Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5568
Peer-review started: February 9, 2016
First decision: April 14, 2016
Revised: May 11, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: June 28, 2016
Processing time: 138 Days and 17 Hours
AIM: To investigate the epidemiology, risk factors and clinical course of acute hepatitis E virus (HEV) infection in Israel, an industrialized country.
METHODS: A retrospective analysis of acute HEV cases diagnosed in Israel from 1993 to 2013. Acute HEV was defined by ALT/AST elevation and a positive HEV PCR test or positive anti-HEV-IgM serology. HEV RNA was tested by quantitative reverse transcription PCR. Antibodies to HEV were tested retrospectively using an ELISA assay. HEV-RNA was sequenced using RT-PCR of ORF1 and ORF2 regions to diagnose genotype of the virus. Epidemiologic and clinical data were collected by reviewing the clinical files and through a telephone interview according to a structured questionnaire.
RESULTS: Acute HEV was diagnosed in 68 patients. Among the 59 patients who gave an informed consent and were interviewed, 41% of infections were autochthonous (acquired in Israel), 44% travel-related and 15% imported by foreign workers. Autochthonous patients were mainly females (62.5%), more than half of them pregnant, 26% recalled consuming food or water in areas with poor sanitation, 44% ate non-kosher meat. Fulminant hepatitis developed in 3 patients (5%), all of them were females, two of them with post-partum infection, all acquired the disease in Israel (autochthonous). Israeli travelers with imported infection were predominantly males (73%), acquired the disease in the Indian subcontinent (81%), with 100% reporting having consumed fresh vegetables and drinks with ice cubes abroad. Six patients’ sera were tested for genotype and revealed HEV genotype 1 (all cases acquired in the Indian subcontinent).
CONCLUSION: This is the first report which highlights the existence of hepatitis E as an autochthonous infection in Israel. Imported HEV originates mostly from the Indian subcontinent.
Core tip: This is the first epidemiologic report on hepatitis E virus (HEV) in Israel. This report demonstrates the significant presence of autochthonous acute HEV in Israel, serving as an example of occurrence in an industrialized country. Suspected risk factors in Israel include consumption of water and food in areas with poor sanitation, exposure to animals and eating a non-Kosher meat. The high risk group for fulminant hepatitis was pregnant women in their final trimester. Additionally, imported HEV, originating mainly from the Indian subcontinent, is also seen in Israel. Awareness of this disease is important both among physicians in Israel as well as those in other industrialized countries.
- Citation: Erez-Granat O, Lachish T, Daudi N, Shouval D, Schwartz E. Hepatitis E in Israel: A nation-wide retrospective study. World J Gastroenterol 2016; 22(24): 5568-5577
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5568.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5568
Hepatitis E virus (HEV) infection is currently one of the leading causes of viral hepatitis worldwide[1]. The first epidemic of HEV infection was recognized in India, by a retrospective epidemiologic and serologic survey performed in India and the United States in the early 1980s. This led to the recognition of HEV as a water-borne associated hepatitis. Similar epidemics were subsequently identified in Central and Southeast Asia, the Middle East and North Africa[2-4]. The viral genome was later cloned and sequenced using samples of bile obtained from experimentally infected macaques, and the virus was named Hepatitis E[5-8].
HEV is a single strand RNA virus classified in the genus Hepevirus, family Hepeviridae[9]. Identification of four HEV genotypes has subsequently been used to study the molecular epidemiology of HEV infection worldwide. Genotypes 1 and 2, restricted to humans, are mostly seen in developing countries causing large waterborne outbreaks of hepatitis. Genotype 1 is mostly associated with outbreaks in Asia and Africa, whereas genotype 2 has been detected in Mexico and some African countries[10]. In recent years there has been increasing evidence of an autochthonous HEV infection in industrialized countries, contrasting with previous reports of HEV as only an imported infection from endemic, developing countries. In these cases, genotypes 3 and 4 have been identified, and found to be responsible for sporadic cases of autochthonous hepatitis E in industrialized countries[11-15]. In contrast to genotypes 1 and 2, genotypes 3 and 4 infect not only humans but also other mammalian species such as pigs, boars, and deer[11].
HEV is transmitted predominantly via the fecal-oral route, causing a self-limiting disease which resolves spontaneously within 4-6 wk[6]. Occasionally, in immune-suppressed patients and in pregnant women, a fulminant form of hepatitis develops[16]. Chronic infection has been identified almost exclusively among immunocompromised persons, including organ-transplant recipients, patients receiving cancer chemotherapy, and HIV-infected persons[17].
Israel is an industrialized country located amid HEV endemic countries and home to immigrants and refugees from African countries (such as Egypt, Sudan and Ethiopia, all endemic for genotype 1 of the virus). Furthermore, since a portion of Israel’s population eats only kosher food (i.e., avoiding pork, game meat or seafood), the index of suspicion for autochthonous cases has been low. Data obtained through old and non-validated immune-assays regarding sero-epidemiology of hepatitis E in Israel revealed a seroprevalence of 2.81% and 1.81% in the Jewish and Arab population, respectively[18]. However, data regarding acute HEV revealed only one case report of acute HEV infection acquired in Israel[19] while the remaining published cases were travel-related[20,21].
The aim of this study was to identify whether there is a change in the epidemiology of acute HEV in Israel, with cases acquired in Israel (autochthonous cases) and to characterize the epidemiology, risk factors and clinical presentation of all documented acute HEV infections in patients diagnosed in Israel.
A descriptive, retrospective, nation-wide study.
The study included all patients diagnosed with acute HEV infection in Israel from October 1993 to 2013 at the laboratory of the Liver Unit at the Hadassah Medical Center in Jerusalem. During the study period, this laboratory was the only reference laboratory in Israel for HEV detection. Epidemiologic and clinical data were collected by reviewing the clinical files and through telephone interviews in accordance with a structured questionnaire. The study was approved by the Sheba-Medical Centers’ institutional review board.
“Definite acute HEV” was defined as acute hepatitis manifested by ALT/AST elevation and a positive HEV PCR (polymerase chain reaction) test or positive anti-HEV-IgM serology. “Probable acute HEV” was defined as acute non-A, non-B, non-C hepatitis with negative HEV PCR and negative anti-HEV-IgM serology but positive for anti-HEV-IgG serology, where serum samples were taken later in the course of the disease, and with a clinical course that fit HEV infection, and no other proven etiology. “Fulminant hepatitis” was defined as a rapid development of acute liver injury with evidence of coagulation abnormality, an international normalized ratio (INR) > 1.5, and any degree of hepatic encephalopathy in a patient without pre-existing liver disease; and after exclusion of the conventional etiologies for acute liver failure[22].
Serologic detection of anti-HEV antibodies: Antibodies to HEV (IgG and IgM) were tested using an ELISA micro-titer plates assay [DS-EIA-anti-HEV-G, DS-EIA-anti-HEV-M, DSI S.R.L. Serronno (VA), Milan, Italy] according to the manufacturer’s instructions. Micro-titer plates were coated with HEV peptides able to detect all four genotypes of HEV. Ten μL serum samples were diluted 1:10 with 90 μL diluent and incubated for 30 min at 37 °C and rinsed three times, followed by a second incubation with 100 μL conjugated antibodies for another 30 min at 37 °C. After rinsing three times, 100 μL substrate was incubated for 30 min at room temperature. Finally, 100 μL stop solution was added followed by reading the plates at a 450 nm wave length. Sample reading > 0.2 OD were considered as positive.
A pangenotypic evaluation by CDC of 6 serologic assays for IgM against HEV identified the assay manufactured by diagnostic systems, which was used in this study, as having the best performance characteristics. Its diagnostic sensitivity and specificity were 98% and 95.2%, respectively[23].
We used an assay for the detection of IgG against HEV from the same manufacturer, with a sensitivity of 100% and specificity of 97.5%[24].
Detection of HEV-RNA by Taqman real time PCR: Detection of HEV RNA was performed by quantitative reverse transcription PCR (qRT-PCR)[25]. RNA was extracted from 200 μL serum with TRI reagent (Bio Lab), then diluted in 10 μL DEPC water. A 10 μL RNA aliquot was used for one step RT-PCR assay in a final volume of 20 μL. The region used for the real time assay is a highly conserved region, junction of ORF’s 2/3 of HEV: HEV Forward primer GGTGGTTTCTGGGGTGAC, HEV Reverse primer AGGGGTTGGTTGGATGAA, HEV Probe FAM-TGATTCTCAGCCCTTCGC-BHQ.
HEV sequence: To define HEV genotype in the sera samples, we ran reverse transcription polymerase chain reaction (RT-PCR) of two regions of the virus: ORF1 and ORF2. PCR products were sent for cleaning and sequencing in a service laboratory (HY-lab). We used the programs CHROMAS and CLUSTAL in order to analyze the sequences.
Quantitative variables are presented as mean ± SD or as medians and range. Qualitative variables are presented as frequencies and ratios (percent). The χ2 and the Fisher’s exact tests were applied to assess associations between two qualitative variables. The comparison of quantitative variables between two independent groups was carried out using the two-sample t-test or the non-parametric Mann-Whitney test. All tests applied were two-tailed, and a P-value of 5% or less was considered statistically significant.
During the years 1993 to 2013, 651 patients with presumed acute non-A, non-B, non-C hepatitis were tested for HEV in Israel. Acute HEV was diagnosed in 68/651 patients (10.45%). Among them, 61 patients (90%) were classified as having “definite acute HEV” confirmed by a positive HEV-RNA PCR result (n = 50) or positive anti-HEV-IgM serology (n = 10). One patient had a positive PCR result from a stool sample taken abroad. Probable acute HEV was diagnosed in 7 patients.
Altogether the cohort of acute HEV infection included 68 patients, 58.8% male, with a mean age of 39.4 years. The greatest number of patients were between the ages of 17-40 years (63.5%). Comparing acute HEV positive patients with non-A-non-B-non-C-non-E acute hepatitis patients revealed no significant differences in gender or age distribution (Table 1).
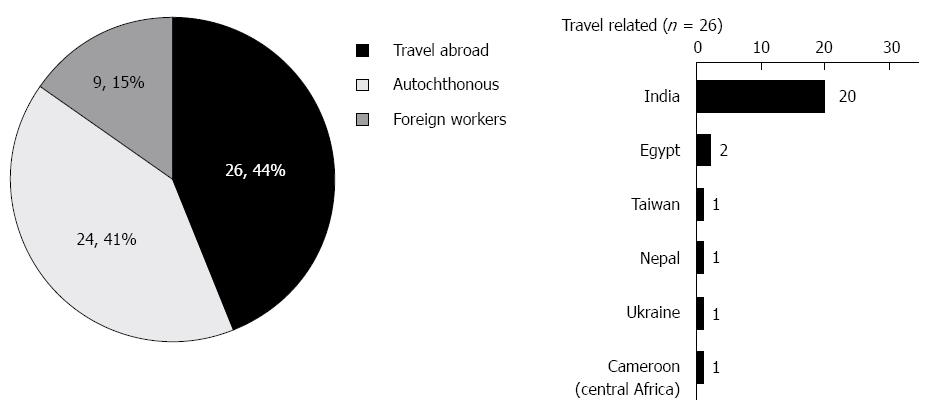
Among this cohort of 68 patients with a history of acute HEV, 59 patients gave an informed consent and were further evaluated. Thirty five patients (35/59, 59%) had “imported” HEV; among them 26 cases (26/59, 44%) were travel-related HEV infections in Israeli patients and 9 cases (9/59, 15%) were diagnosed in foreign workers from HEV endemic countries (Figure 1). The majority (80%) of travel-related HEV cases were acquired in the Indian subcontinent. Finally, 41% of the patients (24/59) did not travel abroad and had no contact with people from endemic areas, and are therefore defined as “autochthonous HEV”.
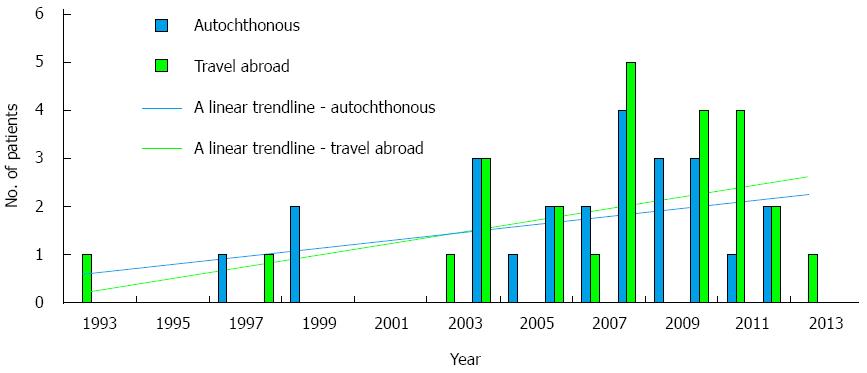
There was a trend of an increasing number of cases diagnosed with acute hepatitis E throughout the study years in both the travel-related and autochthonous groups (Figure 2).
This group consisted of 24 patients, predominantly female (15/24, 62.5%), with a mean age of 42 years old (SD-15, range: 15-69 years old) and without any contact with a foreign worker in Israel. There were, however, 26% (5/19) who recalled consuming food or water from rural settlements and areas of low sanitation (the West Bank, Bedouin villages) during the 6 wk before the onset of symptoms. Other probable risk factors for HEV infection are summarized in Table 2; 44%, (8/18) ate non-kosher meat (14% ate raw meat, 10% consumed sea food); 40% (8/20) reported contact with animals (cats, dogs, chicken, parrots, geese, fish, guinea pigs, horses or a monkey). Five out of the 24 with autochthonous infections (21%) had chronic liver disease before acquiring HEV (chronic HCV, HBsAg carrier, cystic fibrosis of liver or autoimmune hepatitis). Four of them were diagnosed by positive molecular test (PCR), and one by positive anti-IgM serology for HEV. Eight percent (2/24) received immune suppressing medications (Corticosteroids, Azathioprine, Mycophenolate Moftil and Tacrolimus). Among the female patients, 53% (8/15) were pregnant or post-partum at the time of clinical presentation.
| Character | Autochthonous infection (n = 24) | Travel related (n = 26) | P value2 | |
| Demography | Gender: M | 9 (37.5) | 19 (73.1) | 0.011 |
| Age: mean | 41.58 | 37.38 | 0.358 | |
| Range | 15-69 | 20-74 | ||
| Potential risk factors: food related | Eating non-kosher meat | 8 (44.4) | 22 (81.8) | 0.014 |
| Eating raw meat | 3 (14.3) | 3 (13.0) | 1.000 | |
| Eating sea-food | 2 (10.0) | 6 (28.6) | 0.238 | |
| Consuming food/water from areas with poor sanitation | 5 (26.3) | 2 (12.5) | 0.415 | |
| Contact with animals3 | 8 (40.0) | 8 (34.8) | 0.724 | |
| Potential risk factors: others | Pregnancy | 8 (53.3) | 1 (14.3) | 0.165 |
| Immunosuppression4 | 2 (8.3) | 0 (0.0) | 0.225 | |
| Chronic liver disease | 5 (20.8) | 1 (3.8) | 0.064 | |
| Clinical data | Time from onset of symptoms to diagnosis (d) | 59.13 (n = 15) | 25.21 (n = 19) | 0.009 |
| Duration of symptoms (average weeks) | 5.94 (n = 18) | 4.08 (n = 20) | 0.149 | |
| Hospitalization (percent of patients) | 68.2% (15/22) | 20 (80.0) | 0.345 | |
| Duration of hospitalization (d) | 22.15 (n = 13) | 11.11 (n = 19) | 0.195 | |
| Laboratory tests (average) | Bilirubin mg/dL (STD) | 10.95 (10.84) | 9.24 (5.93) | 0.813 |
| GPT (ALT) U/L (STD) | 1169.3 (1279.4) | 2446.4 (1604.3) | 0.043 | |
| GOT U/L(STD) | 1311.7 (2114.6) | 1540.4 (1412.7) | 0.436 | |
| ALKP (STD) | 566.5 (986.1) | 205.6 (54.2) | 0.673 | |
| GGT U/L (STD) | 470.0 (625.1) | 232.2 (243.3) | 0.730 | |
| LDH U/L (STD) | 2613 (6400.5) | 1503 (1511.4) | 0.440 | |
| ALB g/dL (STD) | 3.3 (0.94) | 3.9 (0.42) | 0.241 | |
| INR (STD) | 1.42 (0.8) | 1.21 (0.2) | 0.791 | |
| Outcome | Self-limited | 20 (86.9) | 26 (100) | 0.085 |
| Fulminant hepatitis | 3 (13) | 0 (0) | ||
| Chronic hepatitis | 0 (0) | 0 (0) | ||
Fifteen of the patients with autochthonous HEV infection (15/24, 62.5%) were diagnosed by detection of HEV-RNA in their serum. Unfortunately, we were unable to retrospectively test for the genotype in this group of autochthonous patients due to a breakdown in refrigeration.
This group consisted 26 patients, predominantly male (19/26, 73%), with a mean age of 37.38 years, SD-16.7. Among the females, 1 out of 7 was pregnant (14.3%). Sixty four percent (16/25) developed symptoms after returning to Israel, with a mean time elapsing before symptoms of 16 d (range: 2-28 d). Duration of travel was on average 62.5 d (range: 3-240 d). Thirty six percent (9/25) were symptomatic before flying back to Israel, and among those patients, the average duration of travel was 6.5 mo (range: 1-24 mo).
Behavior during travel, possibly contributing to risk of infection is summarized in Table 3. As described, the vast majority had contact with suspected contaminated water and raw vegetables. The entire group consisted of healthy travelers, none took immunosuppressive drugs or had a known systemic disease associated with immunosuppression, with the exception of one patient who had chronic hepatitis B, diagnosed in the past. Foreign workers from endemic countries (n = 9) were not included in the travel-related HEV group.
| Risk factor | No. of patients (total: n = 26) | Incidence |
| Gender - M | 19/26 | 73.07% |
| Pregnancy | 1/7 | 14.28% |
| Chronic liver disease1 | 1/23 | 4.30% |
| Immunosuppression | 0/23 | 0.00% |
| Eating non-kosher meat | 18/22 | 81.81% |
| Eating raw meat | 3/23 | 13.04% |
| Eating sea-food | 6/21 | 28.57% |
| Drinking tap water abroad | 8/23 | 34.78% |
| Consuming drinks with ice cubes | 23/23 | 100.00% |
| Brushing teeth with tap water | 20/23 | 86.95% |
| Eating fresh vegetables abroad | 23/23 | 100.00% |
| Bath in fresh water | 15/23 | 65.21% |
| Contact with animals2 | 8/23 | 34.78% |
| Contact with travelers having similar symptoms | 2/25 | 8.00% |
Twenty of the patients with travel-related HEV (20/26, 77%) were diagnosed by detection of HEV-RNA in their serum. Among them, we were able to sequence the HEV genome in six of the patients with available sera. All cases revealed genotype 1.
Comparing patients with travel-related infection and autochthonous infection revealed that the autochthonous patients were predominantly female (62.5% vs 27%, P < 0.05), had lower levels of alanine aminotransferase on presentation (mean 1169 U/L vs 2446 U/L, P < 0.05) and the time from onset of symptoms to diagnosis was more than twice as long (59 d vs 25 d; P < 0.01) (Table 2). Although there was no significant statistical difference in outcome between the two groups (due to small sample size), the results may indicate that patients with autochthonous HEV are more prone to fulminant hepatitis than patients with travel-related HEV infection (13% vs 0% respectively). There were no significant differences in age, hospitalization rate and symptom duration between the groups.
In this study, there were nine cases of HEV in pregnant women (9/28 women in the study, 32%). Only one of the women was a returning traveler from India, while the rest were diagnosed with autochthonous infection (8/9, 88.9%). Fulminant hepatitis occurred in two of the pregnant women (2/9, 22%), without fatality or vertical transmission. A detailed description of this cohort, together with other cases of acute HEV in pregnant women from Western countries, was published recently[26].
In the entire cohort of the Israeli patients, the most common signs and symptoms were fatigue and non-obstructive-jaundice in 84% (26/31) and 78% (40/51) of patients respectively; 61% (11/18) of patients developed abdominal pain and 41% (19/46) had fever (> 37.5 °C). Other complaints were nausea (40%, 19/48), diarrhea (21%, 10/48) and headache (24%, 11/45). Nine percent (4/43), developed neurologic abnormalities including epilepsy, encephalopathy or loss of consciousness. Admission to hospital in Israel was reported by 78% of patients, for a mean duration of 15.6 d (range: 1-84 d, SD-19.4). One Israeli traveler was admitted to an Indian hospital while abroad where HEV-RNA was identified in a stool sample by PCR.
Results of conventional liver tests and INR at presentation or later were retrieved in only 22 patients (Table 2). The rate of bilirubin level varied between 0.39 to 27.9 mg/dL. No clinically significant bleeding disorders were reported although coagulopathy was recorded in 41% of the patients (7/17), who presented with prolonged INR up to 3.49. Hepatocellular injury manifested in ALT elevation was significantly higher in patients with travel-related hepatitis compared to autochthonous infection (mean 2246.4 U/dL vs 1169.3 U/dL respectively, P < 0.05).
Ninety four percent (46/49) of the patients with HEV infection had self-limiting disease (Table 2). Among the travelers, none of the patients had a chronic or fulminant course of infection. This included a pregnant woman with HEV infection in her third trimester, who had a self-limiting infection, without any pregnancy or fetal related complications. In contrast, among the patients with autochthonous infection, three patients (3/23, 13%) developed fulminant hepatitis. All three were females in their reproductive years, two of them post-partum and the third had cystic fibrosis.
The current study was designed to review retrospectively the epidemiology and clinical outcome of acute HEV cases diagnosed in Israel in the past 20 years. During this period, 68 patients were diagnosed with acute HEV infection, and from those patients, 59 gave their consent for evaluation. In this cohort, 44% were travel related, 15% affected foreign workers and surprisingly 41% were autochthonous infection. This is the first study which recognizes the existence of a relatively large cohort of autochthonous HEV infections in Israel, while previous reports suggest that almost all cases of hepatitis E were travel related as summarized in Table 4.
| Ref. | Year of follow-up | No. of patients | Travel related/autochthonous | Diagnosis |
| Schwartz et al[20] | 1992-1998 | 5 | Travel related (all cases acquired in the Indian subcontinent) | Serology tests (Abbott Laboratories, Abbott Park, IL, United States) |
| Lachish et al[21] | 1997-2012 | 19 | Travel related (84% acquired in the Indian subcontinent) | Molecular or Serology (IgM/IgG) tests (EIA, Abbott Laboratories, Abbott Park, IL, United States) |
| Mechnik et al[19] | 2001 | 1 | Autochthonous | Molecular test (HEV-RNA pos. in serum sample) |
Recently, reports on autochthonous HE in industrialized countries have become more frequent. The distribution of autochthonous vs travel-related cases varies widely between different countries[13,14,27-30], as can be seen in Table 5. In Israel about half of the cases are autochthonous.
As shown in this report (Figure 2), the number of HEV cases increased during the study years in both travelers and the autochthonous groups. This may reflect an increase in infection rates, but may also be the consequence of improved diagnostic tools and growing awareness among physicians in Israel of HEV infection.
Previous studies in industrialized countries linked sporadic cases of HEV to consumption of undercooked pork[1,12,31], raw game meat, shellfish[32] and to blood transfusions[33,34]. In autochthonous HEV in Israel, the most frequent risk factors included eating non-kosher meat and being exposed to animals. Furthermore, about a quarter of the patients reported that during the 6 wk that preceded the symptoms, they consumed food and/or water from the West Bank or other rural areas in Israel known to have relatively lower hygienic standards as compared to the rest of the country.
The other major risk group susceptible to HEV was the Israeli travelers (44% of acute HEV in Israel). In a prospective observational study from Israel, 1% of ill-returning Israeli travelers were diagnosed with acute hepatitis (1997-2012), while HEV has become the most common hepatitis, with a prevalence of 39%, of all hepatitis cases. In the aforementioned study, 84% of the travel-related HEV cases were “imported” from the Indian subcontinent[21]. In this study, 80.7% of travel associated cases were acquired in the Indian subcontinent. In travel-related HEV, in contrast to autochthonous HEV, the majority of patients were young adult males (73% male, average age of 37 years). Only a minority of patients had chronic liver disease (3.8%). Regarding risk behaviors during travel in this population, although the majority of patients followed pre-travel instructions and did not drink tap water abroad, all patients ate raw vegetables, used ice cubes, brushed their teeth with tap water and bathed in fresh water (Table 3). A control group of travelers without HEV was not available for this study.
The comparison of characteristics of autochthonous and travel-related acute HEV infection, revealed significant differences between the two groups (Table 2); the majority of infected travelers were male, while in the autochthonous group, the majority were female. Time to diagnose was significantly longer in the autochthonous group (on average, more than twice longer), suggesting a delay in diagnosis. ALT levels were significantly higher in infected travelers than in patients with autochthonous infection.
HEV is generally a self-limiting disease, although on rare occasions it can be fulminant[6,16]. In the present study, most of the patients with acute HEV infection had a self-limited disease. Patients who had fulminant hepatitis reported one of the following risk factors: pregnancy (including post-partum women), immune-suppression or chronic liver disease.
There is scarce evidence about the mortality of pregnant women with acute HEV infection in industrialized countries[35-38]. Our previous report indicated that in an industrialized country such as Israel, pregnant women had a high risk of fulminant hepatitis during their final trimester (2/9, 22.2%), though with no mortality or vertical transmission[26].
Patients with underlying chronic liver disease who develop hepatitis E have a poor prognosis, as they frequently develop acute or sub-acute liver failure[17]. In a study of a large cohort of patients in India with chronic liver disease, patients who developed HEV associated liver failure had a significantly worse prognosis than patients who decompensated due to other causes. In this cited cohort, the 12-mo mortality with HEV infection reached 70%[39]. In industrialized countries, smaller studies have also shown a poor prognosis for HEV infected patients with underlying chronic liver disease[40,41]. In our current study, evaluation of the entire group of 69 patients with acute HEV infection, revealed 7 patients with underlying chronic liver disease (chronic HCV, chronic HBV, cystic fibrotic liver, autoimmune hepatitis, idiopathic cirrhosis). Six of the patients survived and were interviewed and only one patient with chronic HBV infection and cirrhosis died 4 years after the diagnosis of chronic hepatitis B and 3 years after the diagnosis of acute HEV infection.
The differential diagnosis of autochthonous HEV infection includes drug induced liver injury (DILI). Two reports from the United Kingdom and the United States revealed that 21.4% and 3%, respectively, of the patients with an initial diagnosis of DILI, had autochthonous HEV. Such misdiagnosis is particularly common in elderly populations with autochthonous HEV taking various potentially hepato-toxic medications and herbs[42,43]. In the present cohort, two patients treated with Isoniazide and Ketoconazole who presented with acute hepatitis, were initially misdiagnosed as DILI and subsequently were found positive for HEV RNA by PCR.
In the industrialized world, HEV genotypes 3 and 4 are responsible for sporadic cases of autochthonous HEV infection, where the disease is zoonotic. Imported, travel related, cases are usually genotypes 1 and 2. In this study, genotyping of 6 travel-related cases with available serum revealed genotype 1 in all cases, a genotype which is prevalent in India where all cases were acquired. One case of hepatitis E in a foreign worker from Nepal who spent one month in Israel, revealed genotype 1 as well.
Israel is located amid countries endemic for HEV, with evidence for genotype 1 in most of the cases reported from those countries. For example, isolation of HEV circulating in Egypt, a country with HEV seroprevalence among the highest in the world, revealed HEV genotype 1 in acute HEV cases, both from rural and urban areas[44-46]. Studies of North African countries revealed autochthonous infection with genotype 1 as well[2]. Data on the HEV-genotype circulating in Lebanon, Syria, and Jordan is lacking.
Unfortunately, we were unable to retrospectively test for the genotype in the autochthonous group of patients. The cause included the retrospective design of this study which did not enable storage of large volumes of suspected sera and the physical state of some of the stored sera which were thawed and frozen several times prior to an attempt of sequencing.
However, a recent Israeli study confirmed the presence of HEV in 8.2% of the 169 sewage samples tested throughout the country during 2013-2015[47]. Sequencing revealed genotype 3 in all sewage samples positive for HEV. These data along with the evidence for acute autochthonous HEV in Israel shown in the present study, suggest autochthonous infection with HEV genotype 3, as seen in Europe and in contrast to the circulating HEV genotype in Israel bordering countries.
The present study has several limitations. The information of exposure related to different risk factors, was based on patients’ recollections of events that happened years before the survey, and consequently might be biased. And as mentioned, we were unable to retrospectively test for the HEV genotypes in the autochthonous group of patients. Thus phylogenetic analysis of HEV confirmed autochthonous cases in Israel remains an undertaking for the future.
This report, along with previous reports from Western countries mentioned above, provides evidence for the presence of sporadic autochthonous or travel-related acute HEV cases in the industrialized world. At present, treatment of active HEV infection with viremia with an effective anti-viral agent remains an elusive goal[48].
To date, two types of recombinant HEV vaccine have been developed[49-51], but neither of them is commercially available in the Western countries. Additionally both vaccines are genotype 1-based, and although they would be very useful in pregnant women and travelers to endemic regions, their efficacy in preventing HEV infection in non-endemic areas (where other genotypes predominate) needs to be investigated.
Hepatitis E virus (HEV), one of the leading causes of viral hepatitis worldwide, is transmitted predominantly via the fecal-oral route and responsible for epidemics of acute hepatitis in developing countries. However studies have shown that HEV is an emerging infection in industrialized countries as well, with increasing evidence of autochthonous cases (contracted locally) in addition to imported infection among immigrants and travelers from endemic countries. Israel is an industrialized country located amid endemic countries and absorbs immigrants from African countries, however a significant portion of Israel’s population eats only kosher food (i.e., do not eat pork, game meat or seafood), therefore the existence of autochthonous cases is not obvious.
This study is a nation-wide epidemiological study of acute HEV cases diagnosed in Israel between the years 1993-2013. The aim of this manuscript was to identify whether there is any evidence of acute autochthonous HEV in Israel and to characterize the epidemiology, risk factors and clinical presentation of all documented acute HEV infection in Israel.
This report demonstrates, for the first time, the significant presence of autochthonous acute HEV in Israel (41% of all cases were autochthonous). Additionally, the imported HEV cases were from travelers and foreign workers acquiring the disease mainly in the Indian subcontinent.
The presence of autochthonous cases in Israel highlights the need for medical practitioners to be acquainted with the disease, even in non-endemic countries, and accurate diagnostic means should be easily accessible.
“Autochthonous infection” is an infection contracted in the area where reported. Autochthonous HEV infection in Israel relates to patients with acute hepatitis E, with no history of travel during the last six months before disease onset, and who are not immigrants. In contrast, imported infection is one contracted in a country endemic for HEV, and occurs among travelers and immigrants.
The manuscript represents an interesting study of the etiology of acute hepatitis HEV in Israel reporting well documented data of interest to the scientific community.
P- Reviewer: Rodriguez-Frias F, Shenoy SM S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Ma S
| 1. | Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 739] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 2. | Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-824. [PubMed] |
| 4. | Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet. 1980;2:876-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 259] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23-31. [PubMed] |
| 6. | Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, Bradley DW. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 570] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 808] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Sarin SK, Kumar M. Hepatitis E. Boyer TD, Manns MP, Sanyal AJ. Zakim and Boyer’s Hepatology: a Textbook of liver disease. 6th ed. Chapter 33 2012; 605-628. |
| 10. | Mirazo S, Ramos N, Mainardi V, Gerona S, Arbiza J. Transmission, diagnosis, and management of hepatitis E: an update. Hepat Med. 2014;6:45-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 482] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Wichmann O, Schimanski S, Koch J, Kohler M, Rothe C, Plentz A, Jilg W, Stark K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Chalupa P, Vasickova P, Pavlik I, Holub M. Endemic hepatitis E in the Czech Republic. Clin Infect Dis. 2014;58:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Romanò L, Paladini S, Tagliacarne C, Canuti M, Bianchi S, Zanetti AR. Hepatitis E in Italy: a long-term prospective study. J Hepatol. 2011;54:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Dalton HR, Kamar N, Izopet J. Hepatitis E in developed countries: current status and future perspectives. Future Microbiol. 2014;9:1361-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Teo CG. Fatal outbreaks of jaundice in pregnancy and the epidemic history of hepatitis E. Epidemiol Infect. 2012;140:767-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 459] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 18. | Karetnyi YV, Favorov MO, Khudyakova NS, Weiss P, Bar-Shani S, Handsher R, Aboudy Y, Varsano N, Schwartz E, Levin E. Serological evidence for hepatitis E virus infection in Israel. J Med Virol. 1995;45:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Mechnik L, Bergman N, Attali M, Beergabel M, Mosenkis B, Sokolowski N, Malnick S. Acute hepatitis E virus infection presenting as a prolonged cholestatic jaundice. J Clin Gastroenterol. 2011;33:421-422. [PubMed] |
| 20. | Schwartz E, Jenks NP, Van Damme P, Galun E. Hepatitis E virus infection in travelers. Clin Infect Dis. 1999;29:1312-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Lachish T, Tandlich M, Schwartz E. Acute hepatitis in israeli travelers. J Travel Med. 2013;20:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The management of Acute Liver Failure: Update 2011. Hepatology. 2011;55:965-967. |
| 23. | Drobeniuc J, Meng J, Reuter G, Greene-Montfort T, Khudyakova N, Dimitrova Z, Kamili S, Teo CG. Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: pangenotypic evaluation of performances. Clin Infect Dis. 2010;51:e24-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Ditah I, Ditah F, Devaki P, Ditah C, Kamath PS, Charlton M. Current epidemiology of hepatitis E virus infection in the United States: low seroprevalence in the National Health and Nutrition Evaluation Survey. Hepatology. 2014;60:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 659] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 26. | Lachish T, Erez O, Daudi N, Shouval D, Schwartz E. Acute hepatitis E virus in pregnant women in Israel and in other industrialized countries. J Clin Virol. 2015;73:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Norder H, Sundqvist L, Magnusson L, Østergaard Breum S, Löfdahl M, Larsen LE, Hjulsager CK, Magnius L, Böttiger BE, Widén F. Endemic hepatitis E in two Nordic countries. Euro Surveill. 2009;14:pii 19211. [PubMed] |
| 28. | Ramalingam S, Smith D, Wellington L, Vanek J, Simmonds P, MacGilchrist A, Bathgate A, Simpson K, Johannessen I. Autochthonous hepatitis E in Scotland. J Clin Virol. 2013;58:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Drobeniuc J, Greene-Montfort T, Le NT, Mixson-Hayden TR, Ganova-Raeva L, Dong C, Novak RT, Sharapov UM, Tohme RA, Teshale E. Laboratory-based surveillance for hepatitis E virus infection, United States, 2005-2012. Emerg Infect Dis. 2013;19:218-222; quiz 353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Mansuy JM, Abravanel F, Miedouge M, Mengelle C, Merviel C, Dubois M, Kamar N, Rostaing L, Alric L, Moreau J. Acute hepatitis E in south-west France over a 5-year period. J Clin Virol. 2009;44:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 504] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 32. | Said B, Ijaz S, Kafatos G, Booth L, Thomas HL, Walsh A, Ramsay M, Morgan D. Hepatitis E outbreak on cruise ship. Emerg Infect Dis. 2009;15:1738-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, Teo CG. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Matsubayashi K, Kang JH, Sakata H, Takahashi K, Shindo M, Kato M, Sato S, Kato T, Nishimori H, Tsuji K. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 182] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Anty R, Ollier L, Péron JM, Nicand E, Cannavo I, Bongain A, Giordanengo V, Tran A. First case report of an acute genotype 3 hepatitis E infected pregnant woman living in South-Eastern France. J Clin Virol. 2012;54:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Andersson MI, Hughes J, Gordon FH, Ijaz S, Donati M. Of pigs and pregnancy. Lancet. 2008;372:1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Thoden J, Venhoff N, Miehle N, Klar M, Huzly D, Panther E, Jilg N, Kunze M, Warnatz K. Hepatitis E and jaundice in an HIV-positive pregnant woman. AIDS. 2008;22:909-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, Kumar Panda S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387-394. [PubMed] |
| 40. | Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. Locally acquired hepatitis E in chronic liver disease. Lancet. 2007;369:1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Péron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, Dupuis E, Izopet J, Vinel JP. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Dalton HR, Fellows HJ, Stableforth W, Joseph M, Thurairajah PH, Warshow U, Hazeldine S, Remnarace R, Ijaz S, Hussaini SH. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665-1672.e1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 44. | Blackard JT, Rouster SD, Nady S, Galal G, Marzuuk N, Rafaat MM, Daef E, El Din SS, Purcell RH, Emerson SU. Genotypic characterization of symptomatic hepatitis E virus (HEV) infections in Egypt. J Clin Virol. 2009;46:140-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Tsarev SA, Binn LN, Gomatos PJ, Arthur RR, Monier MK, van Cuyck-Gandre H, Longer CF, Innis BL. Phylogenetic analysis of hepatitis E virus isolates from Egypt. J Med Virol. 1999;57:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Delarocque-Astagneau E, Abravanel F, Moshen A, Le Fouler L, Gad RR, El-Daly M, Ibrahim EM, El-Aidy S, Lashin T, El-Hoseiny M. Epidemiological and virological characteristics of symptomatic acute hepatitis E in Greater Cairo, Egypt. Clin Microbiol Infect. 2012;18:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Ram D, Manor Y, Gozlan Y, Schwartz E, Ben-Ari Z, Mendelson E, Mor O. Hepatitis E virus genotype 3 in sewage and genotype 1 in sporadic acute hepatitis cases in Israel. Am J Trop Med Hyg. 2016;61:331-335. |
| 48. | Hepatitis E vaccine: why wait? Lancet. 2010;376:845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Haffar S, Bazerbachi F, Lake JR. Making the case for the development of a vaccination against hepatitis E virus. Liver Int. 2015;35:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Shrestha MP, Scott RM, Joshi DM, Mammen MP, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 51. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 551] [Article Influence: 36.7] [Reference Citation Analysis (1)] |