Published online May 21, 2016. doi: 10.3748/wjg.v22.i19.4662
Peer-review started: December 1, 2015
First decision: December 31, 2015
Revised: January 11, 2016
Accepted: January 17, 2016
Article in press: January 18, 2016
Published online: May 21, 2016
Processing time: 174 Days and 7.2 Hours
AIM: To determine if expression of colonic tryptophan hydroxylase-2 (TPH2), a surrogate marker of neuronal 5-hydroxytryptamine, is altered in Hirschsprung’s-associated enterocolitis.
METHODS: Entire resected colonic specimens were collected at the time of pull-through operation in children with Hirschsprung’s disease (HSCR, n = 12). Five of these patients had a history of pre-operative Hirschsprung’s-associated enterocolitis (HAEC). Controls were collected at colostomy closure in children with anorectal malformation (n = 10). The distribution of expression of TPH2 was evaluated using immunofluorescence and confocal microscopy. Protein expression of TPH2 was quantified using western blot analysis in the deep smooth muscle layers.
RESULTS: TPH2 was co-expressed in nitrergic and cholinergic ganglia in the myenteric and submucosal plexuses in ganglionic colon in HSCR and healthy controls. Co-expression was also seen in submucosal interstitial cells of Cajal and PDGFRα+ cells. The density of TPH2 immuno-positive fibers decreased incrementally from ganglionic bowel to transition zone bowel to aganglionic bowel in the myenteric plexus. Expression of TPH2 was reduced in ganglionic bowel in those affected by pre-operative HAEC compared to those without HAEC and healthy controls. However, expression of TPH2 was similar or high compared to controls in the colons of children who had undergone diverting colostomy for medically refractory HAEC.
CONCLUSION: Altered TPH2 expression in colonic serotonergic nerves of patients with HSCR complicated by HAEC may contribute to intestinal secretory and motor disturbances, including recurrent HAEC.
Core tip: Despite optimal surgery, children with a history of Hirschsprung’s disease (HSCR) complicated by Hirschsprung’s-associated enterocolitis (HAEC) are at higher risk of long-term colonic dysfunction. Tryptophan hydroxylase-2 (TPH2) is a surrogate marker for neuronal 5-hydroxytryptamine (5-HT). We hypothesized that expression of TPH2 is altered in the colon of children with HAEC. We found the density of serotonergic nerves to be differentially reduced in the ganglionic colon of children with HSCR and HAEC compared to those without HAEC compared to controls, although expression is normalized in those with diverting colostomy. Abnormal neuronal 5-HT expression may contribute to post-operative colonic dysfunction in HSCR.
- Citation: Coyle D, Murphy JM, Doyle B, O’Donnell AM, Gillick J, Puri P. Altered tryptophan hydroxylase 2 expression in enteric serotonergic nerves in Hirschsprung’s-associated enterocolitis. World J Gastroenterol 2016; 22(19): 4662-4672
- URL: https://www.wjgnet.com/1007-9327/full/v22/i19/4662.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i19.4662
Hirschsprung’s-associated enterocolitis (HAEC) is the most serious complication of Hirschsprung’s disease (HSCR) and is the leading cause of disease-related mortality. It occurs in 17%-50% of patients with HSCR and may occur before or after a pull-through operation[1,2]. Although a scoring system for HAEC exists, there is no agreed definition of this condition. It is typically described as an inflammatory disease of the colon leading to a spectrum of symptoms ranging from abdominal distension and loose stools to life-threatening toxic megacolon[3,4]. The etiology and pathogenesis of HAEC are still incompletely understood. It has been proposed that intestinal barrier dysfunction, abnormal innate immunity and the presence of a disturbed microbiome are all potential contributors to its etiology[1]. However, given that the primary abnormality in HSCR is the absence of enteric ganglia in the distal colon, it follows that the enteric nervous system may have a role in the pathogenesis.
5-Hydroxytryptamine (5-HT), also commonly known as serotonin, is a major neuroendocrine signaling molecule. While the gut is the single largest reservoir of 5-HT, the wide range of its functions therein have only been elucidated relatively recently. The majority of enteric serotonin is stored in the mucosa in the enterochromaffin (EC) cells. Approximately 1%-5% of enteric serotonin is stored in the serotonergic enteric nerves, where it acts as a neurotransmitter[5,6]. The rate limiting step in the synthesis of 5-HT is the conversion of L-tryptophan to 5-hydroxytryptophan by tryptophan hydroxylase (TPH). The conversion of 5-hydroxytryptophan to 5-HT then occurs rapidly through the actions of L-amino acid decarboxylase[7]. However, the synthesis pathway of enteric 5-HT differs depending on whether 5-HT is being synthesized in EC cells, where the TPH1 isozyme of TPH predominates, or in serotonergic neurons, where TPH2 predominates. In this way, it is possible to indirectly evaluate the expression of neuronal 5-HT separately from that produced in EC cells[5,7].
5-HT has many roles, including activation of intrinsic reflexes such as peristalsis, vasodilation, and secretion. When released from EC cells, mucosal 5-HT has been demonstrated to promote inflammation - an activity which is counterbalanced by its re-uptake via the serotonin transporter (SERT)[5,8]. Abnormal mucosal 5-HT activity has been demonstrated in inflammatory and functional bowel disorders such as ulcerative colitis and irritable bowel syndrome[9]. Conversely, enteric neuronal 5-HT is anti-inflammatory and neuroprotective, an activity which has obvious importance in the setting of inflammation and enterocolitis, as neuronal damage can frequently result[8]. It has previously been reported that populations of mucosal EC cells are deficient in the ganglionic bowel of children with HSCR who have previously had HAEC. It is unclear if this is a facilitator or an effect of HAEC[10].
We hypothesized that, in children who had previously been treated for HAEC, neuronal 5-HT expression is altered compared to those who did not require treatment for HAEC. In this study we aimed to investigate the distribution of 5-HT in the aganglionic and ganglionic colon of children with HSCR and in healthy controls and to quantify expression of TPH2 in serotonergic enteric nerves of these patients.
The study was approved by the ethics committees of both centers (Our Lady's Children's Hospital Ethics Committee, GEN292.12; Temple Street Children's University Hospital Research and Ethics Committee, 13.003). Informed written consent was obtained from parents/legal guardians prior to specimen collection. The procedures carried out during the study were in conformance with the principles expressed in the Declaration of Helsinki.
Full-length colonic specimens resected during pull-through operations for HSCR were obtained fresh from 12 patients, incorporating aganglionic, transition zone and ganglionic bowel (age range 3 mo-14 mo). Colonic control specimens were similarly obtained from the proximal colostomy limb of 10 patients at the time of descending/sigmoid colostomy closure in children following surgical correction of anorectal malformation (age range 7 mo-21 mo). The level of the most proximal extent of the transition zone was routinely confirmed by 3,3’-diaminobenzidine (DAB) immunohistochemistry probing for protein gene product 9.5 (PGP 9.5), which stains nerve cells. All experiments incorporated comparison of ganglionic bowel in HSCR with transition zone and aganglionic bowel as well as healthy controls.
Colonic sections were embedded in OCT compound [VWR, Ireland (361603E)] and snap frozen in liquid nitrogen. Twenty micron sections were cut and were fixed in 10% neutral buffered formalin (Sigma-Aldrich, Ireland [HT501128-4L]). Cell membranes were permeabilized by rinsing in 1% w/v PBS with 1% Triton X-100. Sections were blocked in 10% bovine serum albumin [BSA, Sigma-Aldrich, Ireland (A2153-50G)] diluted in 1% w/v PBS with 0.05% Tween® [Sigma-Aldrich, Ireland (P1379)] (PBST) for 90 min at room temperature to prevent non-specific antibody binding. Samples were incubated simultaneously in both primary antibodies of interest, diluted in 10% BSA, at 4 °C overnight. Antibodies to the following antigens were used to label specific cell types in the colonic wall: HuD (PGP 9.5) was used to label nerve cells; TMEM16A [anoctamin-1 (ANO1)])was used to label interstitial cells of Cajal (ICCs); platelet derived growth factor receptor-α (PDGFRα) was used to label PDGFRα+ cells, neuronal nitric oxide synthase (nNOS) was used to label nitrergic neurons, vasoactive intestinal peptide (VIP) was used to label peptidergic neurons, and choline acetyltransferase was used to label cholinergic neurons. A detailed description of the primary antibodies used in the study is seen in Table 1. Following incubation in primary antibody solution, samples were rinsed intensively in 1% PBST, following which they were incubated in a solution containing both secondary antibodies specific to the host species of each primary antibody (Table 1), diluted in 10% BSA, for 90 min at room temperature. After intensive rinsing in 1% PBST, samples were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) nuclear counterstain [Thermo Scientific, Ireland (EN62248)]. Sections were mounted with glass coverslips using Mowiol® 4-88 fluorescence mounting medium [Sigma Aldrich, Ireland (81381-50G)], which was constituted according to manufacturer’s specifications. Specimens were visualized using laser scanning confocal microscopy (LSM700 Confocal Microscope, Carl Zeiss MicroImaging GmbH, Jena, Germany). Resulting images were processed, including calculation of TPH2 immuno-positive cell counts, using ImageJ - an open-access software available from http://imagej.nih.gov/ij/.
| Host species | Monoclonal/Polyclonal | Antigen | Product code | Manufacturer | Dilution |
| Rabbit | Polyclonal | Tryptophan hydroxylase 2 | NB-100-74555 | Novus biologicals (Cambridge, United Kingdom) | 1:100 |
| Mouse | Monoclonal | TMEM-16A (ANO1) | Ab190721 | Abcam (Cambridge, United Kingdom) | 1:200 |
| Mouse | Monoclonal | HuD | sc-48421 | Santa-Cruz Biotechnologies (Heidelberg, Germany) | 1:100 |
| Mouse | Monoclonal | Platelet-derived growth factor alpha | sc-21789 | Santa-Cruz Biotechnologies (Heidelberg, Germany) | 1:100 |
| Goat | Polyclonal | Neuronal nitric oxide synthase | ab1376 | Abcam (Cambridge, United Kingdom) | 1:300 |
| Mouse | Monoclonal | Vasoactive intestinal peptide | sc-25347 | Santa-Cruz Biotechnologies (Heidelberg, Germany) | 1:100 |
| Mouse | Monoclonal | Choline acetyltransferase | ab49382 | Abcam (Cambridge, United Kingdom) | 1:100 |
| Mouse | Monoclonal | GAPDH | ab9484 | Abcam (Cambridge, United Kingdom) | 1:2000 |
| Rabbit | Polyclonal | Mouse (secondary) | ab6728 | Abcam (Cambridge, United Kingdom) | 1:10000 |
| Donkey | Polyclonal | Rabbit (secondary) | ab6802 | Abcam (Cambridge, United Kingdom) | 1:10000 |
| Donkey | Polyclonal | Rabbit (Alexa Fluor® 488) | ab150073 | Abcam (Cambridge, United Kingdom) | 1:500 |
| Goat | Polyclonal | Mouse (Alexa Fluor® 594) | ab150116 | Abcam (Cambridge, United Kingdom) | 1:500 |
| Donkey | Polyclonal | Goat (Alexa Fluor® 555) | ab150134 | Abcam (Cambridge, United Kingdom) | 1:500 |
The mucosa was dissected from the deep smooth muscle layers at the time of specimen collection. Protein was extracted from the tunica muscularis layers to limit the probability of unwanted antibody binding to tryptophan hydroxylase 1 (TPH1), of which the mucosa is a substantial reservoir. Bowel tissue fragments were homogenised using a tissue homogeniser in radioimmunoprecipitation (RIPA) buffer containing 1% protease inhibitor cocktail [Sigma Aldrich, Ireland (P2714)]. Soluble and insoluble fractions were then separated by centrifugation at 4 °C at 3000 g over 30 min. The concentration of the supernatant was determined by means of a Bradford assay [Sigma-Aldrich Ltd., Arklow, Ireland (B6916)] using a standard curve generated from known concentrations of BSA. Novex® Bolt® LDS sample buffer and reducing agent [Biosciences, Dublin, Ireland (B0007 and B0009 respectively)] were added to each protein aliquot according to manufacturer’s protocols. The protein concentration of each sample was then equilibrated with the addition of deionised water. Samples were denatured at 70 °C for 10 min and were then loaded onto an SDS polyacrylamide gel [Bolt® Novex 4%-12% Bis-Tris gel: Biosciences, Dublin, Ireland (NW04120BOX)] in NuPAGE® MES SDS running buffer [Biosciences, Dublin, Ireland (NP0002)] and separated by electrophoresis at 150 V. Proteins were then transferred from the gel to a 0.45 μm PVDF membrane at 30 V for 90 min.
Membranes were blocked in 3% dried skimmed milk dissolved in 1% PBST for 1 h to prevent non-specific antibody binding and were then incubated in primary antibody (Table 1) diluted in 10% BSA overnight at 4 °C. Membranes were rinsed in 1% PBST for 4 h and were then incubated in species-specific secondary antibody (Table 1) for 90 min at room temperature. Following further rinses with 1% PBST for a minimum of 1 h, membranes were incubated in chemiluminescent substrate [SuperSignal™ West Pico Chemiluminescent Substrate, Thermo-Fischer, Ireland (34079)] for 5 min at room temperature before transfer to a chemiluminescence cassette for blot visualization. Protein expression levels were semi-quantitatively evaluated by densitometric analysis using the open-access image processing software ImageJ. Statistical analysis was performed using a statistical software package (SPSS v20.0). Non-parametric analysis including Mann-Whitney U-test was utilized in testing for differences in TPH2 protein expression between ganglionic and aganglionic colon in HSCR and healthy controls.
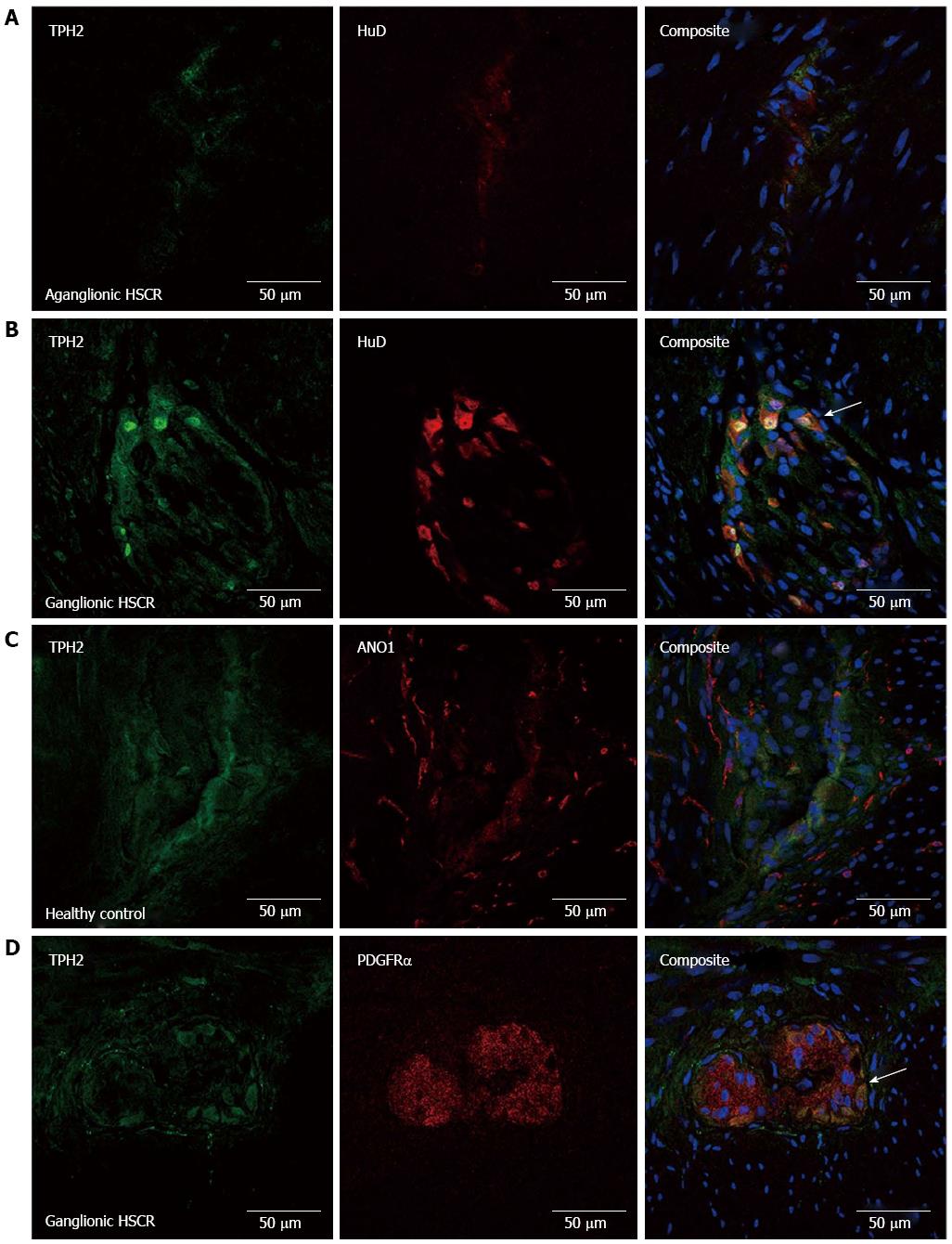
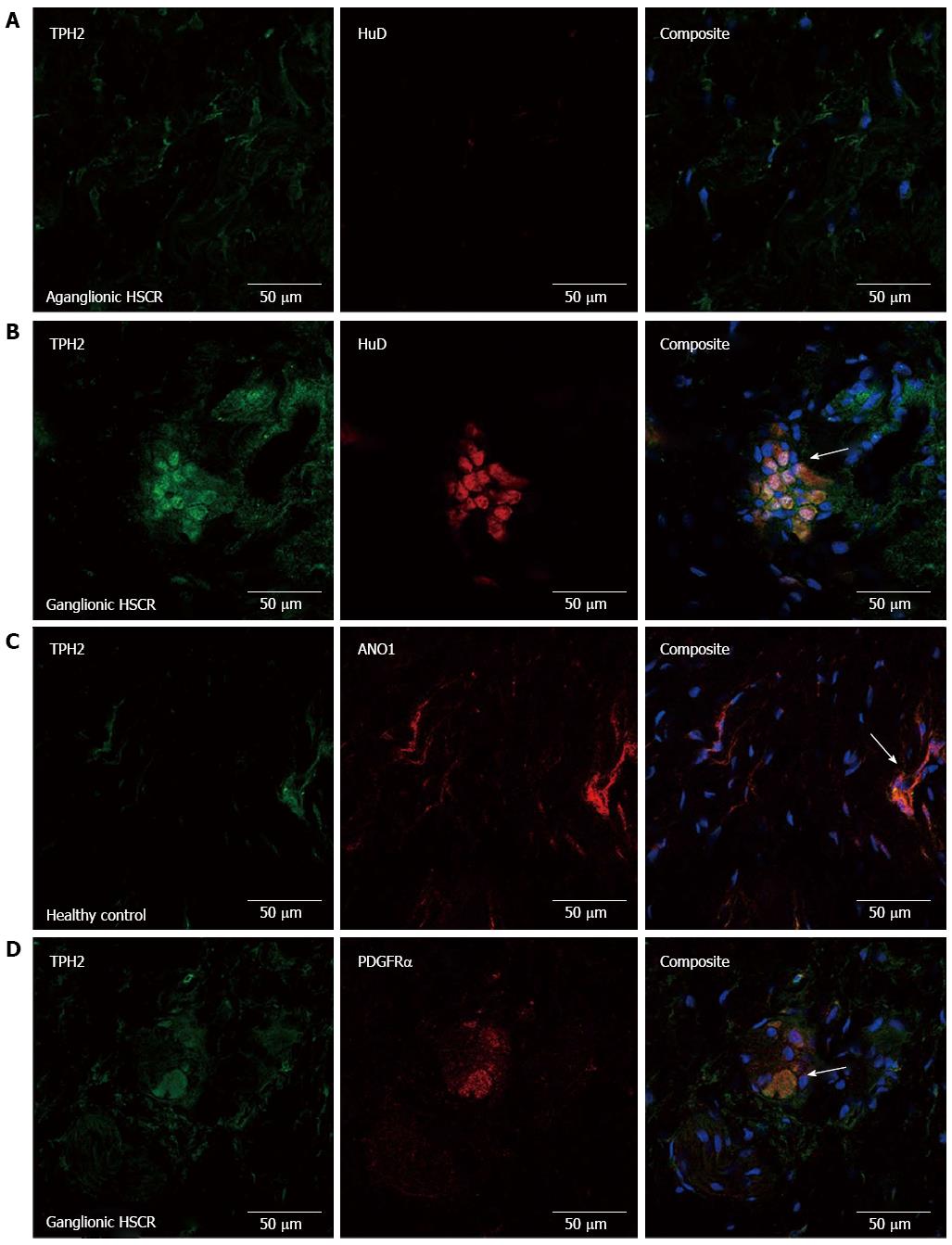
Basic clinical details regarding patients whose specimens were used in this study can be seen in Table 2. The specificity of our antibody to detect neuronal TPH2 was confirmed by the presence of TPH2 co-expression with HuD, a specific marker of nerve cell bodies, seen in the myenteric plexus of normally ganglionated bowel in HSCR and in healthy controls, while no evidence of expression of either was seen in the myenteric plexus of aganglionic bowel in HSCR (Figure 1A and B). There was a dense network of ANO1-immuno-positive ICC fibers seen in the myenteric plexus. There was no co-expression of TPH2 with ANO1 (Figure 1C), although the processes of ICCs did form dense network around ganglia immuno-positive for TPH2. Of interest, there was partial co-expression of TPH2 with PDGFRα in the cell bodies of PDGFRα+ fibroblast-like cells in the myenteric plexus (Figure 1D).
| Characteristic | n = 12 | |
| Gender | Male (n = 11) | Female (n = 1) |
| Median age at pull-through operation | 5 mo | 3-14 mo |
| Associated syndromes | Trisomy 21 | n = 5 (2 with HAEC) |
| Pre-operative HAEC | Yes (n = 5) | No (n = 7) |
| Diverting/levelling stoma | Yes (n = 3) | No (n = 9) |
In the submucosa of aganglionic bowel, HuD-positive nerve cell bodies were absent, although TPH2-positive fibers were still present in reduced density (Figure 2A). Confirmation of the presence of TPH2-immuno-positive nerve cell bodies in the submucosa of ganglionated bowel is seen in Figure 2B. TPH2 was co-expressed with ANO1 in the cell processes of submucosal ICCs (Figure 2C) and with PDGFRα in the cell bodies of PDGFRα+ cells (Figure 2D).
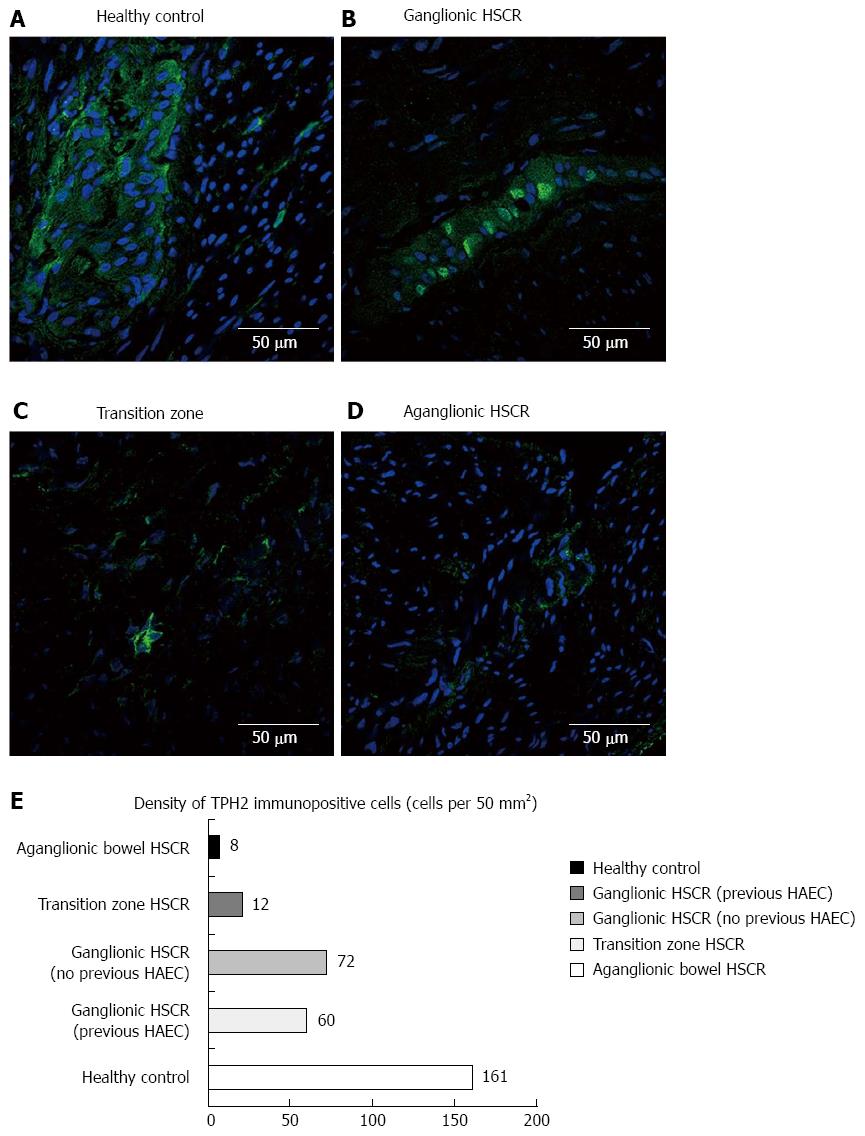
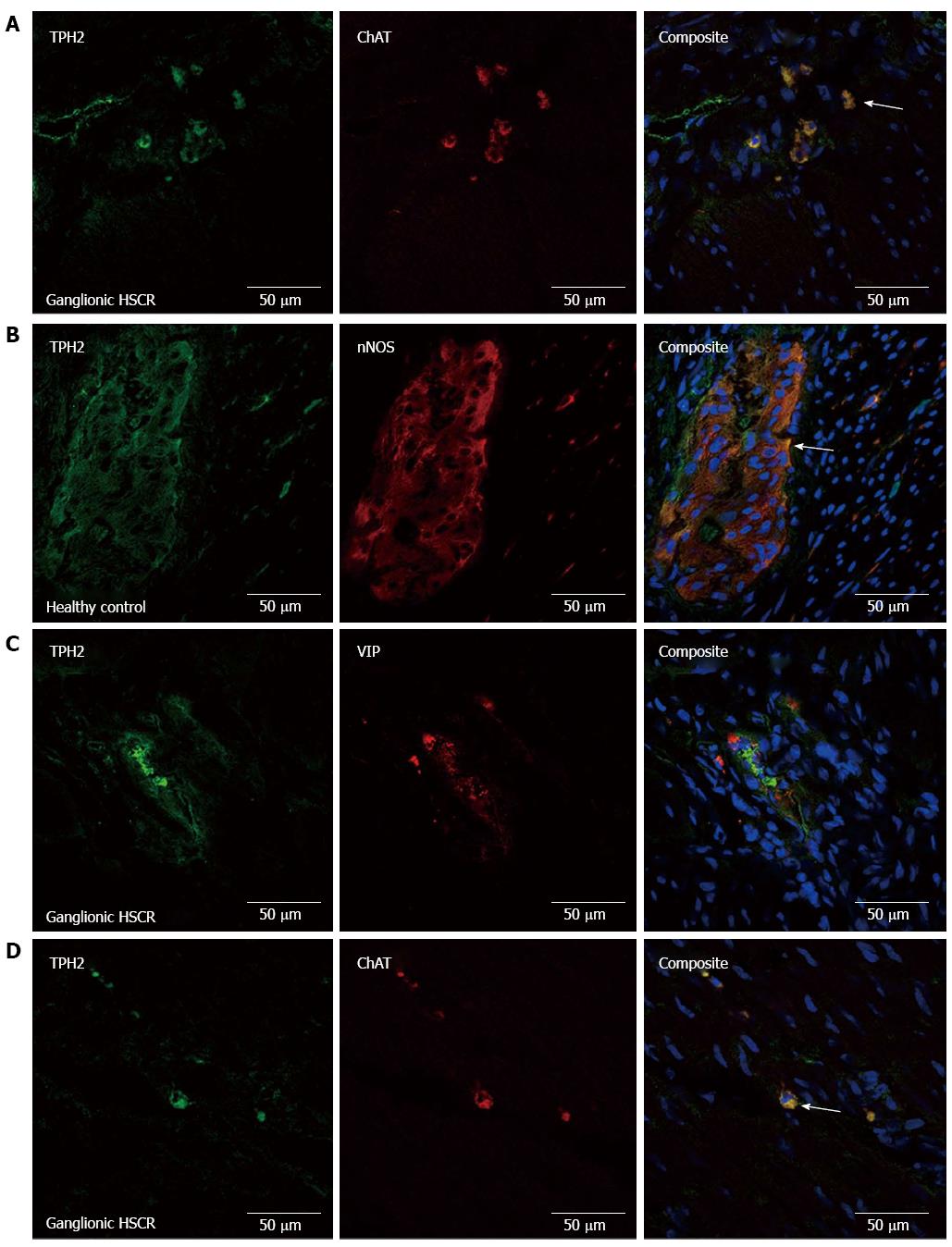
The density of TPH2-immuno-positive cells reduced incrementally from colonic controls (Figure 3A) to ganglionic bowel in HSCR (Figure 3B), transition zone (Figure 3C) and aganglionic bowel (Figure 3D), where expression of TPH2 appeared markedly reduced in the myenteric and submucosal plexuses (Figure 3E). TPH2 was found to be co-expressed with ChAT-positive cholinergic nerve cell bodies in the myenteric plexus (Figure 4A), as well as with nNOS in nitrergic ganglia (Figure 4B). VIP-immuno-positive neurons did not co-express TPH2 in the myenteric plexus (Figure 4C) or in the submucosa (data not shown). Cholinergic nerve fibers in the circular muscle co-expressed TPH2 (Figure 4D).
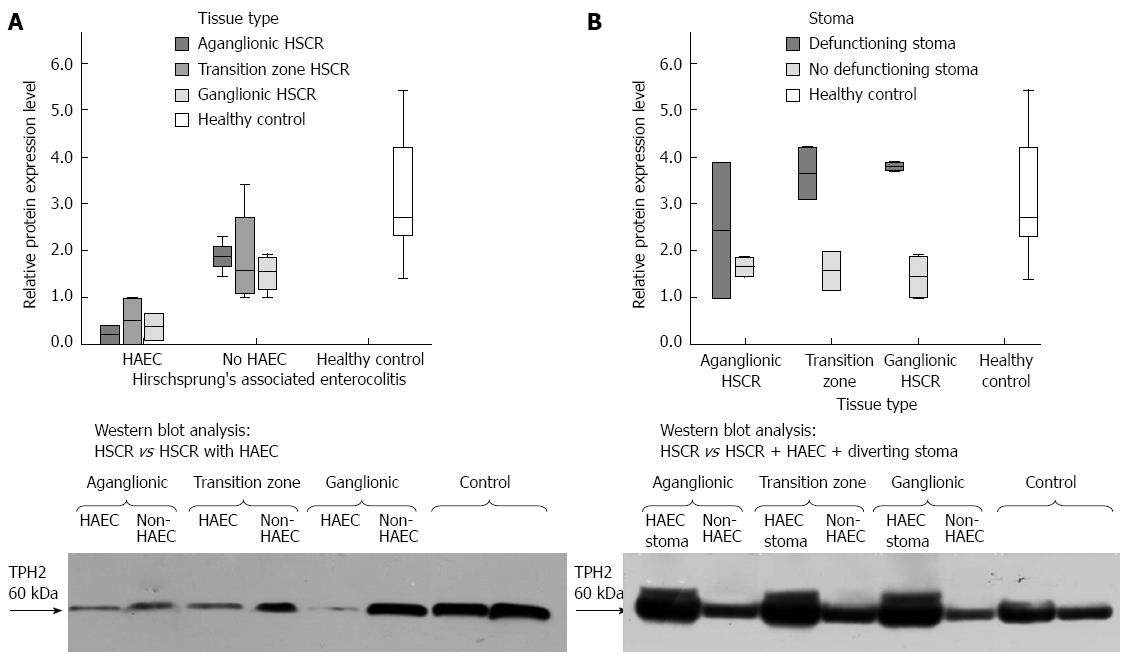
A specific band was detected at approximately 60 kDa, consistent with the molecular weight of TPH2. No doublet bands were observed, confirming specific antibody binding to the TPH2 isozyme, as occurred when Western blot analysis was performed using whole tissue protein extract, which contains significant quantities of TPH1. In patients who had HAEC, TPH2 expression was reduced in the aganglionic bowel, transition zone and ganglionic bowel in HSCR compared to controls (P = 0.044) while there was a trend towards lower expression of TPH2 patients with HSCR who had never been treated for HAEC (n = 7) in aganglionic bowel compared to healthy controls (P = 0.056) (Figure 5A). However, consistent marked recovery of TPH2 expression levels was noted in the colon of patients with HSCR, who had been treated for HAEC, but who had undergone formation of a diverting stoma due to failure of medical management (P = 0.002, n = 3). Such was the recovery that expression levels were even higher than in patients who had HSCR but who did not have a history of pre-operative HAEC (Figure 5B).
Enterocolitis is reported as the presenting feature of HSCR in between 12.5% and 40.5% of cases, with many of these cases occurring in the neonatal period[2,11]. The histopathological changes seen in HAEC range from cryptitis to mucosal ulceration, transmural necrosis and colonic perforation[1]. Only recently has an understanding of the processes contributing to the pathogenesis of enterocolitis been developed. In the colon, the enteric nervous system (ENS) is arranged into two plexuses: a submucosal plexus and a plexus that lies between the two deep smooth muscle layers, known as the myenteric plexus. The role of the myenteric plexus primarily concerns intestinal motility while the submucosal plexus regulates a myriad of epithelial functions such as enteric blood flow, immunity, release of enteric peptides from enteroendocrine cells, and epithelial transport[1,12]. It follows that the absence of a functioning ENS in the colon of those with HSCR will lead to dysregulation of these processes and, consequently, enterocolitis.
Although only 5% of 5-HT in the colon is neuronal in origin, it is notable that, in TPH1 knockout mice, gastrointestinal motility is preserved, even with stripping of the mucosa, indicating that only neuronal 5-HT is involved in gastrointestinal motility[5]. Additionally, animal models have demonstrated that neuronal 5-HT acts as a growth factor during enteric neurogenesis, promoting the development and survival of dopaminergic and GABAergic neurons[5,13]. It is thought that serotonergic neurons are large descending interneurons which, as well as being calbindin-positive, are cholinergic[14]. Our findings concur with this observation, as we observed consistent co-expression of TPH2 with ChAT, a key enzyme in acetyl choline synthesis, in circular muscle nerve fibers.
Serotonergic nerves have previously been shown to make extensive synapses with myenteric ICCs, as well as nitrergic neurons. This indicates a functional role for serotonergic neurons in modulating nitrergic neurotransmission and pacemaker activity. Okamoto et al[15] has also observed that most colonic nitrergic neurons and submucosal ICCs were richly supplied by serotonergic varicosities in murine colon and suggested a potential role for neuronal 5-HT in the regulation of slow wave electrical activity. Liu et al[16] demonstrated that 5-HT acts on the 5-HT3 receptor on ICCs, wherein the receptor functions as a Ca2+-influx mechanism to augment pacemaker activity in ICCs. The intimate spatial arrangement of serotonergic nerves with nNOS-positive nerves is also important as it is thought that, through the intercession of descending inhibitory serotonergic neuronal activity, the release of nitric oxide from nNOS-positive neurons suppresses excitatory cholinergic activity and limits the rate at which colonic migrating motor complexes (CMMC) are propagated in mice[14]. The equivalent peristaltic activity to the CMMC in humans is the high-amplitude propagating contraction.
A close spatial arrangement of myenteric ICC fibers with TPH2-positive ganglia was demonstrated in our study, with co-expression of TPH2 and nNOS in myenteric and submucosal nitrergic ganglia and submucosal ANO-1 positive ICCs. In addition, we have demonstrated co-expression of TPH2 in the relatively recently described PDGFRα+ cells. These fibroblast-like cells share morphological similarities with ICCs but are c-kit negative[17]. It is thought that they play a role in transducing purinergic neurotransmission through the activity of apamin-sensitive small-conductance Ca2+-activated K+ (SK3) channels[18]. Our immunofluorescence findings suggest a possible functional role for serotonergic neurons in modulating PDGFRα+ cell function. It is inferable from the current evidence in the literature, that reduced density of serotonergic nerves in aganglionic and transition zone bowel and, in some patients, the ganglionic bowel, in HSCR, would disturb the inhibitory mechanisms required to maintain normal colonic motility. This is of particular relevance in patients with HAEC.
Mucosal 5-HT, secreted by EC cells, has been demonstrated in animal models to be pro-inflammatory, probably due to its activation of 5-HT receptors on dendritic cells in the lamina propria[8]. In other inflammatory conditions of the bowel, such as ulcerative colitis, reduced levels of the SERT have been reported, leading to increased 5-HT availability[8]. Conversely, neuronal 5-HT has been shown to be neuroprotective. In vitro and in vivo studies have demonstrated that neuronal 5-HT acts via 5-HT2B and 5-HT4A receptors respectively to promote survival of ICCs and enteric neurons respectively[7,8].
It has long been recognized that significant enteric neural damage occurs in severe inflammatory conditions of the colon, particularly in the setting of necrotizing enterocolitis[19-21]. Our finding of reduced expression of TPH2 in the aganglionic, transition zone and ganglionic bowel in HSCR complicated by HAEC, compared with patients unaffected by HAEC and controls, is probably reflective of enterocolitis-mediated neuronal damage. The vicious circle of enterocolitis and loss of neuroprotective serotonergic neurons may thus occur. We have previously reported on the outcomes of children with HSCR complicated by HAEC. It was found that approximately one third of patients in the series experienced enterocolitis both pre- and post-operatively[2]. Disturbances in colonic function were also more common in these patients at long-term follow-up[2]. That our findings represent effect rather than cause is supported by the finding that levels of TPH2 greatly recover in the defunctioned colon of children with HSCR complicated by HAEC who were treated with diverting colostomy due to failure of medical management.
One striking characteristic of the population from whom pull-through specimens were collected for this study is the high proportion of children with trisomy 21 at 41.2%. The incidence of trisomy 21 in Ireland, at 1 in 546 live births, is the highest in Europe[22]. Children with trisomy 21 are recognized to be at a considerably higher risk of developing HAEC (approximately 51%) compared to those without trisomy 21[23]. In our study 2 of the 5 patients who developed pre-operative HAEC had trisomy 21, both of whom were treated with a diverting stoma, indicating the severe nature of their enterocolitis.
In conclusion, we have demonstrated that serotonergic neurons co-express TPH2 with ANO1-immuno-positive ICCs in the submucosa, even in aganglionic bowel. We have also shown, for the first time, co-expression of TPH2 in PDGFRα+ cells, suggesting a possible role for serotonergic modulation of their function. TPH2 expression was reduced in the myenteric plexus and deep smooth muscle layers of aganglionic colon in children with HSCR unaffected by pre-operative HAEC. However, in children with HSCR complicated by HAEC, TPH2 expression was reduced in both aganglionic and ganglionic bowel - a finding that was reversed in the colon of children treated with diverting stoma formation due to HAEC refractory to non-operative strategies. HAEC-mediated serotonergic neuronal damage may contribute to ongoing problems with colonic function and recurrent enterocolitis despite properly performed corrective pull-through surgery.
We wish to thank Dr. Luiz Alvarez and Dr. Caroline King for their experimental support. We also acknowledge the contribution of the Departments of Histopathology in Our Lady’s Children’s Hospital and Temple Street Children’s University Hospital for their logistical support in specimen collection. This study is supported by grants from the National Children’s Research Centre/Children’s Medical and Research Foundation and from the Temple Street Foundation.
Hirschsprung’s disease (HSCR) is the most common congenital gut motility disorder. It may be complicated by a severe pancolitis known as Hirschsprung’s-associated enterocolitis (HAEC). Despite constituting only approximately 1%-5% of total intestinal serotonin (5-HT), neuronal 5-HT plays a key role in modulating gut motility and is thought to have an anti-inflammatory, neuroprotective role, in contrast to mucosal 5-HT which is pro-inflammatory.
Dysregulation of mucosal 5-HT transport has already been implicated in ulcerative colitis and other inflammatory disorders of the human colon. While a previous study has described a reduction of enterochromaffin cells, which produce mucosal 5-HT, in the ganglionic colon of children with a history of HAEC, neuronal 5-HT expression has yet to be evaluated in HSCR. In mice, knockout of tryptophan hydroxylase 2 (TPH2), the key enzyme in the synthesis pathway of neuronal 5-HT, leads to slow gastrointestinal transit and severe intestinal inflammation. Conversely, knockout of TPH1, the key enzyme in the synthesis of mucosal 5-HT, has no effect on gastrointestinal motility.
It is known that patients with HSCR who experience pre-operative HAEC are at an increased risk of poor functional outcome despite optimal surgical treatment, with some patients continuing to experience severe constipation and recurrent enterocolitis even in the absence of a mechanical obstruction. The causes for this have yet to be fully elucidated. Current findings by the authors suggest that neuronal 5-HT is deficient in the ganglionic colon of children with a history of pre-operative HAEC. Given its anti-inflammatory, neuroprotective roles, our findings suggest a mechanism by which post-operative enterocolitis may occur.
5-HT and its receptors are some of the most important current pharmacological targets in the alteration of gut motility. While the authors have indirectly shown a reduction in neuronal 5-HT in the healthy ganglionic colon in HSCR after HAEC, it is unclear if this finding would persist at follow-up after a pull-through operation, as expression of TPH2 appeared to have recovered in patients who underwent levelling or defunctioning colostomy. These findings lay the foundation for future work examining whether the abnormalities detected in this study persist at follow-up, as well an empiric evaluation of the functional outcomes of patients in this study.
Tryptophan hydroxylase 2 (TPH2) is an enzyme which catalyzes the conversion of L-tryptophan to 5-hydroxytryptophan, which is the critical rate-limiting step of neuronal 5-HT synthesis. It is an isozyme of TPH1, which catalyzes the same reaction in the synthesis of mucosal 5-HT in enterochromaffin cells.
This is a clearly presented study, addressing an important aspect of the role of neuronal 5-HT in Hirschprung's disease.
P- Reviewer: De Ponti F, Lee HC S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Austin KM. The pathogenesis of Hirschsprung’s disease-associated enterocolitis. Semin Pediatr Surg. 2012;21:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Menezes M, Puri P. Long-term outcome of patients with enterocolitis complicating Hirschsprung’s disease. Pediatr Surg Int. 2006;22:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Dasgupta R, Langer JC. Hirschsprung disease. Curr Probl Surg. 2004;41:942-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Pastor AC, Osman F, Teitelbaum DH, Caty MG, Langer JC. Development of a standardized definition for Hirschsprung’s-associated enterocolitis: a Delphi analysis. J Pediatr Surg. 2009;44:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 454] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 6. | Gershon MD. Enteric serotonergic neurones ... finally! J Physiol. 2009;587:507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268-280; discussion 280. [PubMed] |
| 8. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 801] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 9. | Galligan JJ. 5-hydroxytryptamine, ulcerative colitis, and irritable bowel syndrome: molecular connections. Gastroenterology. 2004;126:1897-1899. [PubMed] |
| 10. | Soeda J, O’Briain DS, Puri P. Regional reduction in intestinal neuroendocrine cell populations in enterocolitis complicating Hirschsprung’s disease. J Pediatr Surg. 1993;28:1063-1068. [PubMed] |
| 11. | Elhalaby EA, Coran AG, Blane CE, Hirschl RB, Teitelbaum DH. Enterocolitis associated with Hirschsprung’s disease: a clinical-radiological characterization based on 168 patients. J Pediatr Surg. 1995;30:76-83. [PubMed] |
| 12. | Snoek SA, Verstege MI, Boeckxstaens GE, van den Wijngaard RM, de Jonge WJ. The enteric nervous system as a regulator of intestinal epithelial barrier function in health and disease. Expert Rev Gastroenterol Hepatol. 2010;4:637-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998-9009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 14. | Smith TK, Park KJ, Hennig GW. Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. J Neurogastroenterol Motil. 2014;20:423-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Okamoto T, Barton MJ, Hennig GW, Birch GC, Grainger N, Corrigan RD, Koh SD, Sanders KM, Smith TK. Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil. 2014;26:556-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Liu HN, Ohya S, Nishizawa Y, Sawamura K, Iino S, Syed MM, Goto K, Imaizumi Y, Nakayama S. Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS One. 2011;6:e24928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Sigge W, Wedel T, Kühnel W, Krammer HJ. Morphologic alterations of the enteric nervous system and deficiency of non-adrenergic non-cholinergic inhibitory innervation in neonatal necrotizing enterocolitis. Eur J Pediatr Surg. 1998;8:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Wedel T, Krammer HJ, Kühnel W, Sigge W. Alterations of the enteric nervous system in neonatal necrotizing enterocolitis revealed by whole-mount immunohistochemistry. Pediatr Pathol Lab Med. 1998;18:57-70. [PubMed] |
| 21. | Zhou Y, Yang J, Watkins DJ, Boomer LA, Matthews MA, Su Y, Besner GE. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther. 2013;4:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Ni She R, Filan PM. Trisomy 21--incidence and outcomes in the first year, in Ireland today. Ir Med J. 2014;107:248-249. [PubMed] |
| 23. | Frykman PK, Short SS. Hirschsprung-associated enterocolitis: prevention and therapy. Semin Pediatr Surg. 2012;21:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |