3)] |
| 22. | Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 293] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 24. | Kelland LR. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Wogan GN. Impacts of chemicals on liver cancer risk. Semin Cancer Biol. 2000;10:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Williams GM. Chemicals with carcinogenic activity in the rodent liver; mechanistic evaluation of human risk. Cancer Lett. 1997;117:175-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Gonzalez FJ. The peroxisome proliferator-activated receptor alpha (PPARalpha): role in hepatocarcinogenesis. Mol Cell Endocrinol. 2002;193:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Leenders MW, Nijkamp MW, Borel Rinkes IH. Mouse models in liver cancer research: a review of current literature. World J Gastroenterol. 2008;14:6915-6923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Finnberg N, Stenius U, Högberg J. Heterozygous p53-deficient (+/-) mice develop fewer p53-negative preneoplastic focal liver lesions in response to treatment with diethylnitrosamine than do wild-type (+/+) mice. Cancer Lett. 2004;207:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | McGlynn KA, Hunter K, LeVoyer T, Roush J, Wise P, Michielli RA, Shen FM, Evans AA, London WT, Buetow KH. Susceptibility to aflatoxin B1-related primary hepatocellular carcinoma in mice and humans. Cancer Res. 2003;63:4594-4601. [PubMed] |
| 31. | Salguero Palacios R, Roderfeld M, Hemmann S, Rath T, Atanasova S, Tschuschner A, Gressner OA, Weiskirchen R, Graf J, Roeb E. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest. 2008;88:1192-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Farazi PA, Glickman J, Horner J, Depinho RA. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 2006;66:4766-4773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Teoh NC, Dan YY, Swisshelm K, Lehman S, Wright JH, Haque J, Gu Y, Fausto N. Defective DNA strand break repair causes chromosomal instability and accelerates liver carcinogenesis in mice. Hepatology. 2008;47:2078-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Qi Y, Chen X, Chan CY, Li D, Yuan C, Yu F, Lin MC, Yew DT, Kung HF, Lai L. Two-dimensional differential gel electrophoresis/analysis of diethylnitrosamine induced rat hepatocellular carcinoma. Int J Cancer. 2008;122:2682-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Rao KV, Vesselinovitch SD. Age- and sex-associated diethylnitrosamine dealkylation activity of the mouse liver and hepatocarcinogenesis. Cancer Res. 1973;33:1625-1627. [PubMed] |
| 36. | Zender L, Kubicka S. Androgen receptor and hepatocarcinogenesis: what do we learn from HCC mouse models? Gastroenterology. 2008;135:738-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, Jou YS, Chen CW, Yeh S, Chang C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947-955, 955.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Vesselinovitch SD, Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983;43:4253-4259. [PubMed] |
| 39. | Vesselinovitch SD, Koka M, Mihailovich N, Rao KV. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol. 1984;108:60-65. [PubMed] |
| 40. | Vesselinovitch SD. Infant mouse as a sensitive bioassay system for carcinogenicity of N-nitroso compounds. IARC Sci Publ. 1980;645-655. [PubMed] |
| 41. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1488] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 42. | Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 490] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 43. | Bakiri L, Wagner EF. Mouse models for liver cancer. Mol Oncol. 2013;7:206-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 44. | Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Hickman-Davis JM, Davis IC. Transgenic mice. Paediatr Respir Rev. 2006;7:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1973] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 47. | Sell S, Hunt JM, Dunsford HA, Chisari FV. Synergy between hepatitis B virus expression and chemical hepatocarcinogens in transgenic mice. Cancer Res. 1991;51:1278-1285. [PubMed] |
| 48. | Zhu H, Wang Y, Chen J, Cheng G, Xue J. Transgenic mice expressing hepatitis B virus X protein are more susceptible to carcinogen induced hepatocarcinogenesis. Exp Mol Pathol. 2004;76:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 377] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 50. | Tönjes RR, Löhler J, O’Sullivan JF, Kay GF, Schmidt GH, Dalemans W, Pavirani A, Paul D. Autocrine mitogen IgEGF cooperates with c-myc or with the Hcs locus during hepatocarcinogenesis in transgenic mice. Oncogene. 1995;10:765-768. [PubMed] |
| 51. | Cullen JM, Sandgren EP, Brinster RL, Maronpot RR. Histologic characterization of hepatic carcinogenesis in transgenic mice expressing SV40 T-antigens. Vet Pathol. 1993;30:111-118. [PubMed] |
| 52. | Jin YN, Chung HK, Kang JH, Lee YJ, Kimm KI, Kim YJ, Kim S, Chung JK. Radioiodine gene therapy of hepatocellular carcinoma targeted human alpha fetoprotein. Cancer Biother Radiopharm. 2008;23:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Willhauck MJ, Sharif Samani BR, Klutz K, Cengic N, Wolf I, Mohr L, Geissler M, Senekowitsch-Schmidtke R, Göke B, Morris JC. Alpha-fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther. 2008;15:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Klutz K, Willhauck MJ, Wunderlich N, Zach C, Anton M, Senekowitsch-Schmidtke R, Göke B, Spitzweg C. Sodium iodide symporter (NIS)-mediated radionuclide ((131)I, (188)Re) therapy of liver cancer after transcriptionally targeted intratumoral in vivo NIS gene delivery. Hum Gene Ther. 2011;22:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Ma XJ, Huang R, Kuang AR. AFP promoter enhancer increased specific expression of the human sodium iodide symporter (hNIS) for targeted radioiodine therapy of hepatocellular carcinoma. Cancer Invest. 2009;27:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Kim KI, Lee YJ, Lee TS, Song I, Cheon GJ, Lim SM, Chung JK, Kang JH. In vitro radionuclide therapy and in vivo scintigraphic imaging of alpha-fetoprotein-producing hepatocellular carcinoma by targeted sodium iodide symporter gene expression. Nucl Med Mol Imaging. 2013;47:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Park JH, Kim KI, Lee KC, Lee YJ, Lee TS, Chung WS, Lim SM, Kang JH. Assessment of α-fetoprotein targeted HSV1-tk expression in hepatocellular carcinoma with in vivo imaging. Cancer Biother Radiopharm. 2015;30:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Kim KI, Park JH, Lee YJ, Lee TS, Park JJ, Song I, Nahm SS, Cheon GJ, Lim SM, Chung JK. In vivo bioluminescent imaging of α-fetoprotein-producing hepatocellular carcinoma in the diethylnitrosamine-treated mouse using recombinant adenoviral vector. J Gene Med. 2012;14:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Lu X, Guo H, Molter J, Miao H, Gerber L, Hu Y, Barnes EL, Vogel H, Lee Z, Luo G. Alpha-fetoprotein-thymidine kinase-luciferase knockin mice: a novel model for dual modality longitudinal imaging of tumorigenesis in liver. J Hepatol. 2011;55:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Park JH, Kim KI, Lee YJ, Lee TS, Kim KM, Nahm SS, Park YS, Cheon GJ, Lim SM, Kang JH. Non-invasive monitoring of hepatocellular carcinoma in transgenic mouse with bioluminescent imaging. Cancer Lett. 2011;310:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |