Published online May 21, 2016. doi: 10.3748/wjg.v22.i19.4732
Peer-review started: January 19, 2016
First decision: March 7, 2016
Revised: March 16, 2016
Accepted: March 30, 2016
Article in press: March 30, 2016
Published online: May 21, 2016
Processing time: 118 Days and 17.6 Hours
AIM: To evaluate pretreatment hepatitis B virus (HBV) testing, vaccination, and antiviral treatment rates in Veterans Affairs patients receiving anti-CD20 Ab for quality improvement.
METHODS: We performed a retrospective cohort study using a national repository of Veterans Health Administration (VHA) electronic health record data. We identified all patients receiving anti-CD20 Ab treatment (2002-2014). We ascertained patient demographics, laboratory results, HBV vaccination status (from vaccination records), pharmacy data, and vital status. The high risk period for HBV reactivation is during anti-CD20 Ab treatment and 12 mo follow up. Therefore, we analyzed those who were followed to death or for at least 12 mo after completing anti-CD20 Ab. Pretreatment serologic tests were used to categorize chronic HBV (hepatitis B surface antigen positive or HBsAg+), past HBV (HBsAg-, hepatitis B core antibody positive or HBcAb+), resolved HBV (HBsAg-, HBcAb+, hepatitis B surface antibody positive or HBsAb+), likely prior vaccination (isolated HBsAb+), HBV negative (HBsAg-, HBcAb-), or unknown. Acute hepatitis B was defined by the appearance of HBsAg+ in the high risk period in patients who were pretreatment HBV negative. We assessed HBV antiviral treatment and the incidence of hepatitis, liver failure, and death during the high risk period. Cumulative hepatitis, liver failure, and death after anti-CD20 Ab initiation were compared by HBV disease categories and differences compared using the χ2 test. Mean time to hepatitis peak alanine aminotransferase, liver failure, and death relative to anti-CD20 Ab administration and follow-up were also compared by HBV disease group.
RESULTS: Among 19304 VHA patients who received anti-CD20 Ab, 10224 (53%) had pretreatment HBsAg testing during the study period, with 49% and 43% tested for HBsAg and HBcAb, respectively within 6 mo pretreatment in 2014. Of those tested, 2% (167/10224) had chronic HBV, 4% (326/7903) past HBV, 5% (427/8110) resolved HBV, 8% (628/8110) likely prior HBV vaccination, and 76% (6022/7903) were HBV negative. In those with chronic HBV infection, ≤ 37% received HBV antiviral treatment during the high risk period while 21% to 23% of those with past or resolved HBV, respectively, received HBV antiviral treatment. During and 12 mo after anti-CD20 Ab, the rate of hepatitis was significantly greater in those HBV positive vs negative (P = 0.001). The mortality rate was 35%-40% in chronic or past hepatitis B and 26%-31% in hepatitis B negative. In those pretreatment HBV negative, 16 (0.3%) developed acute hepatitis B of 4947 tested during anti-CD20Ab treatment and follow-up.
CONCLUSION: While HBV testing of Veterans has increased prior to anti-CD20 Ab, few HBV+ patients received HBV antivirals, suggesting electronic health record algorithms may enhance health outcomes.
Core tip: Prior to anti-CD20 antibody (Ab) treatment in 2014, 61%-73% of 19304 Veterans had hepatitis B virus (HBV) tests. Of these, 11% tested were positive for hepatitis B surface antigen or core antibody and at risk for reactivation; ≤ 37% of these HBV+ patients received HBV antivirals during anti-CD20 Ab and follow-up. HBV+ patients had significantly higher hepatitis rates than HBV-. Among pretreatment HBV- patients, about 1 in 300 tested suffered acute hepatitis during anti-CD20 Ab and 12 mo follow-up. Electronic health record algorithms to increase HBV testing, antiviral use and vaccination will likely improve outcomes with anti-CD20 Ab treatment.
- Citation: Hunt CM, Beste LA, Lowy E, Suzuki A, Moylan CA, Tillmann HL, Ioannou GN, Lim JK, Kelley MJ, Provenzale D. Veterans health administration hepatitis B testing and treatment with anti-CD20 antibody administration. World J Gastroenterol 2016; 22(19): 4732-4740
- URL: https://www.wjgnet.com/1007-9327/full/v22/i19/4732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i19.4732
In the United States, 4% of the population has had hepatitis B viral (HBV) infection and 0.3% have chronic hepatitis B (with positive hepatitis B surface antigen)[1,2]. Following HBV infection, viral DNA persists in the liver - though its replication is suppressed by B- and T-cells or by HBV antivirals[3,4]. With immunosuppression, HBV can reactivate, even in patients with past or resolved infection. In untreated patients with chronic or prior HBV, nearly 40% of those receiving chemotherapy for hematological malignancies or solid tumors develop HBV reactivation[5]. HBV reactivation frequently interrupts chemotherapy and increases cancer mortality[6] by causing hepatitis (33%), liver failure (13%) and death (5%)[7]. Use of prophylactic HBV antivirals in patients with chronic or prior HBV infection largely prevents reactivation[1,7-11]. As most patients are asymptomatic and unaware of their HBV infection, hepatitis B serology before immunosuppression is the most effective means to identify the potential for reactivation[12]. Due to a higher HBV prevalence in lymphoma patients than the general population, HBV reactivation is a particular concern in lymphoma[13].
Anti-CD20 antibodies (Ab), such as rituximab, ofatumumab and obinutuzumab, are common treatments for non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL), and rheumatoid arthritis. Anti-CD20 Ab act by depleting B-lymphocytes[14]. However, anti-CD20 Ab also decrease host immune suppression of HBV, potentially leading to viral reactivation - identified by increases in HBV DNA and alanine aminotransferase (ALT)[5]. Among patients with lymphoma and prior HBV infection receiving rituximab, 10%-60% exhibit HBV reactivation at a median of 3 mo after the last rituximab dose[8,15]. Despite 2007-8 guidance from the Centers for Disease Control and the American Association for the Study of Liver Disease[16] recommending HBV screening before immunosuppression, low screening rates persist nationally[4,17].
In 2013, the FDA reported 32 anti-CD20 Ab-related HBV reactivation fatalities occurring up to 12 mo post-therapy, in whom only 3 (9%) received prophylactic HBV antivirals during treatment and follow-up[10]. In 2013, the American Society for Clinical Oncology (ASCO) recommended universal HBV screening prior to anti-CD20 Ab; the 2014 ASCO Quality Oncology Practice Initiative reported nearly 70% HBV screening rates[10]. HBV screening and antiviral treatment decrease reactivation 10-fold and yield cost savings[18]. Additionally, antiviral treatment cost-effectively decreases lymphoma- and liver-related deaths in those with HBV infection[6].
With these improved outcomes with HBV antivirals, 2015 ASCO recommendations prior to anti-CD20 Ab include: (1) hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (HBcAb) screening; (2) treating patients with chronic HBV with entecavir or tenofovir during anti-CD20 Ab and 6-12 mo following; and (3) use of either prophylactic or prompt on-demand HBV antivirals for HBV reactivation (identified by increased HBV DNA or ALT on every 3 mo testing) in those with prior HBV (HBsAg-, HBcAb+)[10]. While ASCO does not specify care of resolved (HBsAg-, HBcAb+, HBsAb+) HBV, prospective controlled lymphoma studies of anti-CD20 Ab report 20% HBV reactivation rates in resolved HBV without use of prophylactic HBV antivirals, resulting in chemotherapy interruptions, hepatitis, and reverse seroconversion (i.e., HBsAg reappears)[1,11]. These events were prevented with prophylactic entecavir treatment[1]. Overall, prophylactic antivirals are associated with lower HBV reactivation, liver failure and death rates compared to on-demand antivirals[8,10].
The VHA is the largest single-system United States health care provider. Compared to the general United States population[2], Veterans exhibit a 2 to 3-fold higher prevalence of chronic HBV infection[19,20]. We aimed to identify all VA patients initiating anti-CD20 Ab (2002-2014) to assess the use of HBV serologic testing, vaccination, antivirals and the rate and timing of hepatitis B-associated complications during treatment.
We performed a retrospective cohort study using the VHA Corporate Data Warehouse (CDW), a national repository of VA electronic medical record data[19]. We ascertained patient demographics, inpatient and outpatient visits, laboratory results, procedures (including hepatitis B vaccination), vital signs, pharmacy data, and vital status. VA Vital Status mortality data is highly accurate, exhibiting 98% exact agreement with dates in the National Death Index[21].
All patients initiating anti-CD20 Ab (2002-2014) were identified using VHA pharmacy data. Among these, those who were followed to death or for at least 12 mo after completing anti-CD20 Ab were analyzed in this study. The analysis was exempted by the Durham VAMC Institutional Review Board from review as it was performed for VHA quality improvement. Informed consent was not needed as only anonymized patient information was used in this national quality improvement analysis.
Pretreatment hepatitis B vaccination at any prior time was obtained from CDW vaccination records. Pretreatment HBV testing was quantified at any preceding time, within the study period, and within 6 mo of anti-CD20 Ab initiation. However, we identified HBV disease categories by serologic testing during the study period only[22]. Most (about 90%) VHA HBV assays were qualitative (or categorical), and as normal ranges were not provided in CDW, numerous serology results were indeterminate. We divided the study population into six pretreatment HBV disease categories: definite chronic HBV infection (HBsAg+ for more than 6 mo regardless of HBV DNA), likely chronic HBV (single pretreatment HBsAg+), past HBV (HBsAg-, HBcAb+, HBsAb-)[10], resolved HBV (HBsAg-, HBcAb+, HBsAb+)[1,11], likely prior vaccination (isolated HBsAb+, HBsAg-, HBcAb-), HBV negative (HBsAg-, HBcAb-) or unknown (with no pretreatment HBV serology or those who could not be categorized). Reverse seroconversion was defined as the reappearance of HBsAg or HBeAg in patients with past or resolved HBV[10]. As earlier reported, the “high-risk period” was defined as the period of anti-CD20 Ab treatment and 12 mo follow-up[15]. In patients negative for HBV (HBsAg-, HBcAb-) before treatment, acute HBV was defined by the appearance of HBsAg+ in the high-risk period. Patients with acute, chronic, past or resolved HBV were categorized as HBV positive, while HBV negative or likely vaccinated patients were categorized as HBV negative.
During the high-risk period, HBV antiviral use (adefovir, entecavir, lamivudine, tenofovir, and telbivudine) was identified using the pharmacy data (yes vs no). HBV antiviral treatment was termed “prophylactic” when administered within 3 mo of anti-CD20 Ab initiation and “on demand” following this period. Due to very limited quantitative HBV DNA and HBeAg data, we were unable to identify HBV reactivation by published definitions[4,5,8,15]. The rates and timing of health outcomes in the high-risk period included hepatitis events, liver failure and death (overall, cancer-, liver-, or HBV-related). Outcomes were compared among the pretreatment HBV disease categories and by HBV antiviral use. Hepatitis events were defined as ALT > 2 × baseline (ALT immediately preceding anti-CD20 Ab) and ALT > 2 × upper limit normal (ULN) in the high-risk period[8], while liver failure was defined as hepatitis and an INR ≥ 1.5[23]. Information on death and cause of death in the high-risk period was retrieved from 2014 vital status information. Hepatitis B-associated death met the liver failure definition and had no other apparent cause of death. Liver-related death was identified by International Classification of Diseases, 9th Edition (ICD-9) prior to death[24], as was NHL/CLL cancer related death (ICD-9 codes 200, 202, and 204.12).
Age, gender, race, baseline comorbidities, and the anti-CD20 Ab indication were ascertained at the time of anti-CD20 Ab initiation. Baseline comorbidities were determined using ICD-9 codes related to cirrhosis, decompensated liver disease, hemodialysis-dependent renal failure, human immunodeficiency virus (HIV), sexually transmitted disease, and alcohol and substance abuse.
A biomedical statistician performed the statistical analyses and completed pre-submission statistical review. Baseline patient characteristics were tabulated. Statistical analyses were performed using Stata MP-64 version 13.1 (StataCorp LP, College Station, Texas), and differences were considered statistically significant when the P-value was less than 0.05. Cumulative hepatitis, liver failure, and death after anti-CD20 Ab initiation were compared by HBV disease categories (6 pretreatment HBV disease categories plus acute HBV: chronic, past, resolved, acute, negative, vaccinated, and unknown) and differences compared using the χ2 test. Mean time to hepatitis peak ALT, liver failure, and death relative to anti-CD20 Ab administration and follow-up were also compared by HBV disease group.
We identified 19304 patients who received anti-CD20 Ab in the VA from 2002-2014, of whom 14887 had at least 12 mo follow-up after anti-CD20 Ab. Most patients were older white males receiving anti-CD20 Ab with NHL (66%), CLL (24%), or rheumatoid arthritis (12%) (Table 1). Comorbid illnesses included alcohol or substance abuse, hepatitis C, cirrhosis, decompensated liver disease or hemodialysis-dependent renal failure (Table 1).
| Baseline characteristics | |
| Males | 18464 (96) |
| Mean age (range, SE) | 66.6 yr (20.3-97.5, 0.0813) |
| Median, at risk (range) | 478 d (365-4083) |
| Race | |
| White | 14520 (76) |
| Black or African-American | 2460 (12) |
| Hispanic or Latino | 878 (5) |
| Native Hawaiian or Pacific Islander | 171 (1) |
| American Indian or Alaska Native | 148 (1) |
| Asian | 71 (0) |
| Missing | 1056 (5) |
| Indication for anti-CD20 antibody treatment | |
| Non-hodgkin’s lymphoma | 11384 (66.2) |
| Chronic lymphocytic leukemia | 4,110 (23.9) |
| Rheumatoid arthritis | 2,151 (12.5) |
| Wegener’s granulomatosis | 174 (1) |
| Microscopic polyangiitis | 54 (0.3) |
| Baseline comorbidities | |
| Alcohol abuse | 4286 (24.9) |
| Substance abuse | 1485 (8.6) |
| Hepatitis C | 1369 (8) |
| Cirrhosis | 808 (4.7) |
| Decompensated liver disease | 660 (3.8) |
| Hemodialysis-dependent renal failure | 597 (3.5) |
| HIV | 234 (1.4) |
| Sexually transmitted disease | 25 (0.1) |
| Total number of patients | 19304 (100) |
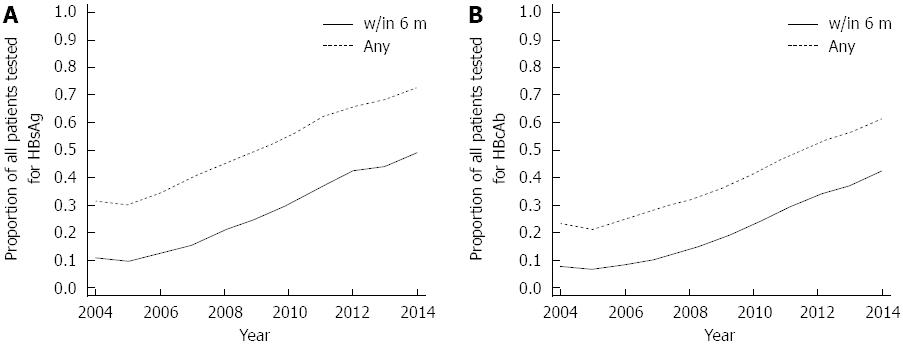
Prior to anti-CD20 Ab treatment, 61%-73% had HBsAg and HBcAb tested at any time pretreatment in 2014 (Figure 1). During the study period, the rates of HBsAg and HBcAb testing increased more than two-fold (Figure 1) with overall pretreatment HBsAg and HBcAb measured in 53% (10224/19304) and 41% (7903/19304), respectively. In 2014, 43%-49% of patients had pretreatment HBsAg and HbcAb screening within 6 mo of anti-CD20 Ab initiation (Figure 1). During the high-risk period for reactivation, < 2% (261/14880) had HBV DNA measured.
In those tested, hepatitis B disease categories (7 categories including “unknown”) were assessed as: definite chronic HBV in 40/10224 (0.4%), likely chronic HBV in 127/10224 (1.2%), past HBV in 326/7903 (4%), resolved HBV in 427/8110 (5%), HBV negative in 6002/7903 (76%), likely prior HBV vaccination in 628/8110 (7%), and acute HBV in 0.3% (16/4947) appearing during or after anti-CD20 Ab treatment. The remaining 11723/19304 (61% of overall) patients were termed “unknown,” as missing serology or not otherwise categorized. Pretreatment HBV DNA was tested in 2% (403/19304) of patients, of whom 2% (9/403) were positive and 24% (97/403) were indeterminate. At pretreatment baseline, HBeAg was tested in 2% (474/19304), of whom 3% (12/474) were positive and 29% (139/474) were indeterminate. In all HBV categories, 17% or fewer received pretreatment HBV vaccination (as determined by vaccination records).
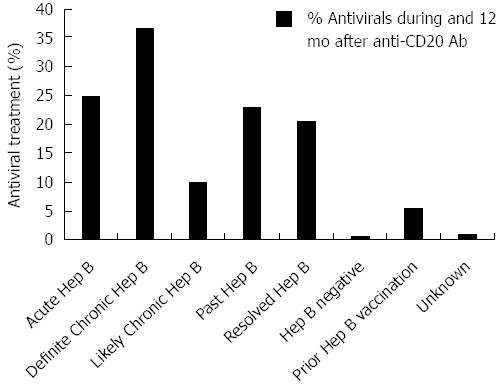
Across all HBV disease categories, few patients receiving HBV antiviral treatment in the high-risk period had concomitant HIV infection (ranging from 1 in 59 to 2 in 9, or 2% to 22%). Overall HBV antiviral use throughout the high risk period ranged from 10%-37% in HBV positive patients at risk for reactivation (Figure 2); the highest rate of HBV antiviral use was 37% in those with definite chronic HBV. In the high-risk period, HBV positive patients exhibited low and variable rates of HBV antiviral treatment throughout the study period (data not shown), although most (80%) HBV antivirals were administered prophylactically (i.e., started within 3 mo of anti-CD20 Ab initiation).
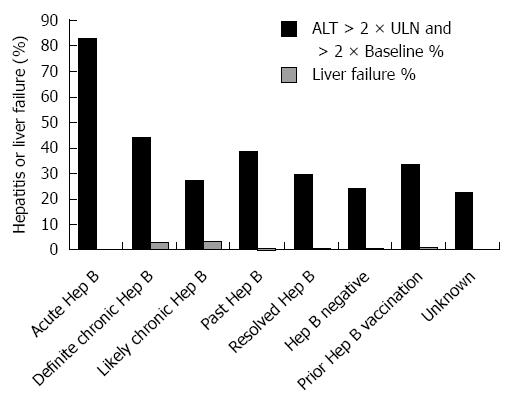
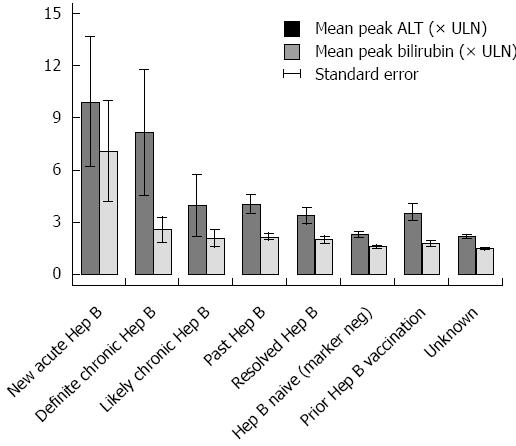
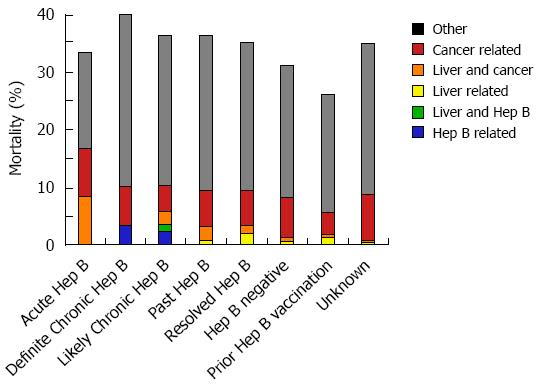
Among 16 pretreatment HBV negative patients acquiring acute HBV during the high-risk period, the mean peak ALT and bilirubin were 10 × ULN (+/- 13 × ULN) and 7 × ULN (+/- 10 × ULN), respectively (Figure 3). In the 25% (3/12) with acute HBV receiving HBV antivirals, the mean peak ALT was 19 × ULN (vs 7 × ULN in those not receiving antivirals). Those with acute HBV exhibited the highest rates of hepatitis [83%) 10/12] among all HBV positive patients, and experienced a 33% (4/12) all-cause mortality (Figure 4). Patients with acute HBV exhibited hepatitis and death at a mean time of 327 d or more following anti-CD20 Ab initiation.
During the high-risk period, 37% (11/30) patients with definite chronic HBV received HBV antivirals and exhibited a mean peak ALT and bilirubin of 8 × ULN (+/- 19 × ULN) and 3 × ULN (+/- 4 × ULN), respectively. In contrast, 10% (9/88) with likely chronic HBV received antivirals and had a mean peak ALT 4 × ULN (+/- 16 × ULN) (Figure 3). Among chronic HBV positive patients, those with definite chronic HBV exhibited the highest rates of hepatitis [(43%) 13/30], liver failure [(3%) 1/30] and all-cause mortality [(40%) 12/30], while those with likely chronic HBV exhibited lower rates of hepatitis [(27%) 24/88], liver failure [(3%) 3/88] and overall mortality [(35%) 31/88] (Figures 4 and 5). Patients with chronic HBV experienced hepatitis and death at a mean time of more than 210 d after anti-CD20 Ab initiation.
Of patients with past and resolved HBV infection, 23% (59/256) and 21% (64/311) received HBV antivirals, respectively, and exhibited a mean peak ALT 3-4 × ULN (+/- 8 × ULN) (Figure 3). Among these patients, those with past HBV exhibited higher rates of hepatitis [(39%) 99/256], liver failure [(1%) 3/256] and overall mortality [(36%) 93/256], while those with resolved HBV exhibited lower rates of hepatitis [(30%) 93/311], liver failure [(0.6%) 2/311] and all-cause mortality [(35%) 109/311] (Figures 4 and 5). Patients with past or resolved HBV developed hepatitis and death at a mean time of 278 d or more following anti-CD20 Ab initiation.
In HBV negative patients, 7% (422/6022) had received prior HBV vaccination. HBV antiviral use was associated with concomitant HIV infection. HBV negative patients experienced a mean peak ALT of 2 × ULN (+/-8 × ULN) and the lowest rates of hepatitis [(24%) 994/4143] and liver failure [(0.6%) 27/4143] (Figure 4). These patients had a relatively low all-cause mortality [(31%) 1292/4143] (Figure 5).
Patients likely vaccinated against hepatitis B (with isolated HBsAb+) exhibited 2-6-fold higher rates of baseline liver-related comorbidities (24% hepatitis C, 11% cirrhosis and 8% decompensated liver disease), relative to those of unknown HBV status (data not shown). During the high risk period, they had a mean peak ALT of 4 × ULN (+/-10 × ULN), low rates of hepatitis [(34%) 140/416], liver failure [(1%) 5/416], and the lowest overall mortality [(26%) 109/416] (Figures 4 and 5).
Patients with unknown HBV infection status (as serology missing or incomplete) exhibited the lowest rates of baseline comorbidity and pretreatment HBV vaccination rates [(2%) 252/11718], a mean peak ALT of 2 × ULN (+/-8 × ULN), low rates of hepatitis [(22%) 2163/9631], liver failure [(0.6%) 53/9631], and a moderate overall mortality of [(35%) 3363/9631] (Figures 4 and 5).
Patients with acute, chronic, past or resolved HBV infection were categorized as HBV positive, and are at risk of HBV reactivation due to the persistence of HBV DNA. When compared to HBV negative or likely vaccinated patients, the HBV positive patients exhibited significantly higher rates of hepatitis (χ2 = 27.8, P = 0.001), and nonsignificantly higher rates of liver failure and overall mortality. The small numbers of patients on HBV antiviral treatment precluded a planned analysis of health outcomes by HBV disease category in the presence or absence of antiviral treatment.
Patients with likely prior hepatitis B vaccination (isolated HBsAb+ pretreatment) had the lowest overall mortality rates (26% [108/416]) (Figure 5). In contrast, pretreatment HBV negative patients who developed acute HBV during the high-risk period experienced a 33% (4/12) mortality rate.
In this first 12 year retrospective national VHA analysis, we evaluated HBV testing, vaccination, treatment and outcomes in nearly 20000 Veterans receiving anti-CD20 Ab treatment, largely for NHL or CLL. Rates of pretreatment HBV screening within 6 mo of anti-CD20 Ab initiation more than doubled over the study period. By 2014, most Veterans receiving anti-CD20 Ab had recent pretreatment HBsAg and HBcAb testing and the large majority had testing at any time, which compares favorably with the rates reported in ASCO quality oncology practices[10]. However, few patients susceptible to reactivation had HBV DNA testing during anti-CD20 Ab treatment and follow-up, limiting detection of HBV reactivation. Among those with pretreatment HBV testing, 1 in 9 were HBV positive and at risk for HBV reactivation - yet, only 21% received HBV antivirals during anti-CD20 Ab treatment and follow-up. As a result, HBV positive patients experienced a significantly higher rate of hepatitis than those HBV negative - with most events occurring within one year of treatment initiation. This data aligns with published data reporting the high risk period during anti-CD20 Ab treatment and 12 mo follow-up[15]. These hepatitis events, as well as related morbidity and costs, can be largely prevented with the use of safe, effective prophylactic antivirals in all HBV positive patients throughout the high-risk period of anti-CD20 Ab treatment and 12 mo follow-up[1,7,8,11].
Unexpectedly, we identified 16 cases of acute hepatitis B in the high-risk period arising in patients negative for HBsAg and HBcAb prior to anti-CD20 Ab initiation. These appear to be the first published reports of acute HBV arising de novo during anti-CD20 Ab therapy - likely as a result of the prolonged B cell suppression compromising host immune defense[3]. Hepatitis B vaccination substantively decreases the risk of acute HBV, even in high-risk adults[25,26]. Yet, in the current study, only 2% of the nearly 12000 “at risk” HBV unknown and 7% of the 6000 HBV negative patients had pretreatment hepatitis B vaccination. These rates are comparable to the 6% and 9% HBV vaccine immunity rates in Veterans and United States adults age 50 or older, respectively[2].
The strengths of this analysis include its large size, national scope, reliable pharmacy data, relatively high rate of HBV testing, identification of acute HBV risk, diverse indications for anti-CD20 therapy, and 12 mo follow-up of the large majority of patients after anti-CD20 Ab administration. Study limitations include the lack of VHA standardization of HBV serology resulting in some indeterminate results, and the predominantly qualitative HBV serologies. While HBV reactivation is generally identified by logarithmic increases in HBV DNA, reverse seroconversion (newly appearing HBeAg or HBsAg), or increases in ALT[27], the very limited quantified HBV DNA and HBeAg data required us to focus our evaluation on hepatitis - which occurs less frequently than HBV DNA increases in reactivation[4]. Additionally, the effect of HBV antivirals on health outcomes was limited by low antiviral treatment rates.
Automated clinical reminders and decision support have earlier been demonstrated to increase HBV screening and antiviral prophylaxis prior to immunosuppressive therapy. For example, to increase HBV screening and antiviral prophylaxis in a Spanish medical center, computerized physician order entry prompts for HBV screening when ordering biologic therapies yielded > 90% screening rates, while appropriate consultation and prophylactic HBV antiviral treatment prevented HBV reactivation[28]. As computerized recommendations and follow-on treatment algorithms are highly effective in influencing physician behavior and prescribing[29], computerized decision support may decrease HBV-related disease with anti-CD20 Ab treatment in the VHA.
In conclusion, the VHA now screens most patients for HBV before anti-CD20 Ab treatment, yet seldom measures HBV DNA during treatment and therefore, likely under-diagnoses HBV reactivation. Increasing VHA hepatitis B vaccination rates should diminish the risk of acute hepatitis B[26] and its complications during anti-CD20 Ab treatment and followup. In HBV positive patients, universal use of HBV antiviral treatment throughout anti-CD20 Ab treatment and 12 mo follow-up will likely decrease mortality and enhance quality of life.
The authors wish to thank David Ross for his support and Anna S.F. Lok, Christina D. Williams, Hashem B. El-Serag, and Fasiha Kanwal for valuable input to the design of this analysis.
Among patients with lymphoma and prior hepatitis B virus (HBV) infection receiving rituximab, 10%-60% exhibit HBV reactivation at a median of 3 mo after the last rituximab dose. Pre-rituximab HBV testing and anti-viral treatment reduces HBV reactivation 10-fold and decreases lymphoma- and liver-related deaths in those with prior HBV infection.
While the American Society for Clinical Oncology guidelines recommend HBV testing and treatment of patients with prior hepatitis B infection during and up to 12 mo following anti-CD20 antibody therapy, it is unclear how commonly these guidelines are followed in the United States Veterans Health Administration.
This 12 year retrospective cohort study analyzed 19304 Veterans in the United States Veterans Health Administration receiving anti-CD20 antibody therapy. The authors found that pre-treatment HBV testing increased over the study period, yet 37% or fewer received HBV antiviral treatment during anti-CD20 antibody treatment and 12 mo follow-up.
Results of this analysis can be shared with providers and used to develop electronic health record algorithms to enhance HBV testing and antiviral treatment with anti-CD20 antibody therapy and followup.
This retrospective study presented by Hunt et al demonstrates the necessity to screen patients for HBV before anti-CD20 Ab treatment, and most likely, prior to the administration of any immunosuppressive treatment; in order to determine if the patient will benefit from HBV vaccination or preventive antiviral treatment. This simple measure will reduce the number of HBV-related deaths occurring in a number of patients. It is an interesting and relevant study.
P- Reviewer: Fernandez-Rodriguez CM, Gonzalez-Aseguinolaza G, McQuillan GM S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, Yang MH, Tzeng CH, Lee PC. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Roberts H, Kruszon-Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB, Holmberg SD. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1212] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 4. | Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Yeo W, Chan HL. Hepatitis B virus reactivation associated with anti-neoplastic therapy. J Gastroenterol Hepatol. 2013;28:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Saab S, Dong MH, Joseph TA, Tong MJ. Hepatitis B prophylaxis in patients undergoing chemotherapy for lymphoma: a decision analysis model. Hepatology. 2007;46:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519-528. [PubMed] |
| 8. | Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (1)] |
| 9. | Kim SJ, Hsu C, Song YQ, Tay K, Hong XN, Cao J, Kim JS, Eom HS, Lee JH, Zhu J. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer. 2013;49:3486-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Hwang JP, Somerfield MR, Alston-Johnson DE, Cryer DR, Feld JJ, Kramer BS, Sabichi AL, Wong SL, Artz AS. Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol. 2015;33:2212-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, Kao WY, Chiu CF, Lin SF, Lin J. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Lok AS, Ward JW, Perrillo RP, McMahon BJ, Liang TJ. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Marcucci F, Mele A, Spada E, Candido A, Bianco E, Pulsoni A, Chionne P, Madonna E, Cotichini R, Barbui A. High prevalence of hepatitis B virus infection in B-cell non-Hodgkin’s lymphoma. Haematologica. 2006;91:554-557. [PubMed] |
| 14. | Shah A. Obinutuzumab: a novel anti-CD20 monoclonal antibody for previously untreated chronic lymphocytic leukemia. Ann Pharmacother. 2014;48:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, Dai MS, Chiu BC, Fintel B, Cheng Y. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 17. | Hay AE, Meyer RM. Hepatitis B, rituximab, screening, and prophylaxis: effectiveness and cost effectiveness. J Clin Oncol. 2012;30:3155-3157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, Feld JJ. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol. 2012;30:3167-3173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Backus LI, Belperio PS, Loomis TP, Han SH, Mole LA. Screening for and prevalence of hepatitis B virus infection among high-risk veterans under the care of the U.S. Department of Veterans Affairs: a case report. Ann Intern Med. 2014;161:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among U.S. veterans with hepatitis B: A national cohort study. Hepatology. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [PubMed] |
| 22. | Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197. [PubMed] |
| 24. | McDonald SA, Hutchinson SJ, Bird SM, Robertson C, Mills PR, Graham L, Dillon JF, Goldberg DJ. The growing contribution of hepatitis C virus infection to liver-related mortality in Scotland. Euro Surveill. 2010;15:1-5. |
| 25. | Chou R, Dana T, Bougatsos C, Blazina I, Khangura J, Zakher B. Screening for hepatitis B virus infection in adolescents and adults: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;161:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | LeFevre ML. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 28. | Sampedro B, Hernández-López C, Ferrandiz JR, Illaro A, Fábrega E, Cuadrado A, Iruzubieta P, Menéndez S, Cabezas J, Crespo J. Computerized physician order entry-based system to prevent HBV reactivation in patients treated with biologic agents: the PRESCRIB project. Hepatology. 2014;60:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1526] [Article Influence: 76.3] [Reference Citation Analysis (0)] |