Published online Jun 28, 2016. doi: 10.3748/wjg.v22.i24.5512
Peer-review started: February 17, 2016
First decision: March 31, 2016
Revised: April 11, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 28, 2016
Processing time: 126 Days and 18.1 Hours
AIM: To explore the expression of transient receptor potential vanilloid 4 (TRPV4) and its physiological meaning in mouse and rat gastric epithelia.
METHODS: RT-PCR and immunochemistry were used to detect TRPV4 mRNA and protein expression in mouse stomach and a rat normal gastric epithelial cell line (RGE1-01), while Ca2+-imaging and electrophysiology were used to evaluate TRPV4 channel activity. ATP release was measured by a luciferin-luciferase assay. Gastric emptying was also compared between WT and TRPV4 knockout mice.
RESULTS: TRPV4 mRNA and protein were detected in mouse tissues and RGE1-01 cells. A TRPV4-specific agonist (GSK1016790A) increased intracellular Ca2+ concentrations and/or evoked TRPV4-like current activities in WT mouse gastric epithelial cells and RGE1-01 cells, but not TRPV4KO cells. GSK1016790A or mechanical stimuli induced ATP release from RGE1-01 cells while TRPV4 knockout mice displayed delayed gastric emptying in vivo.
CONCLUSION: TRPV4 is expressed in mouse and rat gastric epithelium and contributes to ATP release and gastric emptying.
Core tip: A mechano-sensitive ion channel, transient receptor potential vanilloid 4 (TRPV4), is expressed in gastric epithelium and contributes to ATP release and gastric emptying. These findings suggest that gastric distension stimulates TRPV4 on gastric epithelium and released ATP stimulates sub-epithelial nerve fibers or acts on visceral smooth muscles. TRPV4 might be a promising novel diagnostic and therapeutic target for functional gastric disorders.
- Citation: Mihara H, Suzuki N, Boudaka AA, Muhammad JS, Tominaga M, Tabuchi Y, Sugiyama T. Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia. World J Gastroenterol 2016; 22(24): 5512-5519
- URL: https://www.wjgnet.com/1007-9327/full/v22/i24/5512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i24.5512
The transient receptor potential vanilloid 4 channel (TRPV4) is a non-selective cation channel that is involved in various cellular functions[1] and is activated by several physical and chemical stimuli, including mechanical stimuli, endogenous arachidonic acid metabolites (epoxyeicosatrienoic acids)[2], and heat. TRPV4 is also activated by the specific agonist GSK1016790A that elicits whole-cell currents in mouse and rat TRPV4-expressing cells with EC50 values of 18.5 and 10 nmol/L, respectively[3]. TRPV4 is widely expressed throughout the body, including gastrointestinal tract epithelium and the esophagus[4]. Although the physiological function of TRPV4 expression in intestinal epithelial cells is unknown, TRPV4 activation in these cells causes increases in intracellular calcium concentrations, chemokine release, and incidence of colitis[5], as well as increased paracellular permeability[6]. Furthermore, TRPV4 antagonists are promising therapeutic options for colitis[7,8]. However, TRPV4 expression in the gastric epithelium awaits evaluation.
In addition to its function as an intracellular energy donor, ATP is recognized as an important signaling molecule that mediates diverse biological effects via cell surface receptors: the purinergic receptors[9]. ATP is released by neurons of the central, peripheral, and enteric nervous system[10,11], and acts as a non-adrenergic non-cholinergic (NANC) neurotransmitter that causes different responses or effects (either excitatory or inhibitory depending on the P2 receptor subtype upon which they act as well as the animal species under study). Several studies showed that purinergic neurotransmission (assuming that gut neurons are the sole source of released ATP) affects gastric motility[12]. Recent reports showed that ATP is also released from non-neuronal tissues and has an effect on tissue function. Moreover, we found that ATP release in the esophagus and urothelium was mediated by TRPV4 stimulation[4,13,14]. However, there are no data concerning whether TRPV4 is expressed in the stomach and, if so, whether TRPV4 stimulation plays a role in mediating ATP release. Therefore, this study explored the morphological (RT-PCR and immunostaining) and functional (Ca2+-imaging, patch clamp and gastric emptying) expression of TRPV4 in mouse and rat stomach with special focus on gastric epithelium.
Eight week-old male C57BL/6NCr (SLC) and TRPV4-knockout (TRPV4KO) mice[15] weighing between 23 and 25 g were housed in a controlled environment (12-h light/12-h dark cycle; room temperature, 22-24 °C; 50%-60% relative humidity) with free access to food and water. All procedures involving the care and use of animals were approved by The Institutional Animal Care and Use Committee of the National Institutes of Natural Sciences.
RGE1-01 is an immortalized rat gastric mucosal cell line that shows distinct cell differentiation types and preserves some epithelial cell characteristics. RGE1-01 cells were maintained at 34 °C in Dulbecco’s modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/mL streptomycin and 100 U/mL penicillin with the addition of ITES (see reference[16] for details).
WT and TRPV4KO mice were sacrificed by cervical dislocation. The stomachs were washed in cold (4 °C) PBS (-) and then incubated in trypsin solution (Invitrogen) at 4 °C for 1 h. Gastric epithelial cells were harvested and plated on CELL-TAK (BD Biosciences)-coated glass cover slips and used for Ca2+-imaging and patch clamp experiments.
RT-PCR was performed as previously described[4,17]. Total RNA (1 μg) was isolated using the RNeasy Mini Kit (Qiagen, Courtaboeuf, France) and measured with a NanoDrop device (Thermo Fisher Scientific Inc., Wilmington, United States). Genomic DNA was eliminated in the process of reverse transcription (QuantiTect Reverse Transcription Kit, QIAGEN). PCR was performed using rTaq DNA polymerase (TaKaRa) in an iCycler (Bio-Rad) with specific primer sets (Table 1).
| Primer name | Sequence (5'→3') |
| mTRPV4-F | ACAACACCCGAGAGAACACC |
| mTRPV4-R | CCCAAACTTACGCCACTTGT |
| mGAPDH-F | TGAAGGGTGGAGCCAAAAGG |
| mGAPDH-R | GGAAGAGTGGGAGTTGCTGTTG |
| rTRPV4-F | CCTGGCAGGGATCGAGGCCT |
| rTRPV4-R | GGATGGTGGTGGCCCACTGC |
| rGAPDH-F | GCCAAGGCTGTGGGCAAGGT |
| rGAPDH-R | GAGCAATGCCAGCCCCAGCA |
Immunochemistry was performed as previously described[4] using the antibodies summarized in Table 2. For section preparation, mouse stomachs were fixed at 4 °C for 6 h. Tissues were placed in PBS-sucrose and embedded in OCT compound (Tissue Tek, Elkhart, IN, United States). Non-specific antibody binding was reduced by incubation in BlockAce (Yukijirushi, Sapporo, Japan) for 1 h at room temperature prior to antibody exposure. Preparations were analyzed using a confocal laser scanning microscope (LSM 700, Carl Zeiss). For immunocytochemistry, RGE1-01 cells were fixed at 4 °C for 20 min with the same fixative. Bovine serum albumin (3% BSA; Sigma) was used as a blocking solution.
| Tissue antigen/host | Dilution | Source |
| TRPV4/rabbit | 1:500 | B. Nilius, or Abcam |
| Goat anti-rabbit IgG-Alexa488 | 1:1500 | Invitrogen, Inc. |
Fura-2 fluorescence was measured in primary mouse gastric epithelial cells and RGE1-01 cells with a standard bath solution containing 140 mmol/L NaCl, 5 mmol/L KCl, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 10 mmol/L HEPES, and 10 mmol/L glucose at pH 7.4 (adjusted with NaOH) at 25 °C. Results are presented as ratios of fluorescence intensities obtained with fura-2 emissions at 340 nm and 380 nm. GSK1016790A[3] and ionomycin (both from Sigma) were used as a TRPV4 agonist and a positive control, respectively. F340/F380 was calculated and acquired with an image processing system (IP-Lab, Scanalytics Inc., Rockville, MD or AQUA COSMOS, Hamamatsu Co., Japan) and ImageJ software (http://rsb.info.nih.gov/ij/). Changes in ratio (Δ) were calculated by subtracting the mean basal values from peak values. Since the degree of responses (strong, weak, or no response) to GSK varied from cell to cell in WT gastric epithelial cells, we expressed the observed changes in all ionomycin-responsive cells as a ratio between WT and TRPV4 knockout mice (Δ). We evaluated 53 and 41 ionomycin-responsive cells from six WT mice and five TRPV4 knockout mice, respectively. Given the variations in response times, and that some WT cells responded 30 s after GSK application, we decided to incubate cells with GSK for 90 s.
The standard bath solution was the same as that used in the Ca2+-imaging experiments. Pipette solutions for whole-cell recordings contained 140 mmol/L KCl, 5 mmol/L EGTA and 10 mmol/L HEPES, pH 7.4. Whole-cell recording data on primary gastric epithelial cells three hours after insolation and RGE1-01 were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software, Molecular Devices, Foster City, CA, United States). Voltage ramp-pulses from -100 mV to +100 mV (500 ms) were applied every 5 s to generate an I-V curve.
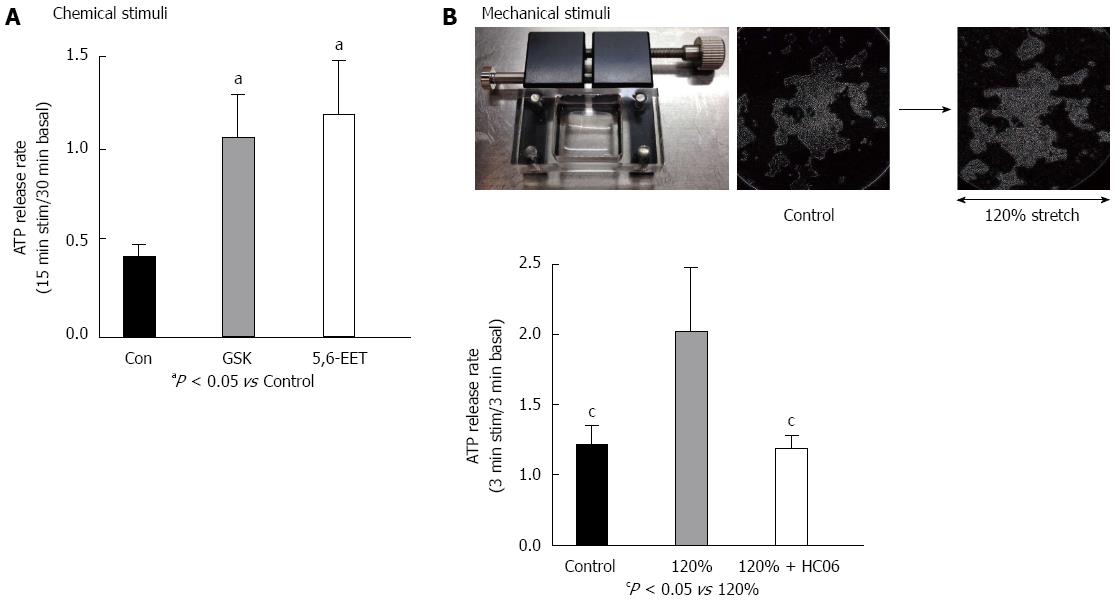
ATP concentrations released from RGE1-01 rat gastric epithelial cells cultured in 12-well plates or stretch silicon chambers (STB-10-04 from STREX Inc., Osaka, Japan) were measured by a luciferin-luciferase assay (ATP Bioluminescence assay kit CLS II, Roche Diagnostics) and a luminometer (Lumat LB 9507, Berthold Technologies, Japan), using a previously described method that was slightly modified[4]. For chemical stimuli, cells cultured to 70%-80% confluence and incubated in 500 μL bath solution for 30 min at room temperature (25 °C) were used to measure basal ATP release. The superfusate was collected and replaced gently with another 500 μL of bath solution with or without the TRPV4 agonists GSK1016790A or 5,6-EET. The superfusate was collected after 15 min and the ratio of released ATP (15 min stimulation/30 min basal condition) was calculated. An aliquot (200 μL) of superfusate was then mixed with 200 μL luciferin-luciferase reagent for luminometric ATP measurements. For mechanical stimuli, stretching was quantitatively applied with a STB-10 stretch machine (STREX Inc.) to RGE1-01 cells cultured on a silicon chamber. Three minutes after chamber placement in the stretch machine, the superfusate was washed away to exclude artificial ATP release and replaced with 500 μL of bath solution for basal ATP measurement. After a further 3 min, the superfusate was collected and replaced, whereupon mechanical stimuli was applied for 3 min and the ratio of released ATP (3 min stimulation/3 min basal condition) was calculated. To block TRPV4 channels, cells were pre-treated with the specific TRPV4 antagonist HC 067047 (Sigma, 1 μmol/L) for 3 min[18].
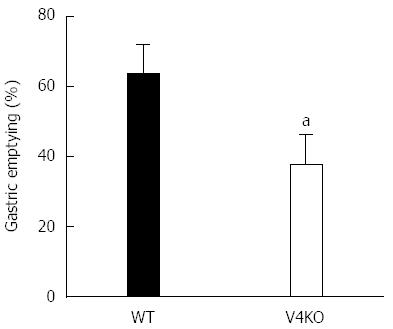
Eight-week-old WT and TRPV4KO male mice were used with a modified version of previously reported methods[17]. Gastric emptying was determined by transit of a test meal containing phenol red. Mice were fasted for 14 h with ad libitum water before the experiment. Five mg/kg (200 μL) of the test meal was administered into the stomach using a feeding needle. Fifteen minutes later, the mice were euthanized by cervical dislocation and the gastrointestinal tract was removed. The stomach was minced and the remaining phenol red concentration was measured. Gastric emptying was expressed as mean ± SEM for each group.
Values for Ca2+-imaging, patch-clamp experiments, ATP measurements, and gastric emptying are presented as mean ± SEM from three or more independent experiments. A Student’s t-test or non-parametric Bonferroni-type multiple comparison was used. Significance was accepted for P < 0.05.
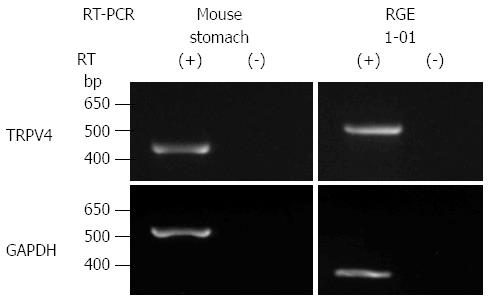
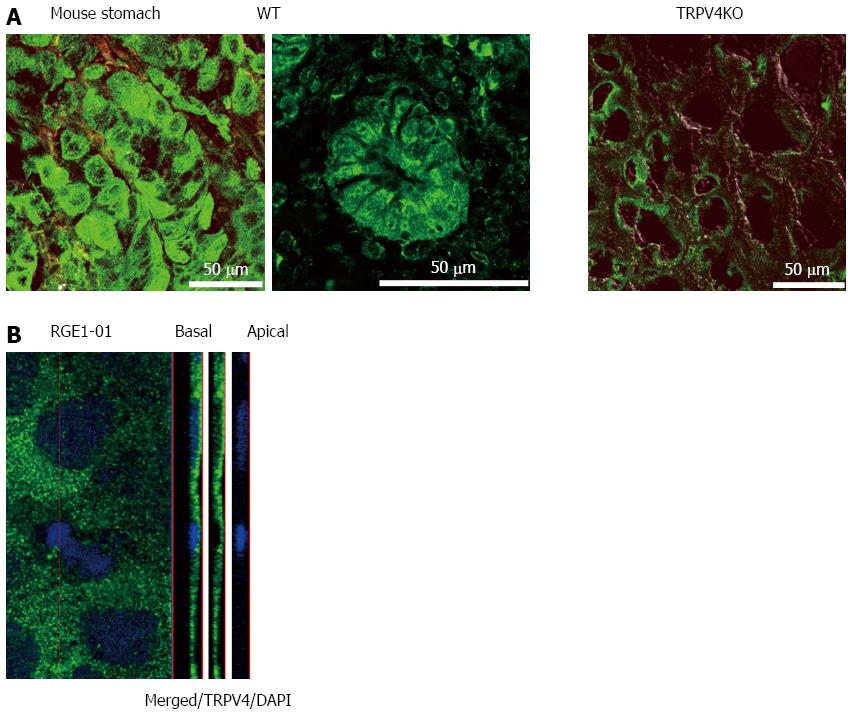
Given that TRPV4 was shown to be expressed in the esophagus and intestinal epithelia[4-6,19,20], we examined TRPV4 mRNA expression in mouse stomach and a rat gastric epithelial cell line, RGE1-01. TRPV4 mRNA was detected in mouse stomach and RGE1-01 cells (Figure 1). We next examined TRPV4 protein expression in mouse and the RGE1-01 cells. A strong homogenous immunofluorescent signal was confined to the epithelial cell layer of the WT mouse gastric corpus and antrum but not in cells from TRPV4KO mice (Figure 2A). Meanwhile, Z-stack images obtained by confocal microscopy of RGE1-01 cells displayed apical TRPV4 expression (Figure 2B).
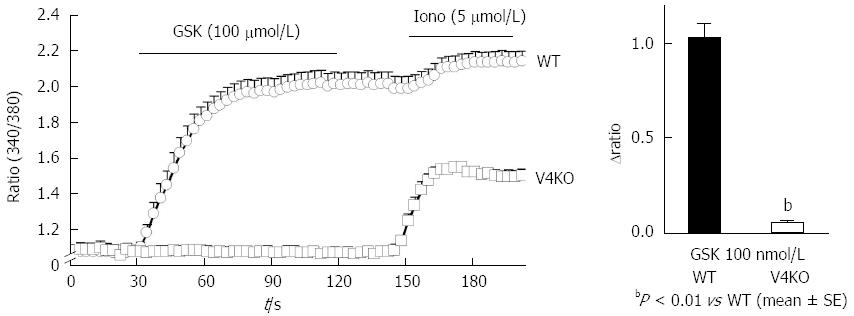
To confirm functional TRPV4 expression in primary gastric epithelial cells and RGE1-01 cells, we examined the response to the reported specific TRPV4 agonist, GSK1016790A (GSK)[3], using a fluorescent Ca2+-imaging system (10 μmol/L, fura-2/AM). Response traces of [Ca2+]i for WT and TRPV4KO gastric epithelial cells in the presence of GSK (100 nmol/L) showed that almost all cells isolated from WT stomach responded to GSK (Figure 3A) and the [Ca2+]i increases were significantly larger in WT cells compared to TRPV4KO cells (Figure 3B). This finding suggests that the majority of gastric epithelial cells expressed TRPV4 and [Ca2+]i responses to GSK were TRPV4 specific.
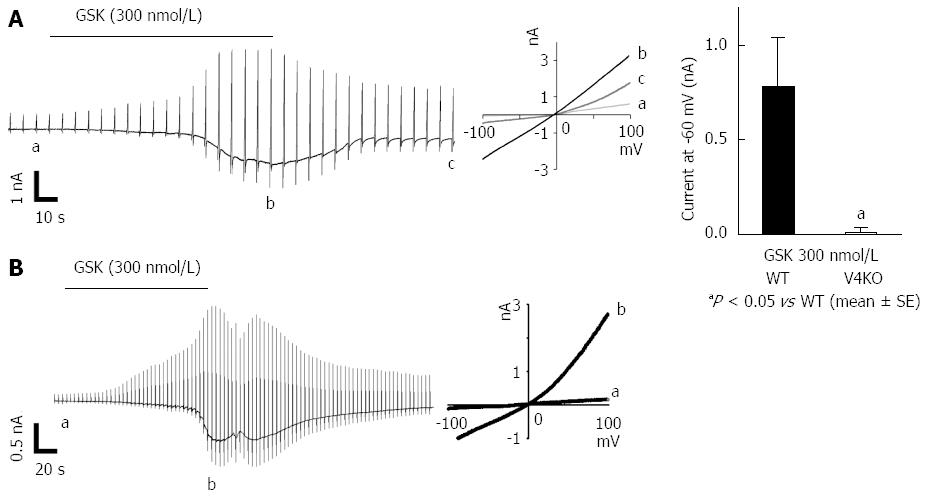
We next performed patch-clamp experiments with acute isolated mouse gastric epithelial cells in the presence of GSK (300 nmol/L) and observed inward current responses with an outwardly rectifying IV-relationship in WT but not TRPV4KO cells (Figure 4A)[3]. Current responses were observed in all 5 trials with WT gastric epithelial cells but were completely absent with TRPV4KO cells, which indicates that the majority of gastric epithelial cells expressed TRPV4. Similar chemical stimulation with GSK (300 nmol/L) induced TRPV4-like current responses in the rat gastric epithelial cell line, RGE1-01 (Figure 4B). These data strongly indicated functional expression of TRPV4 in mouse and rat gastric epithelial cells.
Mechanical stimuli reportedly activate TRPV4 expressed in esophageal keratinocytes that in turn leads to increased ATP release[4]. To examine whether TRPV4 stimulation has a similar effect in gastric epithelium, we measured ATP release in chemically- or mechanically-stimulated RGE1-01 cells using a luciferin-luciferase assay. TRPV4 agonists GSK1016790A (GSK, 100 nmol/L) or 5,6-EET (500 nmol/L)[2] significantly increased ATP release in RGE1-01 cells (Figure 5A, 2- to 3-fold higher vs control, P < 0.05). Additionally, 120% lateral stretch applied for 3 min to RGE1-01 cells cultured on a silicon chamber induced significantly higher amounts of ATP release (Figure 5B, about 2-fold vs without stretch (control), P < 0.05), and these responses were inhibited by the specific TRPV4 inhibitor HC 067047 (1 μmol/L) (stretched cells showed no detachment over the 3-min stretch period). These results suggested that chemical and mechanical stimuli-induced ATP release in RGE1-01 cells was mediated by TRPV4 channel activation.
Since TRPV4 has been shown to sense chemical and mechanical stimuli and contribute to ATP release from gastric epithelial cells, we hypothesized that TRPV4KO mice would exhibit altered gastric motility. To evaluate the physiological role of TRPV4 expressed in the gastric epithelium, we performed an in vivo experiment to compare gastric emptying rates of WT and TRPV4KO mice. Gastric emptying rates in TRPV4KO mice were about 2/3 of those in WT (Figure 6), suggesting that TRPV4 contributes to gastric motor function.
We identified morphological and functional TRPV4 expression in mouse gastric epithelial cells as well as the rat gastric epithelial cell line RGE1-01 (Figures 1-4). Furthermore, we demonstrated that chemical and mechanical stimuli can induce TRPV4-dependent ATP release from RGE1-01 cells (Figure 5), and that stimulation of gastric epithelial TRPV4 enhances gastric emptying in vivo (Figure 6). Using immunohistochemistry, Ca2+ imaging, and electrophysiology, we found that the majority of mouse gastric epithelial cells exhibited abundant TRPV4 expression and responded to TRPV4 agonists, suggesting that TRPV4 is a candidate mechanoreceptor in gastric epithelial cells. In fact, ATP release was several hundred nmol/L in our in vitro study, suggesting that the corresponding ATP concentration in vivo might be estimated to be several μmol/L, which would be sufficient to activate the P2 receptor present in the wall of mouse stomach[21,22]. These results suggested the hypothesis that luminal distension stimulates TRPV4 on gastric epithelial cells that in turn release ATP. The released ATP either stimulates sub-epithelial sensory nerve fibers that form the afferent limb of short or long gastrointestinal reflex arcs or acts directly on visceral smooth muscles expressing purinergic receptors. The first possibility is supported by morphological evidence showing that purinergic receptors, mainly P2X2 and P2X3, were identified on putative gastric mechanosensing structures, including the vagal afferent intraganglionic laminar endings that are located in close proximity to the epithelium[23,24]. These vagal afferents form the afferent limb of the central vago-vagal reflex (long reflex arc) and are known to increase gastric motility following stimulation[25]. The hypothesis is also supported by findings from a previous study wherein P2X3-knockout mice show a blunted neural response to gastric distension and no differences in distension-evoked ATP release between knockout and control mice[26]. The released ATP could also trigger a local reflex arc intrinsic to the stomach wall, which is supported by results from a previous study wherein ATP was shown to induce tetrodotoxin-sensitive contraction responses mediated by neuronal P2X receptors in an in vitro whole-stomach preparation[27].
The possibility that ATP released from gastric epithelium could directly stimulate purinergic receptors expressed on gastric visceral smooth muscles is rather unlikely considering the short half-life of ATP and the distance that ATP must cross while diffusing from the gastric epithelium to visceral smooth muscles. Moreover, gastric smooth muscles are known to functionally express P2Y receptors that mediate relaxation in response to ATP[27] and would be expected, upon stimulation, to delay gastric emptying, which is opposite to our current findings. This outcome further decreases the likelihood of a direct effect for ATP released from gastric epithelium on smooth muscles. However, the purinergic signaling pathway that mediates gastric distension-induced epithelial TRPV4 stimulation requires further future characterization.
In conclusion, TRPV4 is morphologically and functionally expressed in mouse and rat gastric epithelia and contributes to ATP release and gastric emptying. Our results suggest that TRPV4 could be a promising novel diagnostic and therapeutic target for functional gastrointestinal disorders.
We thank Nilius B (Katholieke Universiteit, Belgium) for his generous gift of antibodies and Uchida K (NIPS) and Kozawa T (U. Toyama) for their technical assistance.
The transient receptor potential vanilloid 4 channel (TRPV4) is a non-selective cation channel that is activated by mechanical stimuli. ATP has been recognized as an important signaling molecule via cell surface ATP receptors. This study explored TRPV4 expression in mouse and rat stomach and whether the stimulation mediated ATP release.
The authors have reported that ATP release in the esophagus and urothelium is mediated by TRPV4 stimulation.
This is the first study showing TRPV4 expression and ATP release by its stimulation in the mouse stomach and/or rat gastric epithelial cells.
These data suggested the hypothesis that luminal distension stimulates TRPV4 on gastric epithelial cells that in turn release ATP. However TRPV4 expression in human gastric epithelium and the purinergic signaling pathway requires further evaluation.
TRPV4 is a non-selective cation channel, that is activated by several physical and chemical stimuli, including mechanical stimuli. The channel activation increases intracellular Ca2+ concentration and elicits whole-cell currents in TRPV4-expressing cells.
The manuscript describes an original research performed in mouse stomach and in rat epithelium cell line focusing on the expression and function of TRPV4 ion channels. The study concept is based on previous findings of the authors regarding TRPV4-mediated ATP release on the esophasgus. The series of experiments in this manuscript demonstrated delayed gastric emptying in TRPV4 knockout mice compared to their WT littermates, as well as TRPV4 expression in mRNA and protein levels in both mouse and rat gastric epithelial cell line. In general, the idea is interesting, the various morphological and functional methods are sophisticated, the figures are demonstrative.
P- Reviewer: Cseko K S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 783] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 3. | Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471-3482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | D’Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, Selves J, Alric L, Vinel JP, Vergnolle N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Yamawaki H, Mihara H, Suzuki N, Nishizono H, Uchida K, Watanabe S, Tominaga M, Sugiyama T. Role of transient receptor potential vanilloid 4 activation in indomethacin-induced intestinal damage. Am J Physiol Gastrointest Liver Physiol. 2014;307:G33-G40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Fichna J, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Małecka-Panas E, Janecka A, Krajewska WM, Storr MA. Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment? Neurogastroenterol Motil. 2012;24:e557-e560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Vergnolle N. TRPV4: new therapeutic target for inflammatory bowel diseases. Biochem Pharmacol. 2014;89:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413-492. [PubMed] |
| 10. | Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist. 2003;9:243-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Glasgow I, Mattar K, Krantis A. Rat gastroduodenal motility in vivo: involvement of NO and ATP in spontaneous motor activity. Am J Physiol. 1998;275:G889-G896. [PubMed] |
| 13. | Suzuki N, Mihara H, Nishizono H, Tominaga M, Sugiyama T. Protease-Activated Receptor-2 Up-Regulates Transient Receptor Potential Vanilloid 4 Function in Mouse Esophageal Keratinocyte. Dig Dis Sci. 2015;60:3570-3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Yoshiyama M, Mochizuki T, Nakagomi H, Miyamoto T, Kira S, Mizumachi R, Sokabe T, Takayama Y, Tominaga M, Takeda M. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: dual analysis of behavior and reflex during the micturition cycle. Am J Physiol Renal Physiol. 2015;308:F1128-F1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Tabuchi Y, Arai Y, Ohta S, Shioya H, Takahashi R, Ueda M, Takeguchi N, Asano S, Obinata M. Development and characterization of conditionally immortalized gastric epithelial cell lines from transgenic rats harboring temperature-sensitive simian virus 40 large T-antigen gene. Cell Struct Funct. 2002;27:71-79. [PubMed] |
| 17. | Mihara H, Suzuki N, Yamawaki H, Tominaga M, Sugiyama T. TRPV2 ion channels expressed in inhibitory motor neurons of gastric myenteric plexus contribute to gastric adaptive relaxation and gastric emptying in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G235-G240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA. 2010;107:19084-19089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 327] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Mizuno H, Suzuki Y, Watanabe M, Sokabe T, Yamamoto T, Hattori R, Gotoh M, Tominaga M. Potential role of transient receptor potential (TRP) channels in bladder cancer cells. J Physiol Sci. 2014;64:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Liedtke W, Zhang JY, Hall RP, Steinhoff M. Keratinocyte growth regulation TRP-ed up over downregulated TRPV4? J Invest Dermatol. 2014;134:2310-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2390] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 22. | Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 413] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 23. | Castelucci P, Robbins HL, Furness JB. P2X(2) purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 2003;312:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Wang ZJ, Neuhuber WL. Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol (Berl). 2003;207:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Travagli RA, Hermann GE, Browning KN, Rogers RC. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G180-G187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | McIlwrath SL, Davis BM, Bielefeldt K. Deletion of P2X3 receptors blunts gastro-oesophageal sensation in mice. Neurogastroenterol Motil. 2009;21:890-e66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Mulè F, Naccari D, Serio R. Evidence for the presence of P2y and P2x receptors with different functions in mouse stomach. Eur J Pharmacol. 2005;513:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |