Published online May 21, 2016. doi: 10.3748/wjg.v22.i19.4695
Peer-review started: January 9, 2016
First decision: February 18, 2016
Revised: March 3, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: May 21, 2016
Processing time: 128 Days and 22.5 Hours
AIM: To investigate the mechanism by which Qinggan Huoxue Recipe (QGHXR) inhibits epithelial-to-mesenchymal transition (EMT) in rats with alcoholic liver fibrosis (ALF).
METHODS: A total of 75 male SD rats were used to induce ALF. Serum biochemical indicators, including alanine aminotransferase, aspartate aminotransferase, laminin and hyaluronidase, were measured. Liver histopathological changes were evaluated using hematoxylin-eosin and Sirius red staining. EMT was examined by analyzing the expression of the epithelial marker E-cadherin and the mesenchymal markers vimentin and fibronectin using RT-PCR and Western blot. The inhibitory effect of QGHXR on EMT markers, as well as its effect on molecules associated with the transforming growth factor (TGF)-β1/Smad signaling pathway, including TGF-β1, Smad3, snail, occludin, ZO-1 and claudin, was also examined.
RESULTS: Compared with normal control rats, ALF rats exhibited a decrease in E-cadherin levels (mRNA: ALF 0.16 ± 0.05 vs control 1.00 ± 0.08; protein: ALF 0.09 ± 0.05 vs control 0.70 ± 0.17, P < 0.01) and an increase in vimentin and fibronectin levels (mRNA: 11.43 ± 0.39 vs 1.00 ± 0.19 and 9.91 ± 0.34 vs 1.00 ± 0.44, respectively, P < 0.01; protein: 1.13 ± 0.42 vs 0.09 ± 0.03 and 1.16 ± 0.43 vs 0.09 ± 0.00, respectively, P < 0.01). This indicates that EMT occurred in ALF rats. In addition, the TGF-β1/Smad signaling pathway was activated in ALF rats, as evidenced by the increase in TGF-β1 and snail levels (mRNA: 1.76 ± 0.12 vs 1.00 ± 0.05 and 6.98 ± 0.41 vs 1.00 ± 0.10, respectively, P < 0.01; protein: 1.43 ± 0.05 vs 0.12 ± 0.03 and 1.07 ± 0.29 vs 0.07 ± 0.02, respectively, P < 0.01) and the decrease in Smad3 levels (mRNA: 0.05 ± 0.01 vs 1.00 ± 0.12, P < 0.01; protein: 0.06 ± 0.05 vs 0.89 ± 0.12, P < 0.01). Furthermore, levels of the tight junction markers occludin, ZO-1 and claudin decreased in ALF rats compared with healthy control rats (mRNA: 0.60 ± 0.09 vs 1.00 ± 0.12, 0.11 ± 0.00 vs 1.00 ± 0.12 and 0.60 ± 0.01 vs 1.00 ± 0.08, respectively, P < 0.01; protein: 0.05 ± 0.01 vs 0.87 ± 0.40, 0.09 ± 0.05 vs 0.89 ± 0.18 and 0.04 ± 0.03 vs 0.95 ± 0.21, respectively, P < 0.01). In ALF rats treated with QGHXR, E-cadherin levels increased (mRNA: QGHXR 0.67 ± 0.04 vs ALF model 0.16 ± 0.05, P < 0.01; protein: QGHXR 0.66 ± 0.21 vs ALF model 0.09 ± 0.05, P < 0.01), and vimentin and fibronectin levels decreased (mRNA: 6.57 ± 1.05 vs 11.43 ± 0.39 and 1.45 ± 1.51 vs 9.91 ± 0.34, respectively, P < 0.01; protein: 0.09 ± 0.03 vs 1.13 ± 0.42 and 0.10 ± 0.01 vs 1.16 ± 0.43, respectively, P < 0.01). In addition, QGHXR inhibited the expression of TGF-β1 and increased the expression of Smad3 (mRNA: 1.03 ± 0.11 vs 1.76 ± 0.12, 0.70 ± 0.10 vs 0.05 ± 0.01, respectively, P < 0.05 and P < 0.01; protein: 0.12 ± 0.03 vs 1.43 ± 0.05 and 0.88 ± 0.20 vs 0.06 ± 0.05, respectively, P < 0.01). QGHXR treatment also reduced the levels of the EMT-inducing transcription factor snail (mRNA: 2.28 ± 0.33 vs 6.98 ± 0.41, P < 0.01; protein: 0.08 ± 0.02 vs 1.07 ± 0.29, P < 0.01) and increased the occludin, ZO-1 and claudin levels (mRNA: 0.73 ± 0.05 vs 0.60 ± 0.09, 0.57 ± 0.04 vs 0.11 ± 0.00 and 0.68 ± 0.03 vs 0.60 ± 0.01, respectively, P < 0.01, P < 0.01 and P < 0.05; protein: 0.92 ± 0.50 vs 0.05 ± 0.01, 0.94 ± 0.22 vs 0.09 ± 0.05 and 0.94 ± 0.29 vs 0.04 ± 0.03, respectively, P < 0.01). The effects of QGR and HXR on the TGF-β1/Smad signaling pathway were similar to that of QGHXR; however, the QGR- and HXR-induced changes in vimentin mRNA levels, the QGR-induced changes in fibronectin mRNA levels and the HXR-induced changes in snail and TGF-β1 mRNA levels were not significant.
CONCLUSION: Qinggan Huoxue Recipe inhibits EMT in ALF rats by modulating the TGF-β1/Smad signaling pathway, suggesting that the mechanism underlying the amelioration of ALF induced by QGHXR is associated with this pathway.
Core tip: Epithelial-to-mesenchymal transition (EMT) is a dynamic process by which mature epithelial cells lose their distinct characteristics and acquire a mesenchymal phenotype. EMT is characterized by loss of the expression of the epithelial marker E-cadherin and up-regulation of the mesenchymal markers α-SMA, collagen I, vimentin and fibronectin. Our study provided evidence that QGHXR inhibits EMT in alcoholic liver fibrosis by regulating the transforming growth factor-β1/Smad signaling pathway and that QGHXR-mediated inhibition of EMT might be a promising approach to ameliorating alcoholic liver injury.
- Citation: Wu T, Chen JM, Xiao TG, Shu XB, Xu HC, Yang LL, Xing LJ, Zheng PY, Ji G. Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway. World J Gastroenterol 2016; 22(19): 4695-4706
- URL: https://www.wjgnet.com/1007-9327/full/v22/i19/4695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i19.4695
Alcoholic liver disease (ALD) has become a major cause of morbidity and mortality worldwide[1]. ALD progresses from a healthy liver to alcoholic steatosis, steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[2]. Although great efforts to explore potential therapeutic targets for alcoholic liver fibrosis (ALF) have been made in recent decades, effective therapies for ALF remain an unmet need.
Epithelial-to-mesenchymal transition (EMT) is a remodeling process that occurs in adult tissues in response to pathological states, such as carcinogenesis and fibrosis[3]. EMT, a phenotypic conversion of the epithelium to a fibroblastic or myofibroblastic phenotype, plays a well-established role in the induction of fibrogenesis[4]. EMT generally begins with the dissociation of adhesions between epithelial cells, the decrease of apical-basal polarity, the reorganization of the actin cytoskeleton and the increase of cell motility[5]. Although multiple studies have reported the significance of the EMT in fibrogenesis, the precise mechanisms underlying EMT in this context are only partially understood.
Numerous studies have demonstrated that cytokines, including transforming growth factor-β (TGF-β), epidermal growth factor (EGF) and hepatocyte growth factor (HGF), can induce EMT[6-8]. In addition, various signaling pathways associated with EMT have been found to be activated in EMT, including the TGF-β/Smad signaling pathway.
The traditional Chinese medicine formula Qinggan Huoxue Recipe (QGHXR) exerts many pharmacological effects that can ameliorate ALD, including reversing steatosis, mediating lipid peroxidation resistance and decreasing the levels of inflammatory cytokines[9,10]. A recent study demonstrated that QGHXR activated the lipopolysaccharide-Kupffer cell (LPS-KC) signaling pathway in rats with ALD by reducing serum alanine transaminase (ALT), aspartate transaminase (AST) levels and modulating CD14, NF-κB, TLR4, ERK and TNF-α expression and that QGHXR alleviated pathological changes associated with ALD[11].
However, the molecular mechanisms by which QGHXR inhibits ALD have yet to be fully elucidated. Here, we investigated the potential physiological and molecular mechanisms underlying QGHXR-mediated inhibition of EMT, especially regulation of the TGF-β/Smad signaling pathway, in an ALF rat model.
QGHXR (bupleurum root 9 g, scutellaria root 9 g, red sage root 15 g, Carapax trionycis 9 g and Radix puerariae 15 g), Qinggan Recipe (QGR: bupleurum root 9 g and scutellaria root 9 g) and Huoxue Recipe (HXR: red sage root 15 g, Carapax trionycis 9 g and Radix puerariae 15 g), were used at concentrations of 4.75, 1.5 and 3.25 g/mL, respectively, and they were generated at the Department of Pharmacy of Longhua Hospital (Shanghai, China). Antibodies against E-cadherin, vimentin, fibronectin, TGF-β1, Smad3, snail, occludin, zona occludens-1 (ZO-1), claudin and GAPDH were purchased from Abcam (Cambridge, MA, United States). Parazole was purchased from Sigma-Aldrich Co. LLC (St Louis, MO, United States). All other chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
All experiments were approved by the Local Ethics Committee for Animal Research Studies at the Shanghai University of Traditional Chinese Medicine. Male specific pathogen-free SD rats were purchased from Slac Laboratory Animal Center, Inc. (Shanghai, China). A rat ALF model was established by treating the rats with an alcohol mixture (500 mL/L alcohol, 8 mL/kg per day; pyrazole, 24 mg/kg per day; corn oil, 2 mL/kg per day) once per day, accompanied with administering intraperitoneal injections with a 25% solution of CCl4 in olive oil (0.25 mL/kg) twice a week for 12 wk as previously described[12]. After 8 wk, the ALF rats were divided into 4 groups: control, QGR, HXR and QGHXR groups (n = 15 rats per group). Rats in the QGR, HXR, and QGHXR groups were administered a daily treatment dose of 137.5, 62.5, 200 mg/kg, respectively, by gastric lavage. An equal volume of normal saline was administered to the control ALF and healthy control groups (n = 15) by gastric lavage. The treatment lasted 4 wk. All rats were then anesthetized with 2% pentobarbital sodium (2 mL/kg) and sacrificed, and blood and liver tissue specimens were subsequently collected. One section of the liver tissue was fixed for histopathology, and another section was used for real-time polymerase chain reaction (PCR) and Western blot assays. Serum biochemical assays including measurements of ALT, AST levels were conducted using an automatic biochemistry analyzer (Hitachi Ltd, Tokyo, Japan). Serum laminin and hyaluronidase levels were detected by Shanghai Adicon Clinical laboratories Inc.
Hematoxylin and eosin (HE) and Sirius red staining were routinely performed following standard procedures. Histological analysis was performed by HE staining of paraffin-embedded liver sections. Fibrosis was assessed in tissue sections stained with Sirius red (0.3%). Briefly, the fresh liver tissue samples were fixed in 10% formalin and embedded in paraffin. The samples were sliced into 4 μm to 5 μm sections and stained with HE staining solution or with a 0.1% Sirius red-picric solution. The latter sections were washed rapidly with acetic acid. The stained sections were observed and photographed under a light microscope at a magnification of × 200.
Total RNA was isolated from rat liver tissues with TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and reverse-transcribed into cDNA using a Reverse Transcription System (Promega, Madison, WI, United States). The thermal cycling conditions were as follows: 95 °C for 3 min followed by 34 cycles at 95 °C for 30 s, 55 °C for 40 s and 72 °C for 40 s. The mRNA expression levels of E-cadherin, vimentin, fibronectin, TGF-β1, Smad3, snail, occludin, ZO-1 and claudin were quantitatively analyzed and normalized to GAPDH levels. All assays were performed in triplicate. The forward and reverse primer sequences are provided in Table 1.
| E-cadherin | Forward 5′-CACACTGATGGTGAGGGTACAAGG-3′ |
| Reverse 5′-GGGCTTCAGGAACACATACATGG-3′ | |
| Vimentin | Forward 5′-ACCGCTTCGCCAACTACATC-3′ |
| Reverse 5′-GCAACTCCCTCATCTCCTCCT-3′ | |
| Fibronectin | Forward 5′-GACTCGCTTTGACTTCACCAC-3′ |
| Reverse 5′-ATCTCCTTCCTCGCTCAGTTC-3′ | |
| TGF-β1 | Forward 5′-GAGGCGGTGCTCGCTTTGTA-3′ |
| Reverse 5′-GCACTGCTTCCCGAATGTCTG-3′ | |
| Smad3 | Forward 5′-ATACGGATGTTCAAGTGTTCG-3′ |
| Reverse 5′-ACTGGGTCCTCTTTGGTTTT-3′ | |
| Snail | Forward 5′-GTCCTTGCTCCACAAACACCA-3′ |
| Reverse 5′-CTGCCTTCCATCAGCCATCT-3′ | |
| Occludin | Forward 5′-AGATGCTGGTTGCTGGAGAAGT-3′ |
| Reverse 5′-TGGAGACAGGAAACGGATGGT-3′ | |
| ZO-1 | Forward 5′-CGGAGCAGAGAGGAAGAGC-3′ |
| Reverse 5′-GGCAGAACACCATCAGAAGG-3′ | |
| Claudin-1 | Forward 5′-AGGTCTGGCGACATTAGTGG-3′ |
| Reverse 5′-TGGTGTTGGGTAAGAGGTTG-3′ | |
| GAPDH | Forward 5′-TGAGGACCAGGTTGTCTC-3′ |
| Reverse 5′-TCCACCACCCTGTTGCTGTA-3′ |
Protein extracts from rat liver tissues were quantified using the bicinchoninic acid (BCA) method, separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia Biotech, Piscataway, NJ, United States). Nonspecific binding was blocked with 5% nonfat milk in TBST (Tris-buffered saline with Tween) buffer for 2 h at room temperature. The membranes were incubated with primary antibody against E-cadherin (1:1000), vimentin (1:1000), fibronectin (1:1000), TGF-β1 (1:1000), Smad3 (1:1000), snail (1:1000), occludin (1:1000), ZO-1 (1:1000) or claudin (1:1000) overnight at 4 °C and then incubated with the horseradish peroxidase-conjugated secondary antibodies. Finally, blots were visualized using an enhanced chemiluminescence (ECL) detection kit (GE Healthcare, Amersham, United Kingdom), and GAPDH was used as a loading control. Each experiment was repeated three times independently.
All the data are presented as the mean ± SD and analyzed by one-way analysis of variance using SPSS17 software (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered statistically significant. Histograms were generated using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
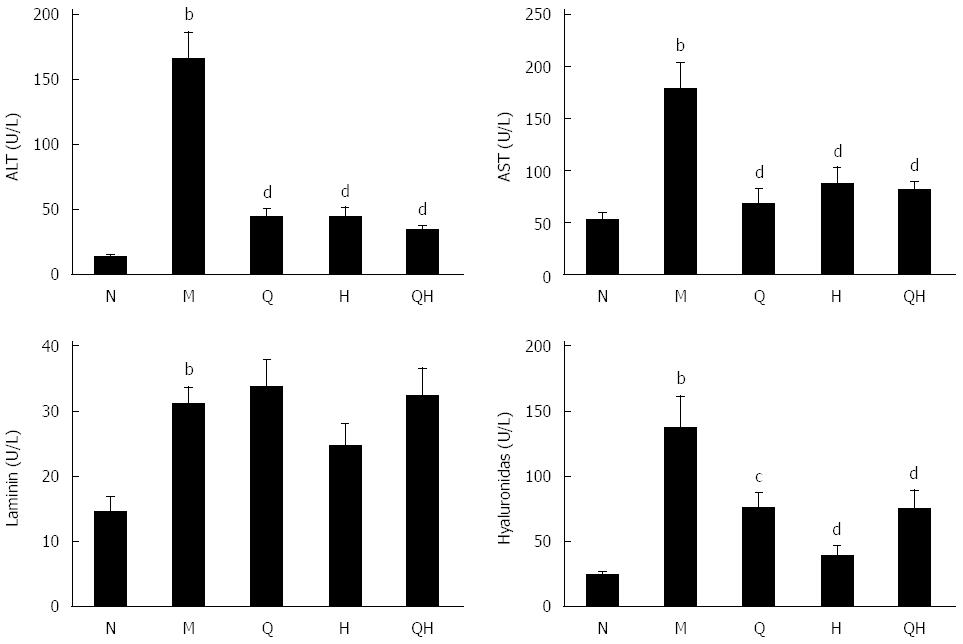
To evaluate the effect of QGHXR on ALF, we generated an alcohol-induced rat model of ALF and measured serum levels of ALT, AST, laminin and hyaluronidase. Rats in the untreated ALF group exhibited signs of alcohol-induced liver injury, such as fibrosis, and the ALT, AST, laminin and hyaluronidase levels in these rats were significantly higher than those in the healthy control group of rats without ALF (Figure 1). Although the QGR, HXR and QGHXR interventions reduced the levels of ALT, AST, and hyaluronidase in rats with ALF, no significant differences in laminin levels were observed (Figure 1).
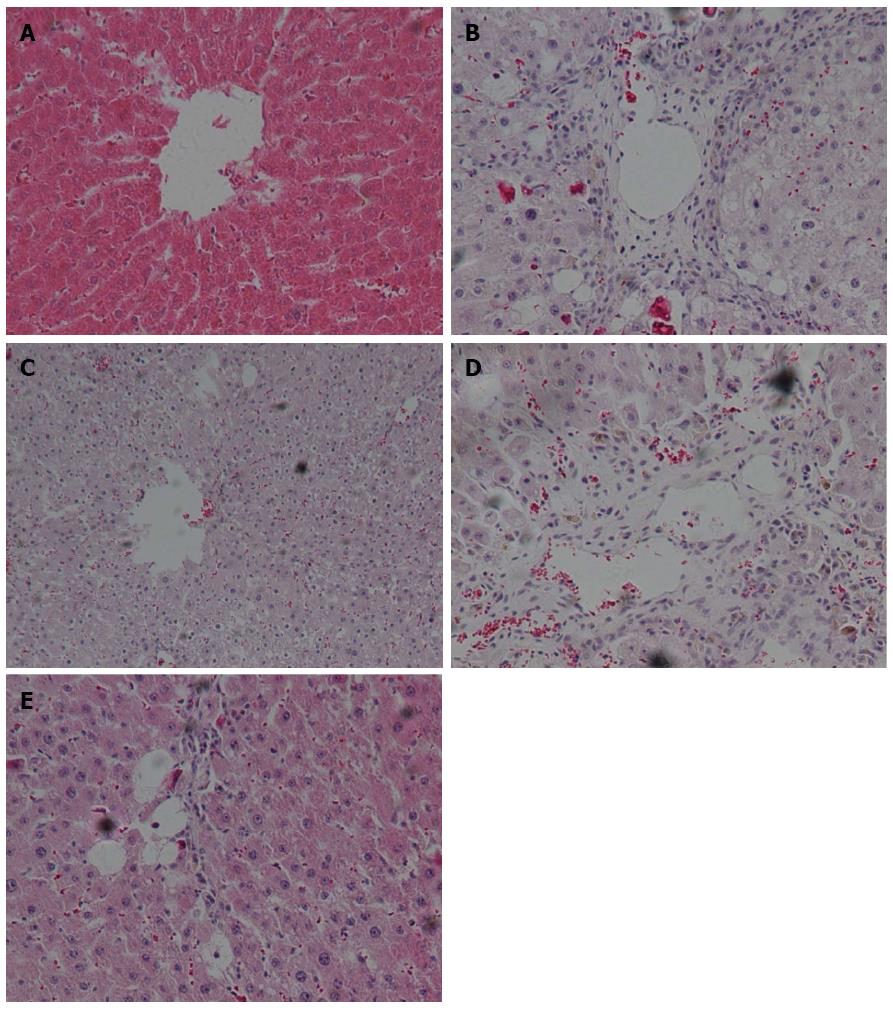
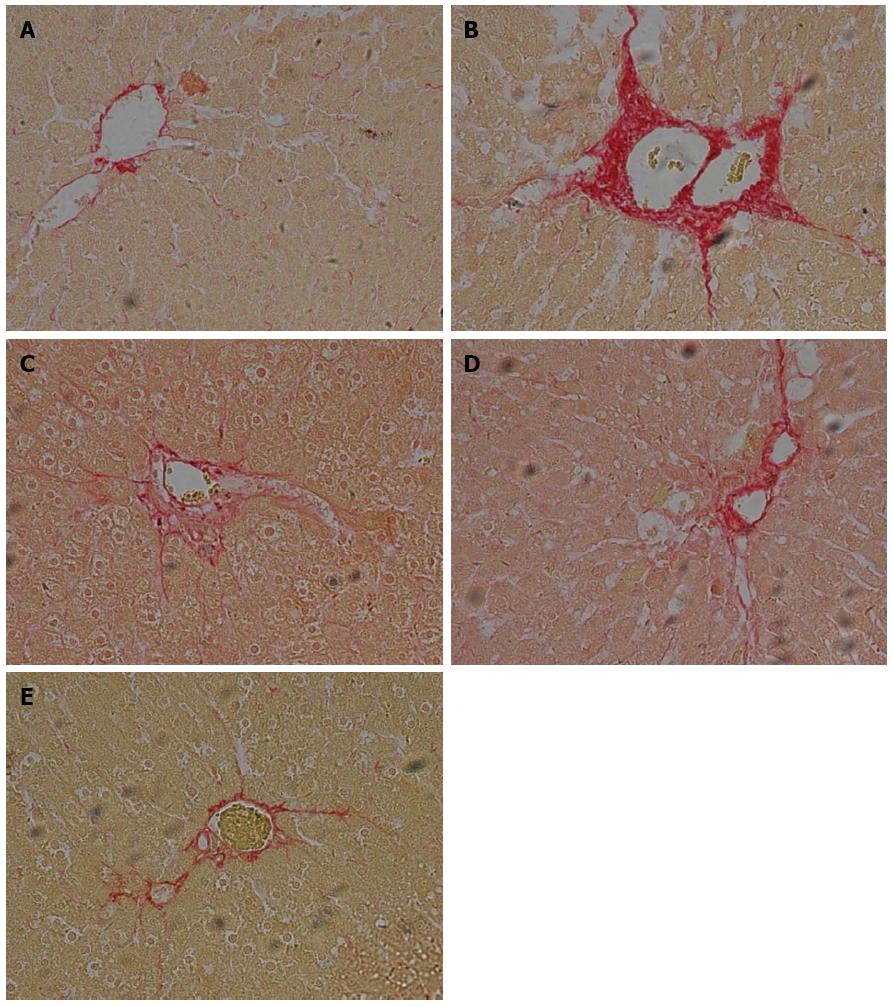
Histological images of liver pathology were also obtained. Liver sections were stained with HE or Sirius staining solution.
In the control group of rats without ALF, no detectable fatty deposits or inflammatory cell infiltrates were observed in images obtained by microscopy. In contrast, relative to the normal control group, the liver sections of rats in the untreated ALF group displayed fat droplet accumulations in hepatocytes and scattered inflammatory cell infiltrates (Figure 2A and B). In addition, collagen fibers surrounding the central vein and portal area, and overt signs of perisinusoidal fibrosis were observed in the untreated ALF group (Figure 3A and B).
Compared with the untreated ALF group, the ALF groups treated with QGR, HXR or QGHXR exhibited improvements in ALF-associated pathological changes, with the greatest improvement observed in the QGHXR-treated group (Figures 2C-E and 3C-E).
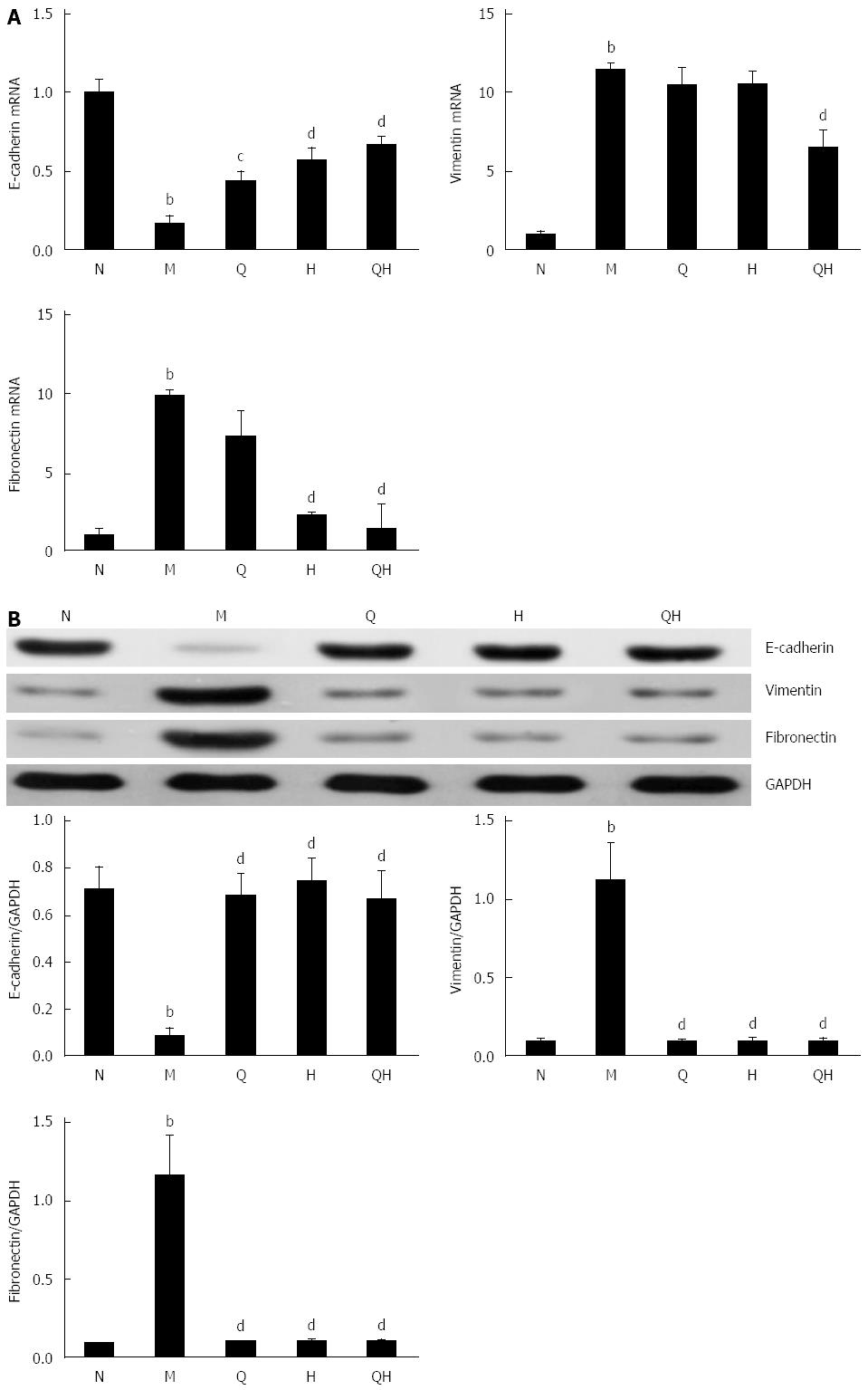
Next, we investigated whether QGHXR ameliorates liver injury in ALF rats by inhibiting TGF-β1-induced EMT. To evaluate EMT, we analyzed the expression of the epithelial marker E-cadherin, and the mesenchymal markers vimentin and fibronectin using PCR and Western blot.
PCR analysis demonstrated that E-cadherin mRNA expression decreased, and vimentin and fibronectin mRNA expression increased in rats with ALF compared with the healthy control group. However, the expression of E-cadherin in ALF rats treated with QGR, HXR or QGHXR gradually returned to the levels observed in the healthy control group (Figure 4A). In addition, a stepwise decrease in the mRNA expression of vimentin and fibronectin was observed in ALF rats treated with QGR, HXR or QGHXR. However, significant changes in the mRNA expression levels of these genes were observed only in the QGHXR-treated group (Figure 4A).
Western blot analysis revealed that E-cadherin protein levels decreased and vimentin and fibronectin protein levels increased in untreated ALF rats than those in the healthy control group, and that treatment with QGHXR, QGR or HXR reversed this effect (Figure 4B).
Collectively, these findings indicate that QGR, HXR and QGHXR inhibited the EMT in ALF rats. However, significant differences in the mRNA and protein levels of the three EMT markers were observed only in the group treated with QGHXR.
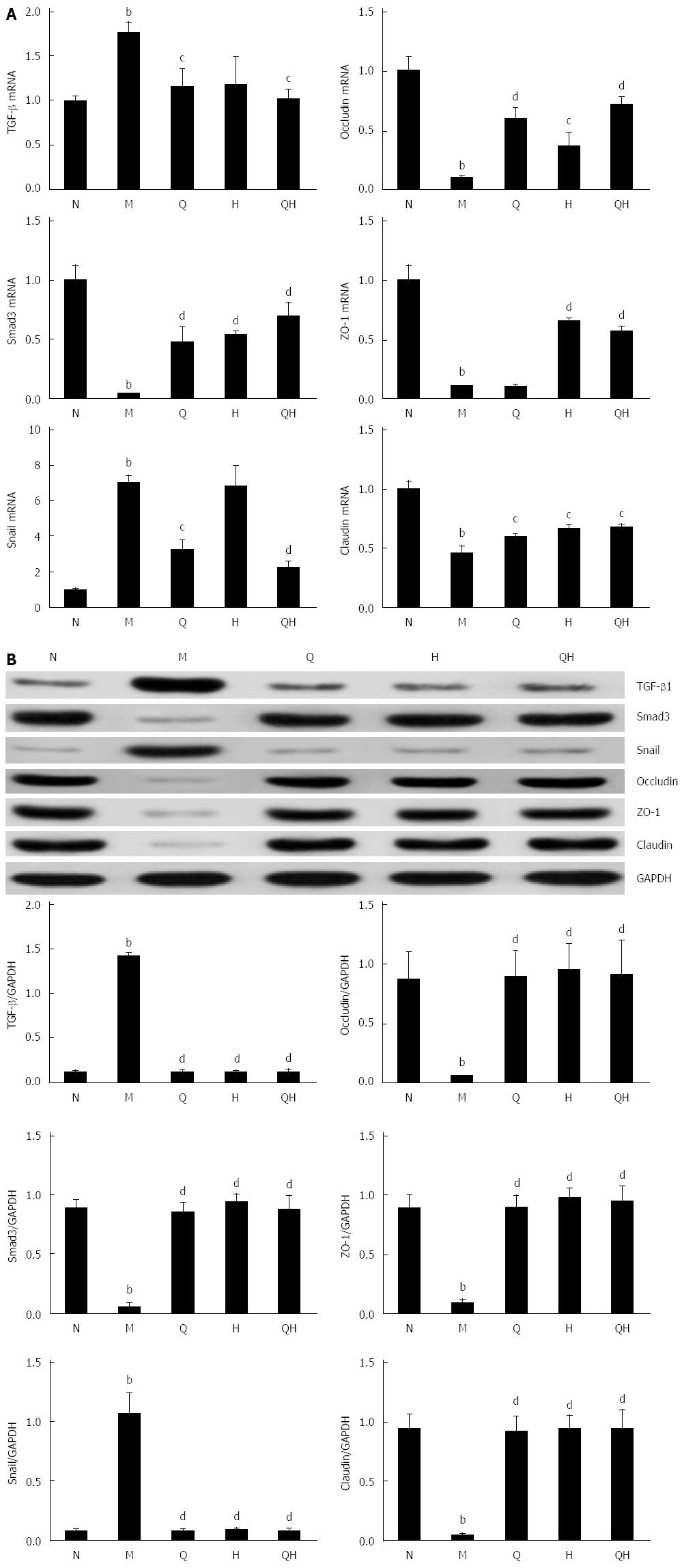
To examine the inhibitory effect of QGHXR on the expression of EMT-associated transcription factors, the expression of Snail, TGF-β1 and Smad3 was evaluated using PCR and Western blot. Compared with the healthy control group, the mRNA and protein expression of Snail was significantly up-regulated in the untreated ALF group (Figure 5); however, treatment with QGR or QGHXR inhibited the Snail mRNA and protein levels, and HXR inhibited the ALF-induced change in Snail protein levels (Figure 5). As the TGF-β/Smad signaling pathway is a critical pathway triggered by the phosphorylation of Smads, we measured the activation status of the Smad signaling pathway. As expected, TGF-β1 levels increased and Smad3 levels decreased in the liver of ALF rats. When QGR, HXR or QGHXR was given to rats with ALF, TGF-β1 and Smad3 protein levels returned to levels similar to those observed in the healthy control group (Figure 5B). The PCR results showed a similar regulation of related molecules; however, in HXR-treated rats, no significant differences in TGF-β1 mRNA levels were observed compared with the untreated control ALF group (Figure 5A). Similar trends in the mRNA and protein expression levels were observed between the three treatment groups.
In addition, the mRNA and protein expression levels of the tight junction markers occludin, ZO-1 and claudin were significantly reduced in the untreated ALF group compared with the healthy group, and treatment with QGR, HXR, or QGHXR reversed these changes (Figure 5).
In the present study, we demonstrated that EMT was observed in rats with ALF and that QGHXR treatment can rescue the mesenchymal phenotype.
EMT is a dynamic process by which mature epithelial cells lose their defining characteristics and acquire a mesenchymal phenotype[13]. During the development of EMT, epithelial cells usually lose their adhesive capability and undergo cytoskeletal rearrangements. Previous studies have reported that EMT is characterized by loss of the expression of the epithelial marker E-cadherin and the up-regulation of the mesenchymal markers α-SMA, collagen I, fibronectin and vimentin[14,15]. In this study, E-cadherin expression decreased and vimentin and fibronectin expression increased in a rat model of ALF, indicating that an EMT model was successfully constructed.
An increasing body of evidence indicates that Chinese medicine formulas have potential value as therapeutic agents or adjuvants in ALD treatment[16]. Our previous studies revealed that QGHXR potentially exerts its therapeutic effect against liver injury via the LPS-KC signal transduction pathway in rats with ALD[11]. In the present study, we demonstrated that ALF rats undergo classic EMT characterized by the up-regulation of the mesenchymal markers vimentin and fibronectin, and the down-regulation of the epithelial marker E-cadherin. We observed that QGR, HXR, and QGHXR ameliorate alcoholic liver injury by reversing EMT, as demonstrated by the increase in E-cadherin expression and the decrease in vimentin and fibronectin expression (except that QGR and HXR showed no significant changes in vimentin mRNA expression, and QGR elicited no changes in fibronectin mRNA expression).
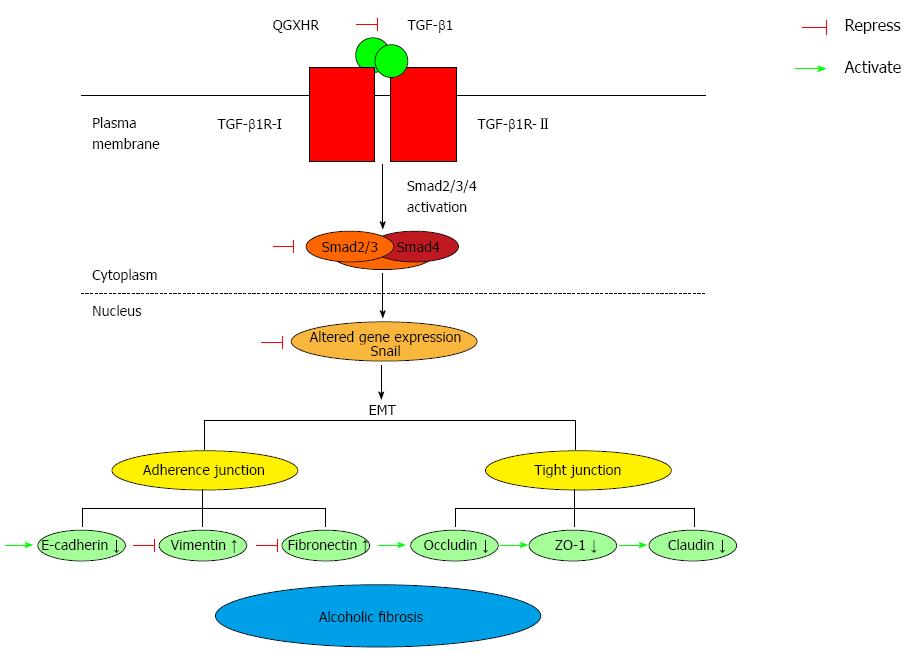
Increasing evidence indicates that TGF-β1 is the primary mediator of EMT and that the TGF-β1/Smad3 signaling pathway is important in the EMT process[17,18]. TGF-β1 binds and phosphorylates cell-surface receptors (TGF-βRI/TGF-βRII), activates TGF-βRI, and phosphorylates Smad2 or Smad3, which subsequently form a complex with Smad4[19,20]. After activation by phosphorylation and partnering with a co-Smad4, the Smad complex translocates to the nucleus and, in conjunction with other transcription factors, directs the activation and repression of genes regulated by TGF-β1[21,22]. Snail, a gene whose expression is regulated by the TGF-β/Smad signaling pathway[23], inhibited E-cadherin expression and promoted EMT. A strong inverse correlation between snail and E-cadherin expression has been reported in a panel of epithelial and dedifferentiated cells derived from carcinomas of different etiologies[24]. In the present study, the protein and mRNA expression levels of TGF-β1 increased in rats with ALF compared with normal rats. These findings are consistent with previous studies reporting that ALF is characterized by excess accumulation of collagen and other extracellular matrix proteins, steatosis, and fibrosis, and the release of the key pro-fibrogenic cytokine TGF-β1, which is mostly produced by bone marrow-derived macrophages and resident Kupffer cells[2,25,26]. The increase in snail and TGF-β1 and the decrease in Smad3 levels observed in the model ALF group further verified that the TGF-β/Smad signaling pathway is activated in ALF rats. In addition, our results demonstrated that the expression of the EMT-inducing transcription factor snail and TGF-β1 was inhibited, and the expression of Smad3 was enhanced in ALF rats treated with QGR, HXR, or QGHXR. However, the differences in snail and TGF-β1 mRNA expression induced by HXR alone were not significant (Figure 6).
EMT is characterized by the downregulation of E-cadherin expression, which results in the disruption of cell-cell junctions and the dissemination of cells from the primary tumor[27]. In hepatocytes, tight junction proteins are critical for maintaining cell polarity. It is well known that claudin proteins are important components of tight junctions in paracellular transport for maintaining the structure and function[28]. Occludin and junction adhesion molecule A are transmembrane proteins that are directly involved in paracellular transport, and ZO-1 is a protein that contains a domain that forms a binding site for other tight junction proteins[29]. In the model ALF group, the mRNA and protein expression of E-cadherin, occludin and ZO-1 decreased compared with the normal healthy control group, which further verified the establishment of EMT in the ALF rat model. QGR, HXR, and QGHXR increased the mRNA and protein levels of tight junction molecules.
There are several deficiencies in our study. First, we did not evaluate the EMT markers E-cadherin, vimentin and fibronectin using immunofluorescence or immunohistochemistry. Second, we did not use gene knockout rats to verify the specific functions of the molecules analyzed in the current study. Third, although liver disease can disrupt endothelial function[30], we did not evaluate the effect of QGHXR on this parameter. Therefore, further studies are needed to verify the findings of this study.
In conclusion, our study provides evidence that QGHXR inhibits EMT in ALF by modulating the TGF-β1/Smad signaling pathway, suggesting that QGHXR ameliorates alcoholic liver injury via this mechanism.
Alcoholic liver disease has become a major cause of morbidity and mortality worldwide. EMT, a well-characterized dynamic process by which epithelial cells transform into a tissue with a fibroblastic or myofibroblastic phenotype, might have a pivotal role in inducing fibrogenesis. Therefore, targeting EMT might be a promising strategy for inhibiting the generation of extracellular matrix, which can provide a new therapeutic target for liver fibrosis.
The TGF-β/Smad signaling pathway plays a pivotal role in EMT, cell proliferation and metastasis. This pathway is currently a hot topic in carcinogenesis and fibrosis studies.
EMT is a dynamic process by which mature epithelial cells lose their defining characteristics and acquire a mesenchymal phenotype. The present study demonstrated that the EMT is characterized by the down-regulation of the epithelial marker E-cadherin and the up-regulation of the mesenchymal markers vimentin and fibronectin in ALF rats. QGHXR reversed the EMT-induced changes in E-cadherin, vimentin and fibronectin levels and inhibited ALF by regulating the TGF-β1/Smad signaling pathway.
The present study provides evidence that QGHXR, a traditional Chinese medicine recipe, inhibits ALF-induced EMT by modulating the TGF-β1/Smad signaling pathway, suggesting that the potential mechanism by which QGHXR ameliorates alcoholic-induced liver injury is associated with this pathway. Thus, EMT represents a novel therapeutic target for ALF and the findings of this study provide new insight into the pathogenesis of ALF.
EMT is a phenotypic conversion of the epithelium to a fibroblastic or myofibroblastic phenotype. EMT begins with the dissociation of adhesions between epithelial cells, the decrease of apical-basal polarity, the reorganization of the actin cytoskeleton and the increase of cell motility.
This study “Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway” is very interesting.
P- Reviewer: Scicchitano P S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4116] [Article Influence: 205.8] [Reference Citation Analysis (3)] |
| 3. | Kong D, Zhang F, Shao J, Wu L, Zhang X, Chen L, Lu Y, Zheng S. Curcumin inhibits cobalt chloride-induced epithelial-to-mesenchymal transition associated with interference with TGF-β/Smad signaling in hepatocytes. Lab Invest. 2015;95:1234-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | van Zijl F, Mair M, Csiszar A, Schneller D, Zulehner G, Huber H, Eferl R, Beug H, Dolznig H, Mikulits W. Hepatic tumor-stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene. 2009;28:4022-4033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Qi HW, Xin LY, Xu X, Ji XX, Fan LH. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J Transl Med. 2014;12:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis. Breast Cancer Res. 2009;11:R68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Tian M, Neil JR, Schiemann WP. Transforming growth factor-β and the hallmarks of cancer. Cell Signal. 2011;23:951-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Tirino V, Camerlingo R, Bifulco K, Irollo E, Montella R, Paino F, Sessa G, Carriero MV, Normanno N, Rocco G. TGF-β1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133+ A549 cell fraction. Cell Death Dis. 2013;4:e620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Chen JM, Wang L, Xing LJ. [Regulatory effects of Qinggan Huoxue Recipe on matrix metalloproteinases of alcoholic liver fibrosis rats]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2011;31:1538-1544. [PubMed] |
| 10. | Wu T, Liu T, Zheng PY, Xing LJ, Ji G. [Effects of Qinggan Huoxue Recipe and its separated recipes on the expression of tumor necrosis factor-alpha in rats with alcoholic liver injury]. Zhong Xi Yi Jie He Xuebao. 2008;6:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wu T, Liu T, Zhang L, Xing LJ, Zheng PY, Ji G. Chinese medicinal formula, Qinggan Huoxue Recipe protects rats from alcoholic liver disease via the lipopolysaccharide-Kupffer cell signal conduction pathway. Exp Ther Med. 2014;8:363-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ji G, Wang L, Zhang SH, Liu JW, Zheng PY, Liu T. Effect of Chinese medicine Qinggan Huoxuefang on inducing HSC apoptosis in alcoholic liver fibrosis rats. World J Gastroenterol. 2006;12:2047-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 1049] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 14. | Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Liu J, Zeng L, Zhao Y, Zhu B, Ren W, Wu C. Selenium suppresses lipopolysaccharide-induced fibrosis in peritoneal mesothelial cells through inhibition of epithelial-to-mesenchymal transition. Biol Trace Elem Res. 2014;161:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Lv XH, Zhou LP, Liu DP, Wang Y, Wang BY, Fu BY, Song M, Liu CR. Traditional Chinese medicine Kang Xian Fu Fang I is effective for prophylaxis and treatment of alcoholic liver disease in rats. Hepatobiliary Pancreat Dis Int. 2007;6:182-187. [PubMed] |
| 17. | Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Wang A, Ziyadeh FN, Lee EY, Pyagay PE, Sung SH, Sheardown SA, Laping NJ, Chen S. Interference with TGF-beta signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am J Physiol Renal Physiol. 2007;293:F1657-F1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3797] [Cited by in RCA: 4298] [Article Influence: 195.4] [Reference Citation Analysis (0)] |
| 20. | Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 1884] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 21. | Geng J, Fan J, Ouyang Q, Zhang X, Zhang X, Yu J, Xu Z, Li Q, Yao X, Liu X. Loss of PPM1A expression enhances invasion and the epithelial-to-mesenchymal transition in bladder cancer by activating the TGF-β/Smad signaling pathway. Oncotarget. 2014;5:5700-5711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Tu B, Peng ZX, Fan QM, Du L, Yan W, Tang TT. Osteosarcoma cells promote the production of pro-tumor cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF-β/Smad2/3 pathway. Exp Cell Res. 2014;320:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Wen SL, Gao JH, Yang WJ, Lu YY, Tong H, Huang ZY, Liu ZX, Tang CW. Celecoxib attenuates hepatic cirrhosis through inhibition of epithelial-to-mesenchymal transition of hepatocytes. J Gastroenterol Hepatol. 2014;29:1932-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer. 2002;86:98-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1555] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 26. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2161] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 27. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7739] [Article Influence: 483.7] [Reference Citation Analysis (0)] |
| 28. | Fénéant L, Ghosn J, Fouquet B, Helle F, Belouzard S, Vausselin T, Séron K, Delfraissy JF, Dubuisson J, Misrahi M. Claudin-6 and Occludin Natural Variants Found in a Patient Highly Exposed but Not Infected with Hepatitis C Virus (HCV) Do Not Confer HCV Resistance In Vitro. PLoS One. 2015;10:e0142539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Hwang I, An BS, Yang H, Kang HS, Jung EM, Jeung EB. Tissue-specific expression of occludin, zona occludens-1, and junction adhesion molecule A in the duodenum, ileum, colon, kidney, liver, lung, brain, and skeletal muscle of C57BL mice. J Physiol Pharmacol. 2013;64:11-18. [PubMed] |
| 30. | Barone M, Viggiani MT, Amoruso A, Schiraldi S, Zito A, Devito F, Cortese F, Gesualdo M, Brunetti N, Di Leo A. Endothelial dysfunction correlates with liver fibrosis in chronic HCV infection. Gastroenterol Res Pract. 2015;2015:682174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |