Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.4988
Peer-review started: January 18, 2016
First decision: February 18, 2016
Revised: March 21, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: June 7, 2016
Processing time: 136 Days and 1.3 Hours
AIM: To provoke persistent/chronic multiorgan inflammatory response and to contribute to stones formation followed by fibrosis in hepatobiliary and pancreatic tissues.
METHODS: Tumor necrosis factor receptors 1 and 2 (TNFR1/R2) deficient mice reared in-house were given dibutyltin dichloride (DBTC) twice within 10 d by oral gavage delivery. Sham control animals received vehicle treatment and naïve animals remained untreated throughout the study. Animals were monitored daily for symptoms of pain and discomfort. The abdominal and hindpaw hypersensitivity were assessed with von Frey microfilaments. Exploratory behaviors were recorded at the baseline, after initiation of treatment, and before study termination. Histopathological changes were examined postmortem in tissues. Collagen accumulation and fibrosis were confirmed with Sirius Red staining.
RESULTS: Animals lost weight after oral administration of DBTC and developed persistent inflammatory abdominal and hindpaw hypersensitivity compared to sham-treated controls (P < 0.0001). These pain related secondary mechanical hypersensitivity responses increased more than 2-fold in DBTC-treated animals. The drastically diminished rearing and grooming rates persisted after DBTC administration throughout the study. Gross as well as micropathology at one month confirmed that animals treated with DBTC developed chronic hepatobiliary injuries evidenced with activation of stellate cells, multifocal necrosis, fatty degeneration of hepatocytes, periportal infiltration of inflammatory cells, and prominent biliary ductal dilation. The severity of hepatitis was scored 3.7 ± 0.2 (severe) in DBTC-treated animals vs score 0 (normal) in sham-treated animals. Fibrotic thickening was extensive around portal ducts, in hepatic parenchyma as well as in lobular pancreatic structures and confirmed with Sirius Red histopathology. In addition, pancreatic microarchitecture was presented with distortion of islets, and parenchyma, infiltration of inflammatory cells, degeneration, vacuolization, and necrosis of acinar cells and distention of pancreatic ducts. Extent of pancreatic damage and pancreatitis were scored 3.6 ± 0.4 (severe) for DBTC-treated in contrast to score 0 (normal) in sham-treated animals. The gall bladder became expanded with ductal distention, and occasional bile stones were detected along with microscopic hepatic lesions. DBTC-treated animals developed splenic hypertrophy with increased weight and length (P < 0.01) along with thymic atrophy (P < 0.001). Finally, colitic lesions and colitis were prominent in DBTC-treated animals and scored 3.4 ± 0.3 (moderately severe) vs 0 (normal) for the sham-treated animals.
CONCLUSION: This is the first report of chronic inflammatory multiorgan hepatobiliary pancreatitis, along with fibrosis and calculi formation induced reliably utilizing oral DBTC administration in TNFR1/R2 deficient mice.
Core tip: Currently there is no reliable model for chronic multiorgan inflammatory and fibrosis. Tumor necrosis factor (TNF)α initiates inflammation through TNFR1/R2. TNFR1/R2 deficient mice administered orally with dibutyltin dichloride (DBTC) developed significant persistent inflammatory and pain related secondary mechanical hypersensitivity. DBTC-animals showed severe chronic hepatobiliary injuries and prominent biliary ductal dilation. Extensive fibrotic thickening was evidenced around portal ducts, in hepatic and pancreatic structures. DBTC-animals had severe pancreatic damage and pancreatitis, hepatic lesions with expansion of gall bladder, bile stones and severe colitis. This is the first report of chronic inflammatory multiorgan hepatobiliary pancreatitis, fibrosis and calculi formation in TNFR1/R2 deficient mice.
- Citation: Oz HS. Multiorgan chronic inflammatory hepatobiliary pancreatic murine model deficient in tumor necrosis factor receptors 1 and 2. World J Gastroenterol 2016; 22(21): 4988-4998
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/4988.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.4988
Fibrogenesis is a required process in wound healing, but persistent inflammatory and fibrotic reaction can lead to devastating symptoms and eventually organ failure[1,2]. Multiorgan fibrosis is typically the end product of various unresolved or repetitive tissue injuries from chronic inflammation, infection, radiation exposure, and abnormal repair outcome. Loss of function contributes to progression of morbidity and mortality. Multiorgan fibrosis is a common complication in cystic fibrosis[3], systemic sclerosis[4] and primary sclerosing cholangitis[5]. Chronic pancreatitis, initiated by idiopathic or recurrent inflammation, is manifested with irreversible destruction of exocrine parenchyma and pancreatic fibrosis. It is a potentially fatal progressive disease leading to diabetes mellitus and pancreatic cancer. Pancreatitis is associated with spontaneous visceral pain as a chief symptom in patients. Neural innervation of the pancreas is pivotal in the instigation and continuation of inflammation and pain response. Cellular destruction leads to activation of pancreatic sensory neurons causing release of neurotransmitters in the spinal cord and neurogenic signaling then back to the pancreas provoking plasma extravasation and neutrophil infiltration[6].
Multifactoral gallstones are one of the most prevalent gastrointestinal complications with serious outcomes such as gallstone pancreatitis and cancer. Gallstone disease is a chronic recurrent hepatobiliary complication which is characterized by formation of gallstones in the hepatic and bile duct, or gallbladder. It is manifested by impaired metabolism of cholesterol, bilirubin and bile acids[7].
Tumor necrosis factor α (TNFα) a proinflammatory cytokine, up-regulates various cytokines/chemokines to initiate acute and chronic stages of inflammation. The biological action of TNFα is chiefly through two gene family receptors, TNFR1 and TNFR2. TNFα is released mainly by activated macrophages, in addition to astroglia, microglia, CD4+ lymphocytes, Natural killer cells (NK), and neurons[8-10]. The complete-length membrane-crossing TNFα (mTNFα) is sliced by the inducible TNF converting enzyme (TACE) to release soluble TNFα (sTNFα) and diffusible peptide[11]. TNFα release is associated with inflammatory response and pain related sensation in patients with pancreatitis, hepatitis and inflammatory bowel disease (IBD), as well as neuropathy[12]. TNFα contributes to development of neuropathic pain[13]. Soluble TNFR1 and R2 neutralize circulating TNFα to alleviate pain related responses to mechanical allodynia, thermal hyperalgesia or peripheral nerve injuries[14-16]. TNFα plays an important function in the pathogenesis of acute pancreatitis. Recent investigations have demonstrated that TNFα inhibition drastically ameliorates the duration of experimental acute pancreatitis[17]. TNFα receptor 1 (TNFR1) gene deletion and etanercept application likewise ameliorated the duration of acute pancreatitis in animal models, suggesting potential of etanercept and anti-TNFα monoclonal antibodies as therapy in clinical pancreatitis[17]. Although, current clinical treatments with these biological agents may diminish inflammation and pain by reducing TNFα and other cytokines, the inflammatory response and pain is likely to re-emerge in most patients with autoimmune disease including arthritis and IBD[10]. In addition, anti-TNFα monoclonal antibodies therapy has potential side effects such as provoking infections with JC virus, fungi and tuberculosis. Currently there is no cure or reliable mouse model for chronic pancreatitis.
Previously we have demonstrated that the baseline mechanical and thermal response to noxious stimulation is similar in TNFR1/R2 deficient mice vs wildtype background mice. However, TNFR1/R2 deficient mice develop more severe responses when similarly treated with various insults[10,16]. Animal models of acute and chronic pancreatitis have been utilized to examine mechanisms of pathogenesis, and to test possible therapeutic interventions. One of the most commonly used pancreatitis models is created by serial intraperitoneal administration of concentrations of caerulein, an ortholog of cholecystokinin[17]. Other chemically induced models have utilized di-n-butyltin dichloride (DBTC). DBTC is a polyvinyl carbonate (PVC) plastic stabilizer/catalyzer additive, insecticide and biocide in agriculture, and antifouling agent in the paint and fabric industry that often contaminates food and water[18]. Tail vein slow injection of DBTC induces relatively unpredictable pancreatitis flares in rats[6]. However, DBTC injection is tedious and minor leakage results in tail necrosis, gangrene and animal loss. We hypothesized that oral administration of DBTC would provoke persistent and chronic pancreatitis in animals deficient in TNF-receptors. Similarly, TNFR1/R2 may accelerate inflammatory response in multiorgans and contribute to stones formation and fibrosis in hepatobiliary and pancreatic tissues. Here we report a chronic persistent DBTC-induced inflammatory model by oral gavage in TNFR1/R2 deficient mice persisting at least one month allowing more clinically relevant studies in this model. Pain related behaviors accompanying this model are characterized.
All animal procedures were approved by the University of Kentucky Institution Animal Care and Use Committee (IACUC). Mice were monitored daily for continued weight gain/loss and general health. Health status and procedures were documented daily on the UK IACUC Standard Operating Procedure (SOP-102) Post-Operative Evaluation form. Experiments were performed using dually deficient TNFR1/R2 mice (Jackson Laboratory) on a B6129SF2/J background inbred at the University of Kentucky animal facilities and provided by Dr. Westlund. Mice were housed in individual cages with a 10 h/14 h dark/light reversed cycle to accommodate behavioral test during their active dark period. Mice were allowed free access to food and water ad libitum, except 2 h before and during behavioral testing.
Chronic persistent pancreatitis was induced in mice utilizing DBTC (Dibutyltin dichloride, Sigma-Aldrich, St Louis, MO). DBTC (10 mg/kg) was dissolved in 95% ethanol (two parts) and then mixed with glycerol (three parts) and given orally. Mice received DBTC by oral gavage (200 μL volume). Intragastric gavage administration was performed by Dr. Oz, an expert veterinarian scientist, in conscious animals, using appropriate bended gavage needles (22 gauge, 1 inch length, 1.25 mm ball diameter). Sham control mice were given the vehicle (95% ethanol + glycerol, 2:3) alone and Naïve control animals remained untreated. Animals were monitored until fully active. In order to induce chronic inflammation, mice received a 2nd treatment by oral gavage within 10 d. Following induction of pancreatitis the animals were monitored daily for activity, appearance, and signs of abdominal discomfort. They were weighed regularly and tested for hypersensitivity on the hindpaw plantar foot pad and the shaved abdominal surface. After completion of the final behavioral testing, one month after induction, the animals were euthanatized with isoflurane overexposure, the thorax opened, blood samples collected by cardiac puncture, and tissue samples collected for histological evaluation.
Pain-related behavior was assessed throughout the study by the determining secondary mechanical threshold to assess hyperalgesia/allodynia. The von Frey test is a standard comparison used in the field of pain research. Day 0, baseline testing to determine footpad nociceptive responses was performed testing hindpaw withdrawal latency to mechanical stimuli with von Frey fibers. Reflex testing for secondary mechanical hyperalgesia/allodynia with von Frey fibers was developed by Max von Frey, who in 1896 identified “pain spots” on human skin. Mechanical nociceptive thresholds were analyzed as described previously[10,19]. Paw withdrawal response latencies were assessed weekly throughout the study. Mice were placed into clear cylindrical plastic enclosures (7 cm × 4 cm × 4 cm) on a smooth metal meshed (3 mm × 3 mm) platform (36 cm × 29 cm × 21.5 cm). Mechanical withdrawal threshold testing was done on the plantar surface of both hindpaws using a set of 8 von Frey monofilaments [(4.74) 6.0 g; (4.31) 2.0 g; (4.08) 1.0 g; (3.61) 0.4 g; (3.22) 0.16 g; (2.83) 0.07 g; (2.36) 0.02 g; (1.65) 0.008 g]. The von Frey filaments were applied perpendicularly to the plantar surface with sufficient force to bend the monofilament slightly and held for about 5 s, and 5 to 10 times with 15 s intervals. A positive response was defined as an abrupt withdrawal (flick response) of the foot during stimulation or immediately after the removal of stimulus. Whenever there was a negative or positive response, the next stronger or weaker filament was applied, respectively. Testing proceeded in this manner until four fibers had been applied after the first one caused a withdrawal response, allowing the estimation of the mechanical withdrawal threshold.
Pain-related behavioral evaluations for abdomen: Prior to induction of inflammation with DBTC administration, baseline testing of abdominal nociceptive responses to mechanical stimuli was performed with von Frey fibers applied to the upper left abdominal quadrant skin of mice as previously described[10,19]. Mechanical hypersensitivity in the abdominal area was quantified by measuring the number of withdrawal events (either abdominal withdraw from the von Frey filament or consequent licking of the abdominal area, or whole body withdrawal) in response to normally innocuous or sub-threshold mechanical stimuli. Testing continued weekly throughout the study.
Evaluation of the pain-related posture: The abnormal posture of each animal with an affected hindlimb was given a single score using a subjective pain-related behavioral scale (spontaneous pain rating score 0-5) i.e. 0- normal; 1- curling of the toes, 2- aversion of the paw; 3- partial weight bearing; 4- non-weight bearing and guarding; and 5- avoidance of any contact with the hindlimb.
Pain-related gait disturbance: Gait disturbances (curling toes, limping, guarding, rearing and grooming were tallied by an observer blinded to treatment group as in our previous studies[10].
Spontaneous visceral pain assessment: The animals were placed individually in the observation chamber for a 25 min recording session. The observation chamber is a 28 cm × 17.5 cm × 12.5 cm see-through plastic home cage with one mirrored side located in an isolated room with constant “white noise”. A digital camera located 0.5 meter from the chamber with an unobstructed view was used to record animals spontaneous visceral pain related behaviors. The camera was linked to a computer recording program for offline data analysis (Logitech Image Studio). The chamber was washed with a detergent disinfectant and dried after each use between animals. Postures defined as statistically significant increase in visceral pain-related behavior in this study included rearing, grooming and licking of the lower abdomen, stretching the abdomen or hindlimb, lowering the abdomen against the floor, and abdomen retractions or arching the back. Recordings were masked and analyzed by the investigator.
Tissue collection: At the end of the one month experiment, animals were deeply anesthetized with isoflurane inhalation. Pancreatic, hepatic, gall bladder tissues were excised and a portion was fixed in cold 4% paraformaldehyde in 0.1 mol/L phosphate buffer saline (PBS). Thymus and splenic tissues were removed, weighed, and fixed in paraformaldehyde. Colonic tissues were removed and flushed with cold PBS, and portion of ascending and descending colon were fixed for histopathological examinations.
Hepatic and pancreatic samples were collected and immerse fixed overnight in 4% paraformaldehyde in 0.1 mol/L PBS, then transferred into 70% ethanol and embedded in paraffin. Sections were cut (5 μm), rehydrated, stained with hematoxylin and eosin for histopathological changes. In order to detect collagen fiber deposits, sections were further stained with Sirius Red (Electron Microscopy Sciences, #26357-02), using routine histological protocols[20].
Pancreatic tissues and a portion of the small intestine along with spleen were removed and processed for histopathological evaluations of the pancreatitis. The severity of lesions was scored on a 0-4 grade on the basis of the histopathological changes as follows: 0 - normal pancreatic microstructure, no inflammatory mononuclear cell infiltration; 1 - slight inflammatory mononuclear cell infiltration, with no detectable parenchymal destruction; 2 - mild pancreatitis, edema, focal parenchymal destruction with mononuclear cell infiltration; 3 - moderate pancreatitis, with diffuse parenchyma destruction, presence of necrosis, and reduced number of islets; and 4 - severe pancreatitis, parenchyma mostly destroyed and replaced with adipose tissues, loss of pancreatic islets, presence of fibrosis and or calculi.
A portion of the right lobe from liver tissues of each mouse was placed in an embedding cassette and fixed in paraformaldehyde as mentioned above. The specimens were dehydrated and embedded in paraffin, and tissue sections of 5 μm were stained with Hematoxylin Eosin. Each slide was evaluated under Ziess light microscopy. Hepatic lesions were graded on a scale of 0 to 4+ based on degeneration, inflammation, and necrosis as follow: Grade 0 - no detectable lesions, degeneration, infiltration of inflammatory cells, normal tissue appearance; Grade 1 - focal infiltration of inflammatory cells in the tissue and hepatocytes degeneration; Grade 2 - mild multifocal infiltration of inflammatory cells, and hepatocytes degeneration; Grade 3 - moderate multifocal infiltration of inflammatory cells and hepatocytes degeneration; and Grade 4 - severe diffuse infiltration of inflammatory cells, necrosis, or fibrosis.
Colonic tissues were flushed with PBS (pH 7.2) and a portion from proximal and distal colonic tissues were fixed for histological examinations. The fixed sections were processed and stained with Hematoxylin Eosin and slides evaluated by Ziess light microscopy. The severity of colitis was assessed with a histological semi-quantitative grading score. The scores were based on histopathological features with a numeric value (0: normal to 4: severe) assigned according to the tissue involvement corresponding to the following criteria[21,22]. Grade 0: No detectable lesions, no inflammatory cells, and normal mucosal appearance; Grade 1: Focal inflammatory infiltrate in the mucosa; Grade 2: Mild multifocal inflammation with moderate expansion into the mucosa; Grade 3: Moderate multifocal inflammation with moderate expansion of the mucosa; and Grade 4: Severe diffuse inflammation with crypt epithelium disruption and ulceration.
All results are expressed as mean and standard error of mean (± SEM) unless otherwise stated. Data were analyzed using paired t-test comparison of groups for histology or analysis of variance (ANOVA) followed by Bonferroni post hoc comparison using GraphPad Prism Software for behavioral testing over time (San Diego, CA, United States). Statistical significance was set at P≤ 0.05.
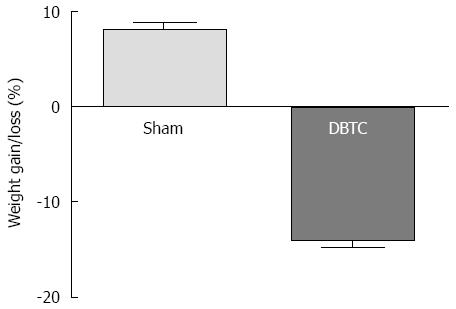
No major differences in body weight, behavioral analysis was detected between sham-treated and naïve control animals. Additionally, sham-treated and naïve control animals and wildtypes did not develop any histopathological lesions. Therefore, only sham-treated controls are reported here. Oral administration of DBTC resulted in weight lost as early as 3 d after treatment which persisted throughout the study, and animals developed persistent inflammatory abdominal and hindpaw hypersensitivity as compared to sham-treated animals. DBTC application induced significant body weight loss (P < 0.001) in compression to weight gain in sham-treated control animals. The major weight loss occurred during the 1st wk of DBTC inoculation when animals lost about 10% of their body weight, compared to weight gain in sham-treated animals. The body weight afterward became stable in DBTC-treated mice until the end of the one month study, but remained less than the sham-treated group (Figure 1).
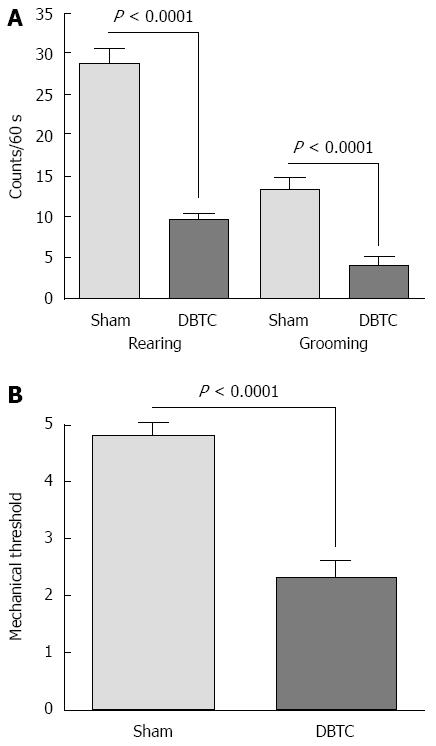
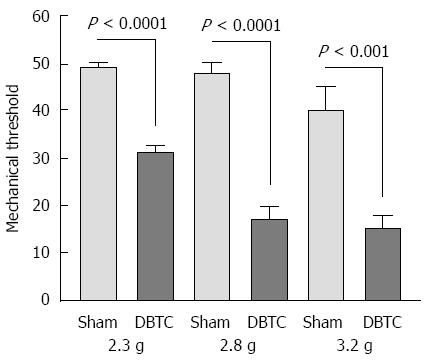
DBTC-treated animals had significant reduction in physical activities such as, cage crossing, rearing and grooming activity (P < 0.0001) compared to naïve sham-treated ones presumably attributed to the abdominal discomfort (Figure 2A). Hindpaw mechanical threshold was significantly decreased in DBTC-treated animals (P < 0.0001) tested using von Frey microfilaments (Figure 2B). In addition, responses to 3 different von Frey fibers with increasing grams force applied to the abdominal skin indicated DBTC-treated animals had significantly decreased mechanical threshold compared to sham-treated control (respectively P < 0.0001 from force 2.3 g, 2.8 g, P < 0.001 to force 3.22 g) (Figure 3). In contrast sham-treated animals demonstrated a partial visceral response to the higher filaments with forces of 3.22 g and above.
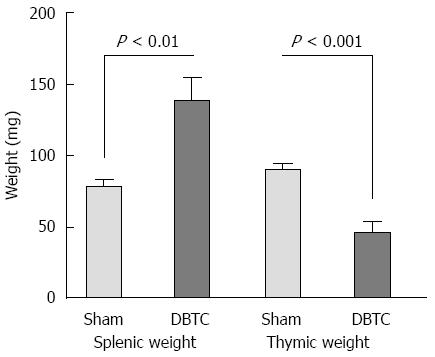
DBTC animals developed splenic hypertrophy with significant increase in weight and length of the spleens (P < 0.01). Splenic histopathologic studies demonstrated loss of medulla, irregular formation of trabecules, with captured trace of blirubin and bile deposits. In contrast, thymic tissues from DBTC-treated animals showed central degeneration and atrophy. The thymus was atrophied, and thymic weight significantly decreased in DBTC-treated animals (P < 0.001) compared to sham-treated animals (Figure 4).
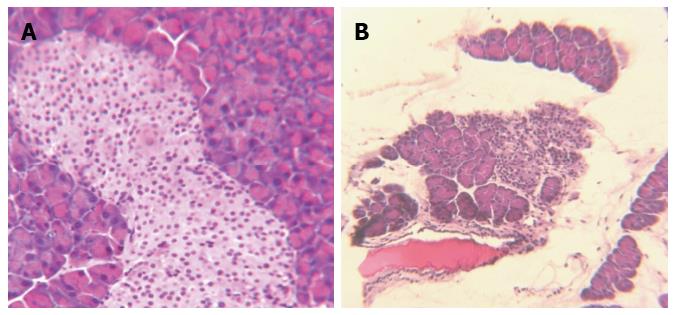
Sham-treated animals demonstrated normal pancreatic structures with prominent islets (Figure 5A). In contrast, DBTC-treated animals developed gross as well as micropathology confirming the moderate to severe chronic pancreatitis. Pancreatic parenchyma presented with edema, congestion, distortion of microarchitecture, and infiltration of inflammatory cells. Pancreatic and acinar cells showed degeneration, fatty necrosis, and fibrosis. The pancreatic ducts became prominent and distended in DBTC-treated animals. These findings were consistent with fibrotic thickening which were particularly prominent in the vicinity of the primary duct, as well in surrounding lobular pancreatic parenchyma as confirmed with Sirius Red histopathological studies.
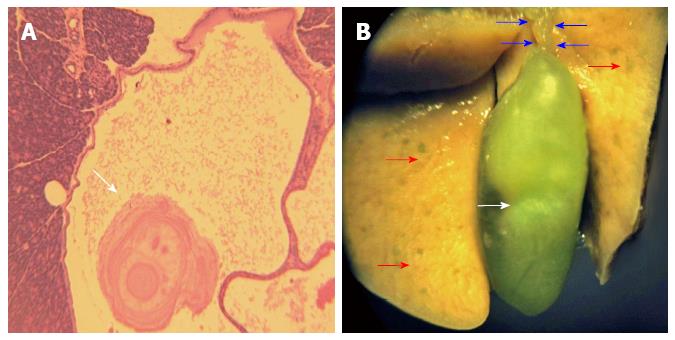
Also noted was loss of microstructure indicated by the presence of irregular and degenerated islets, along with vacuolization and necrosis of β cells accompanied by invasion of inflammatory cells. The sizes as well as the numbers of the pancreatic islets were significantly diminished in DBTC-treated compared with the sham-treated animals (Figure 5B). A few small and shrunken islets were scattered throughout the pancreatic parenchyma, but overall loss of β cells was evident. In addition, pancreatic ducts had become thickened and expanded containing traces of debris and calculi formation (Figure 6A). Extent of pancreatic damage scored (0- normal to 4 most severe) were 3.6 ± 0.4 (severe) in DBTC-treated in contrast to score 0 (normal) for sham-treated animals.
Gall bladder showed extensive expansion with ductal distension and occasional detected bile stones (Figure 6B).
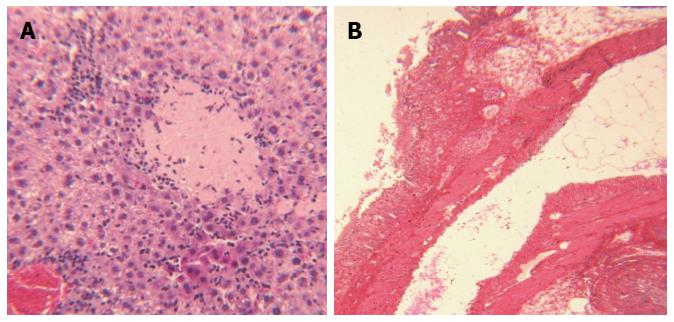
Hepatic tissues became enlarged, friable and pale or yellowish in color with a spotted appearance indicating moderately severe hepatitis. Macroscopic hepatic injuries were evidenced with activation of stellate cells, degeneration of hepatocytes, and multifocal and central necrosis (Figure 7A). Additionally, hepatic structure showed fatty degeneration of hepatocytes, and periportal infiltration of inflammatory cells, along with presence of visible ductal dilation. Fibrotic thickening was prominent in the vicinity of portal ducts, and surrounding lobular hepatic parenchyma as confirmed with Sirius Red histopathological studies. The severity of hepatitis was scored 3.7 ± 0.2 (severe) in DBTC-treated animals compared to the score 0 (normal) in sham-treated animals.
Colonic tissue showed extensive necrosis and loss of intestinal epithelial cells, distortion of cryptic structures, thickening of mucosa due to invasion of inflammatory cells, and some cryptic microabscess formation, presenting advanced colitis (Figure 7B). Colonic lesions and colitis in DBTC-treated animals were scored 3.4 ± 0.3 (moderately severe) compared to 0 (normal) for the sham-treated animals.
Here our findings may provide a new model to better approach investigation of chronic multiorgan inflammatory and visceral pain in murine model and to study possible potential targets in this model for the treatment of chronic hepatobiliary and pancreatitis. Acute pancreatitis is manifested by histopathological transformations, including the presence of inflammatory mediators, acinar atrophy, fat necrosis, intraductal hemorrhage, and stromal proliferation[23]. Chronic pancreatitis is distinguished by recurrent or continuous inflammation of pancreatic progressive atrophy and irreversible fibrosis, with demise of exocrine and endocrine malfunction in severe forms. While, severe and uninhibited abdominal pain is the main aspect of persistent pancreatitis, the mechanism/s by which the pain is induced is poorly explored possibly due to a lack of available appropriate animal model to mimic chronic pancreatitis[24]. In current study the TNFR1/R2 deficient animals displayed significant pain related modifications such as decreases in mechanical threshold after DBTC treatment that persisted through the one month experiment until the end of the study. The significant decreases in mechanical threshold were detected on foot pads and abdomen after the induction of the injury as compared with sham treated animals.
Although, chronic pancreatitis in human is distinguished by irreversible fibrosis, yet pancreatic fibrosis in animal models are mainly reversible[25]. Indeed persistent fibrosis in vital organs results in significant morbidity and mortality worldwide. While, organ fibrogenesis is typically the end product of various non-resolving or repetitive injuries such as chronic infection and radiation exposure; abnormal repair reaction followed by tissue injuries to contribute to the progression of organ fibrosis. Indeed, fibrogenesis is required in natural wound healing process, persistent fibrogenesis in organs can lead to devastating symptoms and organ failure[1,2]. At the cellular level fibrogenesis is remarkably similar progress in different organs and can cause generalized fibrosis in these tissues. Yet, currently there is no appropriate model available to study systemic fibrosis.
Similarly chronic hepatitis and hepatic fibrosis result from excess extracellular matrix produced primarily by hepatic stellate cells. We and other investigators have shown that proinflammatory cytokines (e.g., TNFα) and other inflammatory mediators such as growth factors are regulated by matrix metalloproteinase (MMPs) expressions[20,26]. Further activation of Stellate cells is major event in hepatic fibrosis formation caused by multiple injuries due to chemicals, infectious agents, surgical and/or inflammatory cytokine and chemokines which prompt proliferation and transformation of stellate cells to secret extra cellular matrix. Additionally, the mesenchyme-specific transcription factor forkhead box f1 (Foxf1) in liver is specifically expressed in hepatic stellate cells. Recently, a lipid based liver-specific delivery system also called “dbtc” is reported to be efficient to transfer the Foxf1 siRNA to activated hepatic stellate cells and silence genes expressed in different cell types in liver when used in an acute mouse model of bile duct ligation-induced secondary cholestasis[27].
Pancreatitis models are divided into surgically induced and chemical administration induced hypersecretion of the pancreatic enzymes. The surgical models include ligation and/or cannulation of the biliopancreatic ducts with infusion of variety of solutions, or the formation of closed duodenal loops. Pancreatic fibrosis in bile duct ligated rats is a difficult model to induce and requires increased stimulation. Chemical secretagogues (caerulein or l-arginine) include administration of DBTC to cause a partial blockage of the pancreatic ducts to induce pancreatic disease through enzymic reflux into the gland[28].
Various environmental chemicals have been implicated in the induction of autoimmune responses. Di-n-dibutyltin dichloride is an organotin compound and PVC plastic additive that frequently released to contaminate food and water. As eventually DBTC is degraded in the environment with possible harmful effects on man and animals[29]. Some therapeutic indications of DBTC include at a dose of 10 mg/kg per day for 5 consecutive days effective to eliminate Trypanosoma brucei infection in mice[23]. LD50 of DBTC is reported to be 90 mg/kg[30]. Metabolism of DBTC by cytochrome P450 enzymes plays an important role in the induction of biological effects, as DBTC with affinity for mitochondria depresses respiration and elevates serum enzymatic activities resulting in hepatic injuries[31]. Thymus atrophy noted in the current investigation was similar to that reported as a consequence rather than a cause in DBTC-intraperitoneal injected rats[32]. Increased proliferin expression and promotion of morphological thymic transformation reportedly occurring at similar concentrations most probably are DBTC-induced thymus involution. Indeed, this reaction is due to antiproliferative activity of DBTC, as observed by inhibition of thymidine incorporation of thymocytes isolated from DBTC-treated rats[32]. After administration of 4-61 mg/kg iv or 120-240 mg/kg oral DBTC, a dose dependent reversible reduction of thymus weight and number of thymocytes were observed in mice. Iv administration of DBTC highly increased the level of total bilirubin in serum of these animals. But, the level of bilirubin in serum did not correlate with the thymotoxic effects of DBTC in mice[33]. Of interest, toxicity of DBTC in mice is reported after 3 consecutive daily high doses of 50 mg/kg, killing 75% and the survivors developed severe hepatic and bile duct damage. While 3 daily doses of 20 mg/kg caused only mild bile duct and liver lesions[34].
In another study, mice were given DBTC at 8, 15, or 30 mg/kg per day by gavage on days 0-3 or days 4-7 and sacrificed on day 18 of pregnancy. The incidence of embryonic loss increased on days 0-3 at 15 mg or over and, on days 4-7 with 8 mg/kg bw/d and higher. However, no increase in the rate of fetus malformations was observed after the DBTC administration. A decline in the serum progesterone levels was noted in dams given DBTC at 30 mg/kg per day, which might have affected the pregnancy initiation, maintenance, and loss when administered during early pregnancy[35].
Non-alcoholic steatohepatitis (NASH), the most common hepatic disorder, is manifested with inflammation, hepatocyte injury, cell death, fibrosis and multiorganelle failure, leading to cirrhosis[26]. Previously we reported cytokine/chemokine, extracellular matrix accumulation and metalloproteinase upregulation in a dietary deficient NASH model[20]. RT-PCR measurements showed a significant overexpression of inflammatory cytokines [TNFα, transforming growth factor (TGF-β), interleukin (IL-1β), IL-6], suppressor of cytokines signaling1 and genes involved in tissue remodeling and fibrosis (MMPs, collagen-α1) in the hepatic tissues of rats fed methionine-choline deficient diet[20].
Furthermore, using DBTC tail injection rat model we have shown implication of the endothelin cascade gene expression as a major contributing factor in pancreatic pain in both pancreatitis and potential pancreatic cancer[6]. In the present study oral inoculation of DBTC in TNFR1/R2 deficient mice induced a chronic persistent multiorgan hepatobiliary pancreatitis as confirmed by pathological studies including biliary dilation, loss of hepatic and pancreatic architecture and islets, edema in parenchyma, infiltration of inflammatory cells, degeneration, vacuolization and fibrosis, and pancreatic necrosis of acinar cells. Pain related behaviors were increased in animals with pancreatic inflammation including visceral pain-related behavior and secondary cutaneous mechanical hypersensitivity which increased greater than 2-fold. Here lack of TNF receptors appears to accelerate the inflammatory response in multiple organs and contribute to fibrosis in hepatobiliary and pancreatic tissues. A serum proteome profiling analysis in our previous study in TNFR1/R2 deficient mice with pain related behaviors in an arthritis model revealed high levels of serum inflammatory factors. The inflammatory factors included TNFα, which is regulated by the activation of normally T-cell expressed and secreted (RANTES), chemokine (C-X-C motif) ligand 9 [CXCL9 (MIG)], chemokine (C-X-C motif) ligand 10 [CXCL10 (IP-10)], and chemokine (C-C motif) ligand 2 [CCL2 (MCP-1)][10].
Primary sclerosing cholangitis is a complex hepatic disorder, characterized by chronic inflammation of the biliary epithelium, and cholestasis resulting in multifocal bile duct strictures, fibrosis of hepatic parenchyma and biliary tract leading to cirrhosis and malignancy. The etiology of primary sclerosing cholangitis is not fully discovered and no effective therapy is available[5]. Gallstone, one of the most prevalent gastrointestinal complications, is multifactoral, with serious outcomes such as acute gallstone pancreatitis and gallbladder cancer. Gallstone disease is a chronic recurrent hepatobiliary complication which is manifested by creation of gallstones in the hepatic and bile duct, or gallbladder. It is manifested by dysfunctional metabolism of cholesterol, bilirubin and bile acids[7]. Other factors may involve genetic, environmental and steroids. The prevalence of gallstone disease has increased because of sedentary lifestyle and poor diets. Gallstones are known as a common cause of pancreatitis. From 932 patients with acute pancreatitis 40% had gallstones, and 22% alcohol induced[36]. Further, pancreatitis is frequent amongst IBD patients. Gallstones are reported as the most frequent cause of pancreatitis in IBD patients which cause growing diagnostic challenges[37]. Thus, this model may facilitate study of fibrogenesis and/or fibrosis resolution in multiple vital organs leading to development of novel technologies and therapeutic strategies aimed at lessening organ fibrosis.
In conclusions, this is the first report of a chronic inflammatory multiorgan hepatobiliary pancreatitis along with fibrosis and calculi formation model that can be induced reliably with use of oral DBTC administration in TNFR1/R2 deficient mice. Future studies will utilize this model in investigations of anti-fibrotic and analgesic therapeutics.
Dr. Karin N Westlund, PhD, has provided TNFR1/R2 deficient mice and animal facility funded by R01-NS039041 (KNW). Sabrina McIlwrath, PhD, raised the TNFR1/R2 mouse colony. A portion of this paper is selected for presentation in the Metabolic and Genetic Liver Disease Session at the Digestive Disease Week, DDW2016 abstract number 2441753 in San Diego, CA, United States.
Chronic multiorgan, pancreatitis and hepatobiliary complications manifested with irreversible fibrosis and spontaneous visceral pain in patients. Currently there is no cure or reliable model available for chronic pancreatitis or multiorgan fibrosis other than repeated dosing with chemical caerulein to produce acute flares. An appropriate murine model to mimic the syndrome is desirable. Pancreatic fibrosis in bile duct ligated rats is a difficult model to induce and requires other increased stimulations. The prevalence of gallstone disease has increased because of sedentary lifestyle and poor diets. Dibutyltin dichloride (DBTC) is a biocide, and antifouling agent in the paint and fabric industry. Tail vein injection of DBTC induces unpredictable pancreatitis flares in rats, DBTC injection is tedious, and minor leakage results in tail gangrene and animal loss. TNFα proinflammatory cytokine initiates inflammation through its 2 receptors, TNFR1/R2. We devised a new chronic model of pancreatitis and multiorgan inflammation in TNFR1/R2 deficient mice using oral DBTC.
Currently, there is no cure or reliable model available for chronic pancreatitis and multiorgan fibrosis in mice. Persistent pancreatitis manifests with severe abdominal pain, but the mechanism/s by which induced is/are poorly explored possibly due to lack of appropriate models. Three daily doses of 20 mg/kg DBTC caused only mild bile duct and liver lesions, while 3 consecutive daily doses of 50 mg/kg DBTC were toxic and killed 75% of mice. TNFα proinflammatory cytokine initiates inflammation through its 2 receptors, TNFR1/R2. Proteome profiling analysis in our previous study in TNFR1/R2 deficient mice with persistent pain related behaviors in an arthritis model revealed high levels of serum inflammatory cytokines likely responsible for the multiorgan inflammatory response in this model.
This is the first report of a chronic inflammatory hepatobiliary pancreatitis, colitis and stone formation model that can be induced reliably with use of oral DBTC-administration in TNFR1/R2 deficient mice. These findings provide this new murine model to better approach investigation of chronic multiorgan inflammatory and visceral pain in murine mode. In addition, to facilitate study of fibrogenesis and/or fibrosis resolution in multiple vital organs leading to development of novel technologies and therapeutic strategies aimed at lessening organ fibrosis.
This chronic inflammatory hepatobiliary pancreatitis, colitis/fibrosis and stone formation model can be induced reliably utilizing oral DBTC in TNFR1/R2 deficient mice. The model can be used to investigate chronic multiorgan inflammatory and visceral pain in mice to explore the mechanisms of injury and to study of fibrogenesis and/or fibrosis resolution in multiple vital organs leading to development of novel technologies and therapeutic strategies aimed at lessening organ fibrosis. This study grants ability for further investigation into the use of this model to explore mechanisms of multiorgan injury, the biochemical players and the therapeutic exploration for devastating chronic multiorgn inflammatory and fibrogenesis such as cystic fibrosis and systemic sclerosis as well as cholangitis.
TNFR1/R2 deficient mice are transgenic animals lacking tumor necrosis factor receptor 1 and 2 with constant higher proinflammatory TNFα levels compared to wildtype background animals. Multiorgan damage, when 2 or more organs involved in the course of injury. Fibrogenesis is a process usually followed chronic inflammatory to form fibrous structures in and around tissues. Pain related mechanical hypersensitivity, is measured by von Frey microfilament (an accepted standard procedure), demonstrating decreased tolerance to a simple touch manifested with withdraw to protect against induced excess pressure while normal animals tolerate the mechanical touch with no withdrawal response to the fine microfilament touch.
This is an interesting and well-written paper. The author provided that TNFR1/R2 deficient mice treated with DBTC reveal the severe chronic injury of various internal organs which was proved with usage of histological methods.
P- Reviewer: Khedmat H, Kurzepa J S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | De Langhe E, Lories R. Fibrogenesis, novel lessons from animal models. Semin Immunopathol. 2015;37:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Medley JM, Kaplan E, Oz HS, Sundararaj SC, Puleo DA, Dziubla TD. Fibrin-targeted block copolymers for the prevention of postsurgical adhesions. J Biomed Mater Res B Appl Biomater. 2011;99:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Lavie M, Manovitz T, Vilozni D, Levy-Mendelovich S, Sarouk I, Weintraubv I, Shoseyov D, Cohen-Cymberknoh M, Rivlin J, Efrati O. Long-term follow-up of distal intestinal obstruction syndrome in cystic fibrosis. World J Gastroenterol. 2015;21:318-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Kavian N, Batteux F. Macro- and microvascular disease in systemic sclerosis. Vascul Pharmacol. 2015;71:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (36)] |
| 6. | Oz HS, Lu Y, Vera-Portocarrero LP, Ge P, Silos-Santiago A, Westlund KN. Gene expression profiling and endothelin in acute experimental pancreatitis. World J Gastroenterol. 2012;18:4257-4269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Reshetnyak VI. Concept of the pathogenesis and treatment of cholelithiasis. World J Hepatol. 2012;4:18-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (5)] |
| 8. | Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 254] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2679] [Cited by in RCA: 2879] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 10. | Westlund KN, Zhang L, Ma F, Oz HS. Chronic inflammation and pain in a tumor necrosis factor receptor (TNFR) (p55/p75-/-) dual deficient murine model. Transl Res. 2012;160:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 12. | Uçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 826] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 14. | Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 232] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517-2521. [PubMed] |
| 16. | Ma F, Zhang L, Oz HS, Mashni M, Westlund KN. Dysregulated TNFα promotes cytokine proteome profile increases and bilateral orofacial hypersensitivity. Neuroscience. 2015;300:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Malleo G, Mazzon E, Genovese T, Di Paola R, Muià C, Centorrino T, Siriwardena AK, Cuzzocrea S. Etanercept attenuates the development of cerulein-induced acute pancreatitis in mice: a comparison with TNF-alpha genetic deletion. Shock. 2007;27:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | DeWitt JC, Copeland CB, Luebke RW. An organotin mixture found in polyvinyl chloride (PVC) pipe is not immunotoxic to adult Sprague-Dawley rats. J Toxicol Environ Health A. 2008;71:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Oz HS. Toxoplasmosis complications and novel therapeutic synergism combination of diclazuril plus atovaquone. Front Microbiol. 2014;5:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Oz HS, Im HJ, Chen TS, de Villiers WJ, McClain CJ. Glutathione-enhancing agents protect against steatohepatitis in a dietary model. J Biochem Mol Toxicol. 2006;20:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Oz HS, Chen T, de Villiers WJ. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front Immunol. 2013;4:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Oz HS, Chen TS, Nagasawa H. Comparative efficacies of 2 cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl Res. 2007;150:122-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Schmidt J, Hotz HG, Foitzik T, Ryschich E, Buhr HJ, Warshaw AL, Herfarth C, Klar E. Intravenous contrast medium aggravates the impairment of pancreatic microcirculation in necrotizing pancreatitis in the rat. Ann Surg. 1995;221:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Barreto SG, Saccone GT. Pancreatic nociception--revisiting the physiology and pathophysiology. Pancreatology. 2012;12:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Miyauchi M, Suda K, Kuwayama C, Abe H, Kakinuma C. Role of fibrosis-related genes and pancreatic duct obstruction in rat pancreatitis models: implications for chronic pancreatitis. Histol Histopathol. 2007;22:1119-1127. [PubMed] |
| 26. | Caldwell S. NASH (Nonalcoholic steatohepatitis): A case of multiorganelle failure. Free Radic Biol Med. 2014;75 Suppl 1:S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Abshagen K, Brensel M, Genz B, Roth K, Thomas M, Fehring V, Schaeper U, Vollmar B. Foxf1 siRNA delivery to hepatic stellate cells by DBTC lipoplex formulations ameliorates fibrosis in livers of bile duct ligated mice. Curr Gene Ther. 2015;15:215-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Foster JR. A review of animal models of nonneoplastic pancreatic diseases. Toxicol Pathol. 2014;42:243-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Kobayashi H, Suzuki T, Kasashima Y, Motegi A, Sato I, Matsusaka N, Ono N, Miura A, Saito F, Saito S. Effects of tri-, di- and monobutyltin on synaptic parameters of the cholinergic system in the cerebral cortex of mice. Jpn J Pharmacol. 1996;72:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Shuaibu MN, Ameh DA, Bonire JJ, Adaudi AO, Ibrahim S, Nok AJ. Trypanocidal activity of organotin chlorides on Trypanosoma brucei-infected mice. Parasite. 2000;7:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Ueno S, Kashimoto T, Susa N, Shiota Y, Okuda M, Mutoh K, Hoshi F, Watanabe K, Tsuda S, Kawazoe S. Effects of butyltin compounds on mitochondrial respiration and its relation to hepatotoxicity in mice and Guinea pigs. Toxicol Sci. 2003;75:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Penninks A, Kuper F, Spit BJ, Seinen W. On the mechanism of dialkyltin-induced thymus involution. Immunopharmacology. 1985;10:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Hennighausen G, Lange P. Immunotoxic effects of dialkyltins used for stabilization of plastics. Pol J Pharmacol Pharm. 1980;32:119-124. [PubMed] |
| 34. | Barnes JM, Stoner HB. Toxic properties of some dialkyl and trialkyl tin salts. Br J Ind Med. 1958;15:15-22. [PubMed] |
| 35. | Ema M, Fujii S, Ikka T, Matsumoto M, Hirose A, Kamata E. Early pregnancy failure induced by dibutyltin dichloride in mice. Environ Toxicol. 2007;22:44-52. [PubMed] |
| 36. | Nesvaderani M, Eslick GD, Vagg D, Faraj S, Cox MR. Epidemiology, aetiology and outcomes of acute pancreatitis: A retrospective cohort study. Int J Surg. 2015;23:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Ramos LR, Sachar DB, DiMaio CJ, Colombel JF, Torres J. Inflammatory Bowel Disease and Pancreatitis: A Review. J Crohns Colitis. 2016;10:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |