Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4338
Peer-review started: December 0, 2015
First decision: January 13, 2016
Revised: January 25, 2016
Accepted: March 2, 2016
Article in press: March 2, 2016
Published online: May 7, 2016
Processing time: 142 Days and 22.3 Hours
AIM: To evaluate the correlation of shear wave elastography (SWE) results with liver fibrosis histology and quantitative function reserve.
METHODS: Weekly subcutaneous injection of 60% carbon tetrachloride (1.5 mL/kg) was given to 12 canines for 24 wk to induce experimental liver fibrosis, with olive oil given to 2 control canines. At 24 wk, liver condition was evaluated using clinical biochemistry assays, SWE imaging, lidocaine metabolite monoethylglycine-xylidide (MEGX) test, and histologic fibrosis grading. Clinical biochemistry assays were performed at the institutional central laboratory for routine liver function evaluation. Liver stiffness was measured in triplicate from three different intercostal spaces and expressed as mean liver stiffness modulus (LSM). Plasma concentrations of lidocaine and its metabolite MEGX were determined using high-performance liquid chromatography repeated in duplicate. Liver biopsy samples were fixed in 10% formaldehyde, and liver fibrosis was graded using the modified histological activity index Knodell score (F0-F4). Correlations among histologic grading, LSM, and MEGX measures were analyzed with the Pearson linear correlation coefficient.
RESULTS: At 24 wk liver fibrosis histologic grading was as follows: F0, n = 2 (control); F1, n = 0; F2, n = 3; F3, n = 7; and F4, n = 2. SWE LSM was positively correlated with histologic grading (r = 0.835, P < 0.001). Specifically, the F4 group had a significantly higher elastic modulus than the F3, F2, and F0 groups (P = 0.002, P = 0.003, and P = 0.006, respectively), and the F3 group also had a significantly higher modulus than the control F0 group (P = 0.039). LSM was negatively associated with plasma MEGX concentrations at 30 min (r = -0.642; P = 0.013) and 60 min (r = -0.651; P = 0.012), time to ½ of the maximum concentration (r = -0.538; P = 0.047), and the area under the curve (r = -0.636; P = 0.014). Multiple comparisons showed identical differences in these three measures: significantly lower with F4 (P = 0.037) and F3 (P = 0.032) as compared to F0 and significantly lower with F4 as compared to F2 (P = 0.032).
CONCLUSION: SWE LSM shows a good correlation with histologic fibrosis grading and pharmacologic quantitative liver function reserve in experimental severe fibrosis and cirrhosis.
Core tip: Non-invasive evaluation of liver histology and function reserve is critical for determination of treatment option and prognosis in severe fibrosis and cirrhotic patients. Shear wave elastography (SWE) is a newly emerging elastrographic modality with relatively high resolution and good reproducibility for liver imaging. Lidocaine/monoethylglycinexylidide is also an advanced, laboratory dynamic liver function assay with good diagnostic sensitivity, specificity, and accuracy. This study sheds light on the correlation of SWE imaging results with pharmacologic quantitative liver function for disease severity and function reserve evaluation in patients with severe fibrosis/cirrhosis scheduled for major hepatectomy or liver transplantation.
- Citation: Feng YH, Hu XD, Zhai L, Liu JB, Qiu LY, Zu Y, Liang S, Gui Y, Qian LX. Shear wave elastography results correlate with liver fibrosis histology and liver function reserve. World J Gastroenterol 2016; 22(17): 4338-4344
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4338
Liver fibrosis is a progressive liver disease characterized by replacement of normal liver parenchymal tissue by fibrotic nonparenchymal tissue with or without concomitant abnormal regenerative nodules[1]. The etiologies of liver fibrosis vary among populations worldwide and mainly include viral hepatitis, especially chronic hepatitis B and C in Eastern and Southeastern Asians, alcoholic liver disease in Westerners, and nonalcoholic fatty liver disease[2]. Aside from portal hypertension, reduced or absent liver function reserve is the major pathophysiologic impairment in severely fibrotic and cirrhotic patients. Diagnosis and severity grading of liver fibrosis, especially the evaluation of liver function reserve, are clinically significant for determining the appropriate treatment modality, such as prioritization of liver transplantation and prediction of prognosis in patients with cirrhosis.
Liver ultrasonography is an non-invasive imaging modality most frequently used for routine liver fibrosis screening but suffers from a low sensitivity and specificity, especially for early liver disease or that complicated by another non-fibrotic disease[3]. A variety of elastrographic techniques, including quasistatic elastrography, transient elastography, acoustic radiation force impulse imaging, shear wave elastography (SWE), and magnetic resonance elastography, have been applied or investigated for quantitative evaluation of liver fibrosis[4]. SWE, also called supersonic shear imaging, is a newly emerging elastrographic modality[5] that has been shown to be clinically beneficial for breast[6], thyroid gland, prostate, musculoskeletal, and liver[7] imaging with relatively high resolution and good reproducibility.
Routine liver function biochemistry assays cannot detect compensated liver insufficiency, and thus, liver-based metabolism tests are normally performed for this purpose. The indocyanine green elimination test is a dynamic, liver metabolism-based function assay mainly used for preoperative bedside evaluation of a patient scheduled for liver resection[8] or transplantation[9]. Lidocaine/monoethylglycinexylidide (MEGX) is also an advanced, laboratory dynamic liver function assay based on the metabolization of lidocaine into MEGX by abundant cytochrome P450 in hepatocytes, with good diagnostic sensitivity, specificity, and accuracy, especially for critically ill patients[10].
The primary objective of this study was to assess the correlation of SWE imaging results and liver histology and MEGX liver function test results in an experimental canine model of liver fibrosis. Specifically, the investigation into the correlation of SWE imaging results with pharmacologic quantitative liver function might aid in the evaluation of the liver function reserve in patients with severe fibrosis/cirrhosis scheduled for major hepatectomy or liver transplantation using a bedside, noninvasive modality rather than an invasive laboratory diagnostic modality.
The study protocol was approved by the Animal Research Committee of Beijing Friendship Hospital, Capital Medical University, in accordance with the National Institute of Health Guidelines for Laboratory Animal Care and Use. Fourteen healthy laboratory Beagles (Rixin Technology Co., Ltd., Beijing; license No. SCXK[BJ]2011-0007) weighing 6-8 kg, including 6 males and 8 females, were bred at the Center of Laboratory Large Animal in Jilin Sino-Japan Friendship Hospital and housed in individual cages with free access to high-lipid canine feed and tap water containing 1:10 (v/v) ethanol. Under general anesthesia by subcutaneous injection of 3% phenobarbital (1 mg/kg), 12 animals were given a subcutaneous injection of 60% carbon tetrachloride (1.5 mL/kg; Shanghai Chemical Co., Ltd, China) diluted in commercially available olive oil, at a weekly interval for 24 consecutive weeks after the initial injection to induce experimental liver fibrosis[11]. Two animals were given 1.5 mL/kg olive oil alone using the same protocol as controls.
At 24 wk, the animals were anesthetized and positioned supine with the anterior abdominal wall shaved on the operating table. An Aixplorer color Doppler ultrasound system (SuperSonic Imagine, Aix-en-Provence, France), equipped with a 4-15 MHz probe, was operated by an independent ultrasound technician for SWE imaging. An appropriate right-side intercostal space was located for identifying the optimal liver parenchymal window with gray scale ultrasound imaging. The SWE module was subsequently switched on for elastography of the right lobe parenchyma approximately 1 cm below the liver capsule. Liver stiffness was measured in triplicate from three different intercostal spaces and expressed as the mean elastic modulus (kPa)[12].
Clinical biochemistry assays were performed at the institutional central laboratory for routine liver function evaluation, including serum total protein, albumin, alanine aminotransferase, asparate aminotransferase, gamma glutamyltransferase, alkaline phosphatase, and bilirubin (total, unconjugated, conjugated). After blood sampling for clinical biochemistry assays, lidocaine hydrochloride (1 mg/kg) was injected through the cephalic vein into the anesthetized animals. The femoral vein was punctured for venous blood sampling (2 mL) at 0, 5, 10, 15, 20, 30, 40, 50, and 60 min after lidocaine injection. Plasma concentrations of lidocaine and its metabolite MEGX were determined using high-performance liquid chromatography repeated in duplicate[13]. A plasma MEGX concentration curve was plotted against time.
Percutaneous liver biopsy was performed using an 18-gauge needle by an independent, board-certified interventional ultrasound physician under B-mode ultrasound guidance. Liver biopsy samples were fixed in 10% formaldehyde, and liver fibrosis was graded using the modified histological activity index, i.e., Knodell score (F0-F4), with a higher score indicating more serious liver fibrosis[14].
The statistical software package SPSS 11.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analyses. All continuous data are expressed as median ± interquartile range (IQR), and the medians were compared using the Wilcoxon rank-sum test. Multiple comparisons were performed using the Fisher least significance difference test at an adjusted significance level. Correlations among histologic grading, LSM, and MEGX measures were analyzed with the Pearson linear correlation coefficient. A two-sided P value < 0.05 was considered statistically significant.
All 14 animals survived at 24 wk, and liver fibrosis histologic grading was as follows: F0, n = 2 (control); F1, n = 0; F2, n = 3; F3, n = 7; and F4, n = 2. The liver biochemistry assay results are shown in Table 1. All biochemical measures remained similar among the four histologic groups (P > 0.05); however, the serum albumin level was significantly lower in the F4 group than in the F2 and F0 groups (P = 0.003 and P = 0.021, respectively) but not statistically different among the F3, F2, and F0 groups and between F4 and F3 groups (P > 0.05).
| Variable | F0 control | F2 | F3 | F4 | P value |
| (n = 2) | (n = 3) | (n = 7) | (n = 2) | ||
| Albumin (g/Dl) | 3.9 ± 1.81 | 3.1 ± 0.51 | 2.6 ± 0.91,2 | 1.8 ± 0.52 | 0.021 |
| Total protein (g/dL) | 6.5 ± 1.1 | 7.0 ± 1.7 | 6.1 ± 1.2 | 6.2 ± 1.8 | 0.639 |
| AST (IU/L) | 28 ± 14 | 21 ± 12 | 28 ± 15 | 60 ± 76 | 0.711 |
| ALT (IU/L) | 44 ± 17 | 43 ± 22 | 31 ± 28 | 58 ± 27 | 0.669 |
| GGT (IU/L) | 5.2 ± 2.1 | 5 ± 1.3 | 5.2 ± 2.6 | 6.3 ± 6.2 | 0.938 |
| ALP (IU/L) | 44 ± 25 | 49 ± 8 | 61 ± 19 | 74 ± 31 | 0.140 |
| TBil (mg/dL) | 0.27 ± 0.08 | 0.24 ± 0.07 | 0.25 ± 0.11 | 0.33 ± 0.21 | 0.865 |
| UBil (mg/dL) | 0.13 ± 0.05 | 0.13 ± 0.06 | 0.16 ± 0.10 | 0.17 ± 0.12 | 0.825 |
| CBil (mg/dL) | 0.15 ± 0.03 | 0.09 ± 0.05 | 0.10 ± 0.07 | 0.16 ± 0.09 | 0.318 |
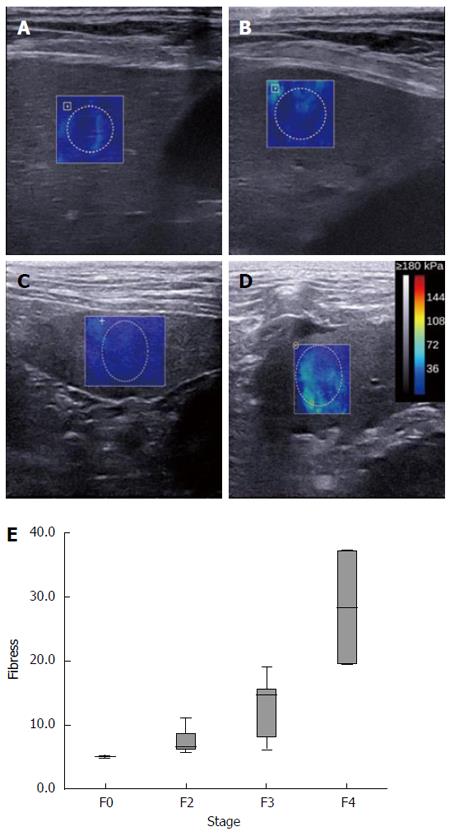
Representative SWE images for F0 and F2-F4 are shown in Figure 1A-D. SWE liver stiffness modulus data are shown in Table 2 and exhibited a significant positive correlation with histologic grading (r = 0.835, P < 0.001) (Figure 1E). Specifically, the F4 group had a significantly higher elastic modulus than the F3, F2, and F0 groups (P = 0.002, P = 0.003, and P = 0.006, respectively), and the F3 group also had a significantly higher modulus than the control F0 group (P = 0.039). However, the elastic modulus was similar between the F3 and F2 groups and the F2 and F0 groups (P > 0.05).
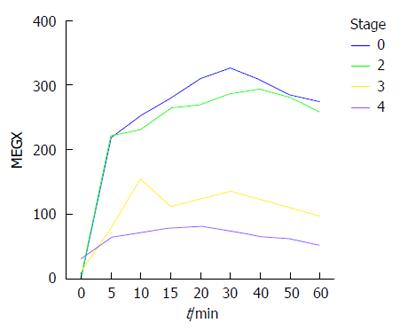
A plasma MEGX concentration vs time plot is shown in Figure 2, and the pharmacokinetics of plasma MEGX are described in Table 3. The four fibrosis grading groups showed no significant differences in all of the pharmacokinetic measures, although a declining trend was observed from F0 to F4, which was statistically significant for plasma MEGX concentrations at 30 min (P = 0.033) and 60 min (P = 0.020) as well as with respect to the area under the curve (P = 0.016). Multiple comparisons showed identical differences in these three measures: significantly lower with F4 (P = 0.037) and F3 (P = 0.032) as compared to F0 and significantly lower with F4 as compared to F2 (P = 0.032), but similar between F3 and F2 and between F2 and F0 (both P values > 0.05). LSM was negatively associated with plasma MEGX concentrations at 30 min (r = -0.642, P = 0.013) and 60 min (r = -0.651, P = 0.012), time to ½ of the maximum concentration (r = -0.538, P = 0.047), and the area under the curve (r = -0.636, P = 0.014).
| Variable | F0 control | F2 | F3 | F4 | P value |
| (n = 2) | (n = 3) | (n = 7) | (n = 2) | ||
| C10 min (ng/mL) | 252 ± 31 | 197 ± 381 | 99 ± 105 | 72 ± 38 | 0.280 |
| C30 min (ng/mL) | 327 ± 561 | 268 ± 3751,2 | 120 ± 312,3 | 73 ± 203,4 | 0.033 |
| C60 min (ng/mL) | 274 ± 711 | 258 ± 3361,2 | 93 ± 262,3 | 51 ± 373,4 | 0.020 |
| Cmax (ng/mL) | 327 ± 56 | 282 ± 332 | 143 ± 106 | 82 ± 22 | 0.069 |
| tmax (min) | 30.0 ± 0.0 | 40.0 ± 20.0 | 30.0 ± 20.0 | 17.5 ± 5.0 | 0.120 |
| t½max (min) | 141.3 ± 110.1 | 151.1 ± 188.3 | 46.9 ± 50.2 | 74.9 ± 75.9 | 0.545 |
| AUC (ng/mL·min) | 16644 ± 31391 | 14421 ± 25151,2 | 6100 ± 22862,3 | 3970 ± 16703,4 | 0.016 |
| Kmax (ng/mL/min) | 43.7 ± 7.2 | 38.1 ± 83.8 | 17.7 ± 22.8 | 6.6 ± 1.5 | 0.358 |
Liver biopsy, usually through the percutaneous approach, is the gold standard diagnostic modality for liver fibrosis/cirrhosis, but this procedure cannot be used and repeated as routine in general clinical practice due to low procedure-associated morbidities, such as bleeding, perforation, and infection[15]. Moreover, histologic grading of fibrosis does not necessarily correlate well with the underlying liver function reserve, but instead may over- or underestimate the disease severity due to the use of a limited liver tissue sample. As an alternative non-invasive diagnostic modality to liver biopsy, liver elastography is an advanced ultrasound or magnetic resonance imaging technique that quantifies liver stiffness by measuring liver tissue distortion (shear wave) and wave transition velocity upon mechanical vibration[16]. Among the elastographic techniques currently available, the FibroScan using transient elastography has been well validated for fibrosis grading in liver fibrosis patients and the results show a good histologic correlation with liver biopsy findings; however, FibroScan measures liver stiffness on a one-dimensional ultrasonograph and also requires an additional designated probe for patients with a narrow rib cage or with complicating fatty liver disease[17]. In contrast, SWE offers a quantitative, real-time, two-dimensional elastography by incorporating an advanced ultrafast imaging technique[5] with a high correlation of histologic fibrosis staging comparable to transient elastography[18]. Our study results add new knowledge to current literature showing that the liver SWE elastic modulus also was well correlated with liver-based drug metabolism in addition to histologic grading. To the best of our knowledge, the present work is the first report regarding correlation of SWE results with fibrosis grading and cytochrome P450-based liver function reserve in experimental liver fibrosis.
Quantitative evaluation of liver function reserve can improve the predictive accuracy for chronic liver disease progression, including chronic hepatitis to liver fibrosis/cirrhosis[19]. The Child-Pugh score[20] and Model for End-Stage Liver Disease score[21] are most often used and based on clinical manifestations and laboratory biochemistry assays; however, these two scales have a variety of confounding factors and only represent a patient’s pre-existing long-term liver function reserve rather than an acute change in liver function. The indocyanine green elimination test is the liver function reserve assay most frequently used in general clinical practice; however, its results may be affected by liver perfusion impairment, biliary obstruction, and complicating hypoalbuminemia[22]. MEGX test is a dynamic, quantitative liver function test that measures cytochrome P450 metabolism of lidocaine in metabolically active hepatocytes and is superior to the indocyanine green elimination test with respect to sensitivity, specificity, accuracy, and reproducibility. The MEGX level has been used for preoperative planning of liver resection[23], prediction of post-hepectomy acute liver failure[24], and survival of decompensated cirrhosis patients on the waiting list for liver transplantation[25]. Moreover, quantitative liver function tests including the MEGX test were reported as independent risk factors for improving the predictability of virologic response and disease progression of chronic hepatitis C virus with antiviral treatment[26]. Our results identified three potential measures, especially the area under the curve of the concentration vs time plot a sensitive and specific indicator of cytochrome P450 metabolic functionality, which could differentiate severe fibrosis or cirrhosis from mild disease.
SWE has been widely used for the evaluation of liver fibrosis/cirrhosis of multiple etiologies or with complicating comorbidities, including chronic hepatitis[27], liver cancer[28], steatohepatitis[29], and biliary atresia[30]. This two-dimensional elastographic technique offers better performance for assessing liver fibrosis as compared to conventional transient elastography, especially regarding the correlation of the LSM with histologic grading, with cutoff values of 8.0 kPa and 13.1 kPa for F2 and F4, respectively[31]. Our preliminary results demonstrated that severe fibrosis and especially cirrhosis had a higher LSM than moderate liver disease although no statistically significant difference was observed between F2 and F0 or between F3 and F2. Moreover, our results showed that the LSM on SWE was negatively correlated with MEGX test measures, including plasma concentrations at 30 min and 60 min, the time to ½ of the maximum concentration, and with respect to the area under the curve. Quantification of liver stiffness in patients with severe fibrosis or cirrhosis who are scheduled for major hepatectomy or liver transplantation may help estimate the risk of postoperative liver insufficiency with an advantage over pharmacologic liver function assays due to its non-invasiveness and technical reproducibility[32].
In conclusion, our results demonstrated that SWE results are well correlated with the histologic grading of experimental fibrosis. Moreover, SWE results also correlated well with quantitative liver function reserve measurements obtained by the lidocaine metabolite MEGX test. Therefore, it is beneficial to employ SWE for assessment of liver disease severity and function reserve in patients with severe fibrosis or cirrhosis as this modality is non-invasive and reproducible in the clinical setting.
Liver ultrasonography is a non-invasive imaging modality most frequently used for routine liver fibrosis screening but suffers from a low sensitivity and specificity, especially for early liver disease or that complicated by another non-fibrotic disease. Routine liver function biochemistry assays cannot detect compensated liver insufficiency, and thus, liver-based metabolism tests are normally performed for this purpose.
Shear wave elastography (SWE) is a newly emerging elastrographic modality that has been shown to be clinically beneficial for liver imaging with relatively high resolution and good reproducibility. Monoethylglycinexylidide (MEGX) is also an advanced, laboratory dynamic liver function assay based on the metabolization of lidocaine into MEGX by abundant cytochrome P450 in hepatocytes, with good diagnostic sensitivity, specificity, and accuracy, especially for critically ill patients.
SWE results are well correlated with the histologic grading of experimental fibrosis and also with quantitative liver function reserve measurements obtained by the lidocaine metabolite MEGX test.
SWE is beneficial for assessment of liver disease severity and function reserve in patients with severe fibrosis or cirrhosis as this modality is non-invasive and reproducible in the clinical setting.
Interesting paper and topic. In the present study, the authors evaluated the correlation of shear wave elastrography results with liver fibrosis histology and liver function reserve. In general, the manuscript is well-written and the methodology is acceptable. Although the correlation of SWE in patients with various liver conditions have been extensively handled in the literature, this study throws light for the first time on its correlation with quantitative liver function reserve measurements obtained by the lidocaine metabolite MEGX test.
P- Reviewer: Dirchwolf M, El-Akabawy G, Scicchitano P S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 2. | Shukla A, Vadeyar H, Rela M, Shah S. Liver Transplantation: East versus West. J Clin Exp Hepatol. 2013;3:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | De Robertis R, D’Onofrio M, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Noninvasive diagnosis of cirrhosis: a review of different imaging modalities. World J Gastroenterol. 2014;20:7231-7241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 4. | Van Beers BE, Daire JL, Garteiser P. New imaging techniques for liver diseases. J Hepatol. 2015;62:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 607] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 6. | Li G, Li DW, Fang YX, Song YJ, Deng ZJ, Gao J, Xie Y, Yin TS, Ying L, Tang KF. Performance of shear wave elastography for differentiation of benign and malignant solid breast masses. PLoS One. 2013;8:e76322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Ferraioli G, Parekh P, Levitov AB, Filice C. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med. 2014;33:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Gao L, Ramzan I, Baker AB. Potential use of pharmacological markers to quantitatively assess liver function during liver transplantation surgery. Anaesth Intensive Care. 2000;28:375-385. [PubMed] |
| 10. | Ercolani G, Grazi GL, Callivà R, Pierangeli F, Cescon M, Cavallari A, Mazziotti A. The lidocaine (MEGX) test as an index of hepatic function: its clinical usefulness in liver surgery. Surgery. 2000;127:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Luo L, Zhou A. Antifibrotic activity of anisodamine in vivo is associated with changed intrahepatic levels of matrix metalloproteinase-2 and its inhibitor tissue inhibitors of metalloproteinases-2 and transforming growth factor beta1 in rats with carbon tetrachloride-induced liver injury. J Gastroenterol Hepatol. 2009;24:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Holdsworth A, Bradley K, Birch S, Browne WJ, Barberet V. Elastography of the normal canine liver, spleen and kidneys. Vet Radiol Ultrasound. 2014;55:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Matsuyama K, Fukuda Y, Miyake H, Yogita S, Tashiro S. Experimental study of the evaluation of liver function on the opposite side during portacaval anastomosis and ligation of the left portal branch. J Med Invest. 2004;51:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Yeh TS, Ho YP, Huang SF, Yeh JN, Jan YY, Chen MF. Thalidomide salvages lethal hepatic necroinflammation and accelerates recovery from cirrhosis in rats. J Hepatol. 2004;41:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Tobkes AI, Nord HJ. Liver biopsy: review of methodology and complications. Dig Dis. 1995;13:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Heller MT, Tublin ME. The role of ultrasonography in the evaluation of diffuse liver disease. Radiol Clin North Am. 2014;52:1163-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | Piscaglia F, Marinelli S, Bota S, Serra C, Venerandi L, Leoni S, Salvatore V. The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives. Eur J Radiol. 2014;83:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Manizate F, Hiotis SP, Labow D, Roayaie S, Schwartz M. Liver functional reserve estimation: state of the art and relevance to local treatments. Oncology. 2010;78 Suppl 1:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Halle BM, Poulsen TD, Pedersen HP. Indocyanine green plasma disappearance rate as dynamic liver function test in critically ill patients. Acta Anaesthesiol Scand. 2014;58:1214-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 23. | Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Kanzler S, Teufel A, Galle PR. Liver function test to predict hepatic failure after liver resection--expensive and without clinical relevance? Zentralbl Chir. 2007;132:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Ravaioli M, Grazi GL, Principe A, Ercolani G, Cescon M, Gardini A, Varotti G, Del Gaudio M, Cavallari A. Operative risk by the lidocaine test (MEGX) in resected patients for HCC on cirrhosis. Hepatogastroenterology. 2003;50:1552-1555. [PubMed] |
| 26. | Everson GT, Shiffman ML, Hoefs JC, Morgan TR, Sterling RK, Wagner DA, Lauriski S, Curto TM, Stoddard A, Wright EC. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 507] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 28. | Honjo M, Moriyasu F, Sugimoto K, Oshiro H, Sakamaki K, Kasuya K, Nagai T, Tsuchida A, Imai Y. Relationship between the liver tissue shear modulus and histopathologic findings analyzed by intraoperative shear wave elastography and digital microscopically assisted morphometry in patients with hepatocellular carcinoma. J Ultrasound Med. 2014;33:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Lu Y, Wei J, Tang Y, Yuan Y, Huang Y, Zhang Y, Li Y. Evaluation of fatty liver fibrosis in rabbits using real-time shear wave elastography. Exp Ther Med. 2014;8:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Leschied JR, Dillman JR, Bilhartz J, Heider A, Smith EA, Lopez MJ. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr Radiol. 2015;45:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Bota S, Paternostro R, Etschmaier A, Schwarzer R, Salzl P, Mandorfer M, Kienbacher C, Ferlitsch M, Reiberger T, Trauner M. Performance of 2-D shear wave elastography in liver fibrosis assessment compared with serologic tests and transient elastography in clinical routine. Ultrasound Med Biol. 2015;41:2340-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Inoue Y, Kokudo N. Elastography for hepato-biliary-pancreatic surgery. Surg Today. 2014;44:1793-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |