Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4403
Peer-review started: January 31, 2016
First decision: March 7, 2016
Revised: March 8, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: May 7, 2016
Processing time: 89 Days and 5.2 Hours
AIM: To determine the efficacy of Mac-2 binding protein (Mac-2bp) for diagnosis of chronic pancreatitis.
METHODS: Fifty-nine healthy volunteers (HV), 162 patients with chronic pancreatitis (CP), and 94 patients with pancreatic ductal adenocarcinoma (PDAC) were enrolled in this study. We measured serum Mac-2bp using our developed enzyme-linked immunosorbent assay kit. Additional biochemical variables were measured using an automated analyzer (including aminotransferase, alanine aminotransferase, γ-glutamyltransferase, alkaline phosphatase, triglyceride, C-reactive protein, and amylase levels) or chemiluminescent enzyme immunoassay (carbohydrate antigen 19-9 and carcinoembryonic antigen). The ability of Mac-2bp to predict CP diagnosis accurately was assessed using receiver operating characteristic (ROC) analyses.
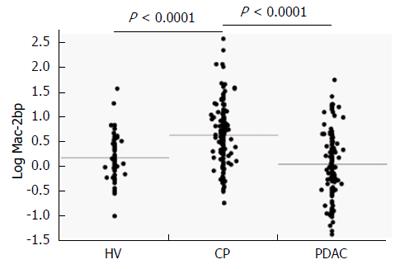
RESULTS: Serum Mac-2bp levels were significantly increased in CP patients compared to HV (P < 0.0001) and PDAC patients (P < 0.0001). Area under the ROC curve values of Mac-2bp for the discrimination of CP from HV and PDAC were 0.727 and 0.784, respectively. Multivariate analyses demonstrated that serum Mac-2bp levels were independent determinants for CP diagnosis from HV and PDAC patients. Immunohistological staining showed that Mac-2bp was expressed faintly in the pancreas tissues of both CP and PDAC patients. Serum aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, alkaline phosphatase, and triglyceride levels were significantly higher in patients with CP or PDAC. Serum Mac-2bp levels were highly correlated with protein levels of alanine aminotransferase, γ-glutamyltransferase, and C-reactive protein, but not amylase, suggesting that the damaged liver produces Mac-2bp.
CONCLUSION: Measurement of serum Mac-2bp may be a novel and useful biomarker for CP diagnosis as well as liver fibrosis in the general population.
Core tip: Serum Mac-2 binding protein (Mac-2bp) levels were significantly increased in chronic pancreatitis patients compared to healthy volunteers and pancreatic ductal adenocarcinoma patients. Therefore, measurement of serum Mac-2bp is a novel method for diagnosing chronic pancreatitis.
- Citation: Maekawa T, Kamada Y, Ebisutani Y, Ueda M, Hata T, Kawamoto K, Takamatsu S, Mizutani K, Shimomura M, Sobajima T, Fujii H, Nakayama K, Nishino K, Yamada M, Kumada T, Ito T, Eguchi H, Nagano H, Miyoshi E. Serum Mac-2 binding protein is a novel biomarker for chronic pancreatitis. World J Gastroenterol 2016; 22(17): 4403-4410
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4403
Chronic pancreatitis (CP) is characterized by progressive destruction of the pancreas tissue and recurrent episodes of intractable abdominal pain accompanied by exocrine and endocrine pancreatic insufficiencies[1,2]. CP is morphologically defined as progressive pancreatic fibrosis and inflammation that is accompanied by atrophy of pancreatic parenchymal cells[1,2]. CP is a strong risk factor for pancreatic ductal adenocarcinoma (PDAC) occurrence[3-5]. Recently, we investigated 76 PDAC patients who underwent surgery and analyzed pancreatic histological changes in specimens of noncancerous lesions[6]. Interestingly, we found that pancreatic steatosis, inflammation, and fibrosis in noncancerous lesions were each significant and independent determinants for PDAC. These histological changes are known as steatopancreatitis, and exactly the same characteristics are observed in the nonalcoholic steatohepatitis (NASH) liver. NASH, a severe form of nonalcoholic fatty liver disease (NAFLD), is a growing medical problem in industrialized countries around the world, and can progress to liver cirrhosis and hepatocellular carcinoma (HCC). We hypothesized that steatopancreatitis-induced CP would exist in the pathological background of PDAC comparable to NASH-related HCC occurrence.
PDAC is one of the most fatal cancers with a poor prognosis[7-11]. Most patients with PDAC are diagnosed at an advanced stage when the tumors are unresectable[7]. Even in the resectable cases, the prognosis of patients with PDAC is very poor because of local recurrence or distant metastasis occurring within a short period after the operation[9-11]. In our previous report, we found none of the 76 PDAC patients had a past history of clinical CP[6]. Therefore, the diagnosis of subclinical CP should be an important predictive factor for the early detection of PDAC. However, no useful diagnostic methods for the clinical diagnosis of CP have been identified.
Recently, we identified Mac-2 binding protein (Mac-2bp) as a novel diagnostic serum biomarker for NASH and fibrosis[12,13]. Mac-2bp is a glycoprotein that has seven potential N-glycosylation sites[14,15]. Serum Mac-2bp concentrations increase in patients with breast and lung cancers, viral hepatitis, and autoimmune diseases[14]. Mac-2bp is rarely detectable in normal liver, but strongly detected in hepatocytes from chronic hepatitis type C (CHC) patients as liver fibrosis progresses[16]. Using proteome analysis, serum Mac-2bp is reported as a potential fibrosis marker in CHC patients[17]. In addition, Wisteria floribunda agglutinin (WFA)-positive Mac-2bp was recently reported as a novel serum fibrosis biomarker for CHC[18,19] and NASH[12,13,20]. Now, serum Mac-2bp is recognized as a novel and useful liver fibrosis biomarker in the clinic.
Therefore, we hypothesized that serum Mac-2bp also would be increased in CP patients with pancreatic fibrosis progression, and could be a useful biomarker for CP diagnosis. In this study, we measured serum Mac-2bp in 59 healthy volunteers (HV), 162 patients with CP, and 94 patients with PDAC, and investigated the availability of serum Mac-2bp as a CP diagnostic biomarker in comparison with other biochemical data.
This study was approved by the ethics committee of Osaka University Hospital (No. 260), and the study was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all subjects at the time of enrollment or blood sampling.
Fifty-nine HV subjects were enrolled in this study from the aMs New Otani Clinic. We defined HV as the subjects who revealed no abnormal values in their laboratory evaluation without ultrasound-diagnosed fatty liver in health check-ups. One hundred sixty-two clinically diagnosed CP patients and 94 PDAC patients were enrolled in this study from Ogaki Municipal Hospital, Japan Community Health Care Organization Osaka Hospital, and Osaka University Hospital. The diagnosis of CP was made according to the guidelines of the Japan Pancreas Society[21]. The exclusion criteria from this study included a history of hepatic disease, such as chronic hepatitis C, chronic hepatitis B (seropositive for hepatitis B surface antigen), autoimmune hepatitis, Wilson’s disease, or hepatic injury caused by substance abuse. Sera from these subjects were collected and frozen at -80 °C until use. Study subjects in this study were enrolled from 2002 to 2013.
Serum biochemical variables [aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), albumin (Alb), total bilirubin (T-Bil), creatinine (Cr), total cholesterol (T-Chol), triglyceride (TG), C-reactive protein (CRP), amylase (AMY)] were measured with a conventional automated analyzer. Serum levels of carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) were determined using a chemiluminescent enzyme immunoassay. We measured serum Mac-2bp using our developed ELISA kit (Immuno-Biological Laboratory Co., Ltd., Fujioka, Japan, code No. 27362) as previously reported[12].
Statistical analysis was conducted using JMP Pro 11.0 software (SAS Institute Inc., Cary, NC). Variables were expressed as the mean ± standard deviation (SD). Statistical analysis included descriptive statistics, analysis of variance, the Wilcoxon and Kruskal-Wallis tests, and Spearman R correlations. As Mac-2bp, AST, ALT, GGT, T-Bil, TG, AMY, CRP, CA19-9, and CEA did not show a Gaussian distribution, these parameters were common log-transformed before analysis. The diagnostic performances of the scoring systems were assessed by analyzing receiver operating characteristic (ROC) curves. The probabilities of true positive (sensitivity) and true negative (specificity) assessments were determined for selected cut-off values, and the area under the ROC curve (AUC) was calculated for each index. The Youden index was used to identify the optimal cut-off points. Differences were considered statistically significant at P < 0.05.
The descriptive characteristics of the study subjects are shown in Table 1. The average age and serum AST, ALT, GGT, ALP, TG, and CEA were significantly lower in HV subjects than in CP and PDAC patients. T-Chol, CRP, AMY, and platelet count were significantly higher in CP patients than in HV subjects and PDAC participants. Serum Alb was significantly lower and average age and CA19-9 were significantly higher in PDAC patients than in CP patients. Serum Mac-2bp levels were significantly higher in CP patients than in both HV subjects and PDAC patients (Table 1, Figure 1).
| HV | CP | PDAC | P value 11 | P value 22 | |
| Number | 59 | 162 | 94 | ||
| Age (yr) | 48.2 ± 8.1 | 60.8 ± 14.5 | 66.7 ± 8.2 | < 0.0001 | < 0.05 |
| Gender (F/M) | 29/30 | 53/109 | 29/65 | < 0.05 | NS |
| AST (U/L) | 18.4 ± 4.4 | 56.1 ± 246.0 | 39.2 ± 39.3 | < 0.0001 | NS |
| ALT (U/L) | 15.3 ± 5.9 | 41.8 ± 156.7 | 42.9 ± 60.5 | < 0.0005 | NS |
| GGT (U/L) | 28.0 ± 33.1 | 132.9 ± 317.0 | 98.8 ± 188.8 | < 0.0001 | NS |
| ALP (U/L) | 179.5 ± 63.0 | 229.4 ± 92.6 | 325.8 ± 281.3 | < 0.0001 | NS |
| Alb (g/dL) | 4.23 ± 0.22 | 4.18 ± 0.39 | 3.83 ± 0.37 | NS | < 0.0005 |
| T-Bil (mg/dL) | 0.79 ± 0.29 | 0.88 ± 0.69 | 1.50 ± 3.26 | NS | NS |
| Cr (mg/dL) | 0.78 ± 0.19 | 0.77 ± 0.28 | 0.71 ± 0.19 | NS | NS |
| T-Chol (mg/dL) | 183.2 ± 26.5 | 202.7 ± 37.5 | 166.2 ± 33.0 | < 0.05 | < 0.0005 |
| TG (mg/dL) | 71.2 ± 25.6 | 120.3 ± 59.5 | 105.6 ± 48.0 | < 0.0001 | NS |
| CRP (mg/dL) | 0.074 ± 0.119 | 1.98 ± 4.08 | 0.89 ± 2.09 | < 0.0001 | < 0.005 |
| AMY (U/L) | 71.5 ± 24.5 | 318.2 ± 1223.0 | 73.0 ± 44.8 | < 0.0001 | < 0.0001 |
| Platelet (× 104/μL) | 20.8 ± 4.7 | 23.9 ± 9.1 | 19.3 ± 6.7 | < 0.05 | < 0.005 |
| CA19-9 (U/mL) | 9.3 ± 7.9 | 38.7 ± 157.9 | 904.1 ± 3064.3 | NS | < 0.0001 |
| CEA (ng/mL) | 1.35 ± 1.07 | 3.57 ± 4.72 | 7.31 ± 16.44 | < 0.0005 | NS |
| Mac-2bp (μg/mL) | 1.33 ± 0.72 | 2.30 ± 1.75 | 1.32 ± 0.95 | < 0.0001 | < 0.0001 |
The results of Pearson’s correlations between serum Mac-2bp levels and other biochemical tests are summarized in Table 2. Mac-2bp levels showed significant positive correlations with AST, ALT, GGT, and CRP.
| Factors | R | P value |
| Age (yr) | 0.1 | NS |
| AST (U/L) | 0.22 | < 0.0005 |
| ALT (U/L) | 0.15 | < 0.05 |
| GGT (U/L) | 0.17 | < 0.01 |
| ALP (U/L) | 0.12 | NS |
| Alb (g/dL) | -0.051 | NS |
| T-Bil (mg/dL) | 0.14 | NS |
| Cr (mg/dL) | 0.035 | NS |
| T-Chol (mg/dL) | -0.028 | NS |
| TG (mg/dL) | 0.16 | NS |
| CRP (mg/dL) | 0.24 | < 0.0005 |
| AMY (U/L) | -0.010 | NS |
| Platelet (×104/μL) | 0.067 | NS |
| CA19-9 (U/mL) | 0.068 | NS |
| CEA (ng/mL) | 0.024 | NS |
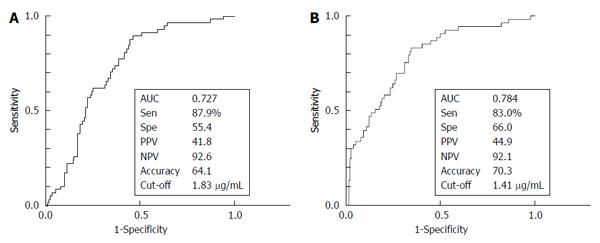
We investigated the ability of serum Mac-2bp levels to distinguish CP patients from HV subjects using the ROC curve (Figure 2A). The AUC value for Mac-2bp in distinguishing CP patients from HV subjects was 0.727, and the cut-off value was 1.83 μg/mL with 87.9% sensitivity and 55.4% specificity. Multiple logistic analysis was performed to assess the CP diagnostic ability between HV subjects and CP patients (Table 3). Clinical variables that were significantly different between HV subjects and CP patients in univariate analysis were included except for ALT, which was strongly correlated with AST (Table 1). Age, TG, CRP, CEA, and Mac-2bp were significant independent variables for CP diagnosis. The odds ratio of Mac-2bp for the diagnosis of CP was 4.04.
| Factors | Odds ratio | 95%CI | P value |
| Age | 1.15 | 1.03-1.34 | < 0.05 |
| Sex (F/M) | 2.30 | 0.25-27.57 | NS |
| AST | 1.16 | 0.93-1.58 | NS |
| GGT | 0.99 | 0.96-1.02 | NS |
| ALP | 0.98 | 0.95-1.01 | NS |
| T-Chol | 1.04 | 0.99-1.11 | NS |
| TG | 1.04 | 1.01-1.07 | < 0.005 |
| AMY | 1.00 | 0.98-1.05 | NS |
| CRP | 229.6 | 3.30-5417218 | < 0.005 |
| CEA | 2.31 | 1.35-4.92 | < 0.05 |
| Mac-2bp | 4.04 | 1.82-11.06 | < 0.05 |
We also investigated the ability of serum Mac-2bp levels to distinguish CP patients from PDAC patients using the ROC curve (Figure 2B). The AUC values for Mac-2bp in distinguishing CP patients from PDAC patients was 0.784, and the cut-off value was 1.41 μg/mL with 83.0% sensitivity and 66.0 % specificity. Multiple logistic regression analysis was also performed to assess the ability to distinguish between CP and PDAC patients (Table 4). Clinical variables that were significantly different between CP and PDAC patients in univariate analysis were included (Table 1). Alb, AMY, Platelet, CA19-9, and Mac-2bp were significant independent variables for CP diagnosis.
| Factors | Odds ratio | 95%CI | P value |
| Age | 0.60 | 0.09-1.25 | NS |
| Alb | 3.7610× 11 | 922.3-1.31 × 1045 | < 0.0001 |
| T-Chol | 1.09 | 1.01-1.60 | < 0.01 |
| CRP | 0.12 | 6.1 × 10-7 - 12.3 | NS |
| AMY | 1.32 | 1.05-2.69 | < 0.005 |
| Platelet | 2.90 | 1.02-300.8 | < 0.05 |
| CA19-9 | 0.93 | 0.71-0.99 | < 0.05 |
| Mac-2bp | 1.80 | 1.34-2.53 | < 0.005 |
In the present study, we found that serum Mac-2bp levels were increased in CP patients compared with in HV subjects and PDAC patients. Multivariate analyses demonstrated that Mac-2bp was an independent and significant determinant for CP discrimination from HV subjects and PDAC patients. Mac-2bp levels are known to increase in chronic liver diseases such as chronic hepatitis type C[18,19] and NASH[12,13,20]. Our results indicate that increased serum Mac-2bp in subjects without liver diseases should be evaluated for CP.
PDAC is one of the most fatal cancers with a poor prognosis, and most PDAC patients are diagnosed at advanced stages[7,9,10]. In advanced stages, PDAC is usually unresectable, and in even resectable cases, the prognosis of PDAC patients is very poor because of local recurrence or distant metastasis. Because CP is identified as a strong risk factor for PDAC occurrence[3-5], detection of CP in patients is important for the identification of early stage PDAC patients. Our present study indicates that the measurement of serum Mac-2bp is a useful strategy for detection of CP patients in the general population.
Very recently, we reported that a dramatic change in oligosaccharides on serum haptoglobin occurs between CP and PDAC patients[22]. In PDAC patients, we previously reported that serum fucosylated haptoglobin levels are significantly increased and could be a useful serum biomarker[23,24]. We measured serum total fucosylated haptoglobin and core-fucosylated haptoglobin by our developed lectin-antibody ELISA systems using Aleuria aurantia lectin (AAL), which recognizes all types of fucosylation, and Pholiota squarrosa lectin (PhoSL), which specifically recognizes core-fucosylation[25], respectively. Using these ELISA systems, we found serum core-fucosylated haptoglobin levels were significantly elevated in CP patients compared with HV subjects and PDAC patients[22]. Although the precise mechanisms through which serum core-fucosylated haptoglobin levels increase in CP patients are still unclear, these findings indicate that core-fucosylation of glycoproteins would be enhanced in CP patients. Mac-2bp is a glycoprotein that we identified as one of the major fucosylated glycoproteins[12]. Therefore, using core-fucosylated Mac-2bp as a better biomarker is consistent with the findings of the present study.
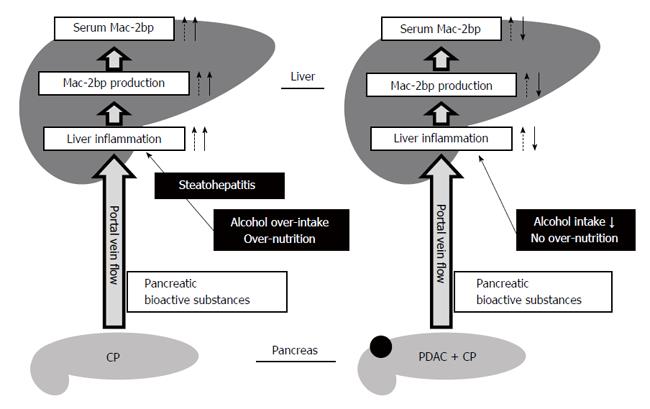
To elucidate what cells produce Mac-2bp in the pancreas, we performed immunohistochemical staining for human Mac-2bp in PDAC and CP patients. However, Mac-2bp was expressed faintly in the pancreas tissues of both PDAC and CP patients (Supplementary Figure 1). These findings indicate that Mac-2bp was not produced in the pancreas. Previous reports demonstrate that tumor necrosis factor α and interferon γ can increase Mac-2bp expression in fibroblasts[26,27]. The blood stream carries abundant bioactive substances produced in the pancreas into the liver through the portal vein. Pancreatic inflammatory cytokines and cancer bioactive substances in CP and PDAC also flow into the liver and hepatic glycoprotein production, including Mac-2bp, would increase. Our previous reports demonstrate that serum Mac-2bp levels are significantly elevated in NASH patients compared to simple steatosis patients[12,13], and we found that serum Mac-2bp levels in CP patients were as high as those in NASH patients in the present study. Steatopancreatitis, induced by alcohol and/or energy over-intake, may promote the development of CP and is related to steatohepatitis[28-30]. Considering these findings, pancreatic bioactive substances would increase Mac-2bp production in the liver, and the presence of steatohepatitis under the condition of alcohol over-intake and/or relative over-nutrition further increases hepatic Mac-2bp production in CP patients. In contrast, no over-nutrition and cessation of alcohol over-intake in PDAC patients would contribute to the improvement of liver injury and might decrease the hepatic production of Mac-2bp (Figure 3). Indeed, our findings demonstrate that serum Mac-2bp levels were significantly and positively correlated with serum liver enzyme levels (AST, ALT, and GGT) and CRP (Table 2), but not with serum AMY levels. These results indicate that the liver may produce the elevated serum Mac-2bp in CP patients.
Our study has some limitations. Firstly, our CP and PDAC patients were clinically diagnosed with or without histological diagnosis. Therefore, the histological changes of noncancerous tissues of CP and PDAC patients were not fully assessed. In our future study, we would like to measure serum Mac-2bp levels in histologically diagnosed PDAC and CP patients. Secondly, age and gender among our study groups were significantly different. Ideally, age and gender would be matched among groups. However, the number of our study subjects was not enough to match these factors. Therefore, we performed multivariate analyses to adjust for these factors and found the significance of Mac-2bp even after adjusting for age and gender (Tables 3 and 4).
In conclusion, we find that serum Mac-2bp levels were significantly increased in CP patients compared with HV subjects and PDAC patients. This finding indicates that serum Mac-2bp can be used as a novel and useful CP biomarker. Measurement of serum Mac-2bp could enable noninvasive screening for subclinical CP as well as chronic liver diseases in the general population.
Pancreatic ductal adenocarcinoma (PDAC) has the worst prognosis of all malignancies, and chronic pancreatitis (CP) is thought to be one of the main precursor diseases for PDAC occurrence. The authors recently found that inflammation and fibrosis are independent, characteristic histological changes in noncancerous lesions in PDAC patients despite the absence of a history of clinical CP. Therefore, the authors hypothesized that cryptogenic CP is an important predictive factor for PDAC occurrence. However, no useful biomarkers for the clinical diagnosis of CP have been identified.
Recently, the authors identified Mac-2 binding protein (Mac-2bp) as a novel nonalcoholic steatohepatitis and fibrosis diagnostic serum biomarker. Mac-2bp is rarely detectable in normal liver, but strongly detected in hepatocytes from chronic hepatitis type C patients as liver fibrosis progresses. In the present study, we found that serum Mac-2bp levels were significantly increased in CP patients compared with healthy volunteers and PDAC patients. This finding indicates that serum Mac-2bp can be used as a CP biomarker.
In the present study, the authors found that serum Mac-2bp levels were increased in CP patients compared with in HV subjects and PDAC patients. Multivariate analyses demonstrated that Mac-2bp was an independent and significant determinant for CP discrimination from HV subjects and PDAC patients. Mac-2bp levels are known to increase in chronic liver diseases such as chronic hepatitis type C and NASH. The present results indicate that increased serum Mac-2bp in subjects without liver diseases should be evaluated for CP.
The present study found that serum Mac-2bp levels were significantly increased in CP patients compared with HV subjects and PDAC patients. This finding indicates that serum Mac-2bp can be used as a novel and useful CP biomarker. Measurement of serum Mac-2bp could enable noninvasive screening for subclinical CP as well as chronic liver diseases in the general population.
Very well written article, scientific content is important as conveying a new message using a novel biomarker for detection of subclinical Chronic pancreatitis. However the use of term subclinical CP in this study has been extrapolated to patient with CP as mentioned in methodology. This needs to be clarified whether these patients had any symptoms of CP or were detected incidentally from imaging findings. Also, author needs to define the term subclinical CP. Authors have also extrapolated their results in NASH patients but not mentioned whether these were same subjects with NASH and CP or PDAC, could they find a correlation?
P- Reviewer: Kitamura K, Shah JA, Sharma SS S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 2. | Ammann RW. A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas. 1997;14:215-221. [PubMed] |
| 3. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1139] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 4. | Talamini G, Falconi M, Bassi C, Sartori N, Salvia R, Caldiron E, Frulloni L, Di Francesco V, Vaona B, Bovo P. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849-852. [PubMed] |
| 6. | Tomita Y, Azuma K, Nonaka Y, Kamada Y, Tomoeda M, Kishida M, Tanemura M, Miyoshi E. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas. 2014;43:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 8. | Brand RE, Tempero MA. Pancreatic cancer. Curr Opin Oncol. 1998;10:362-366. [PubMed] |
| 9. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2205] [Article Influence: 147.0] [Reference Citation Analysis (2)] |
| 10. | Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846-2850. [PubMed] |
| 11. | Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, Kitamura M, Takahashi H, Eguchi H, Ohigashi H. CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol. 2009;16:2531-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Kamada Y, Fujii H, Fujii H, Sawai Y, Doi Y, Uozumi N, Mizutani K, Akita M, Sato M, Kida S. Serum Mac-2 binding protein levels as a novel diagnostic biomarker for prediction of disease severity and nonalcoholic steatohepatitis. Proteomics Clin Appl. 2013;7:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Mori K, Tanaka S, Yamada M, Akita M, Mizutani K. A novel noninvasive diagnostic method for nonalcoholic steatohepatitis using two glycobiomarkers. Hepatology. 2015;62:1433-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, D’Egidio M, Natoli C, Piantelli M, Iacobelli S. 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj J. 2004;19:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Przybyło M, Martuszewska D, Pocheć E, Hoja-Łukowicz D, Lityńska A. Identification of proteins bearing beta1-6 branched N-glycans in human melanoma cell lines from different progression stages by tandem mass spectrometry analysis. Biochim Biophys Acta. 2007;1770:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Artini M, Natoli C, Tinari N, Costanzo A, Marinelli R, Balsano C, Porcari P, Angelucci D, D’Egidio M, Levrero M. Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J Hepatol. 1996;25:212-217. [PubMed] |
| 17. | Cheung KJ, Tilleman K, Deforce D, Colle I, Van Vlierberghe H. The HCV serum proteome: a search for fibrosis protein markers. J Viral Hepat. 2009;16:418-429. [PubMed] |
| 18. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M. A serum ”sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Iacovazzi PA, Cozzolongo R, Lanzillotta E, Frisullo S, Guerra V, Correale M. Serum 90K/Mac-2 binding protein (Mac-2BP) as a response predictor to peginterferon and ribavirin combined treatment in HCV chronic patients. Immunopharmacol Immunotoxicol. 2008;30:687-700. [PubMed] |
| 20. | Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, Hara Y, Hige S, Sakamoto M, Yamada G. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Shimosegawa T, Kataoka K, Kamisawa T, Miyakawa H, Ohara H, Ito T, Naruse S, Sata N, Suda K, Hirota M. The revised Japanese clinical diagnostic criteria for chronic pancreatitis. J Gastroenterol. 2010;45:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Ueda M, Kamada Y, Takamatsu S, Shimomura M, Maekawa T, Sobajima T, Fujii H, Nakayama K, Nishino K, Yamada M. Specific increase in serum core-fucosylated haptoglobin in patients with chronic pancreatitis. Pancreatology. 2016;pii:S1424-3903(16)00009-0. [PubMed] |
| 23. | Matsumoto H, Shinzaki S, Narisada M, Kawamoto S, Kuwamoto K, Moriwaki K, Kanke F, Satomura S, Kumada T, Miyoshi E. Clinical application of a lectin-antibody ELISA to measure fucosylated haptoglobin in sera of patients with pancreatic cancer. Clin Chem Lab Med. 2010;48:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Kamada Y, Kinoshita N, Tsuchiya Y, Kobayashi K, Fujii H, Terao N, Kamihagi K, Koyama N, Yamada S, Daigo Y. Reevaluation of a lectin antibody ELISA kit for measuring fucosylated haptoglobin in various conditions. Clin Chim Acta. 2013;417:48-53. [PubMed] |
| 25. | Kobayashi Y, Tateno H, Dohra H, Moriwaki K, Miyoshi E, Hirabayashi J, Kawagishi H. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J Biol Chem. 2012;287:33973-33982. [PubMed] |
| 26. | Kong W, Li S, Longaker MT, Lorenz HP. Cyclophilin C-associated protein is up-regulated during wound healing. J Cell Physiol. 2007;210:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Kong W, Lin BW, Li S, Longaker MT, Lorenz HP. Cyclophilin C-associated protein/Mac-2 binding protein colocalizes with calnexin and regulates the expression of tissue transglutaminase. J Cell Physiol. 2010;223:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, Pitt HA. Nonalcoholic fatty pancreas disease. HPB (Oxford). 2007;9:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas. 2010;39:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol. 2013;28 Suppl 4:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |