Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4330
Peer-review started: December 30, 2015
First decision: January 28, 2016
Revised: February 11, 2016
Accepted: March 2, 2016
Article in press: March 2, 2016
Published online: May 7, 2016
Processing time: 121 Days and 17 Hours
AIM: To determine the hypothesis that inflating the balloons in the duodenal papilla determines changes in the biochemical markers of pancreatitis.
METHODS: Four groups of pigs were used: Group papilla (GP), the overtube’s balloon was inflated in the area of the papilla; GP + double balloon enteroscopy (GP + DBE), the overtube’s balloon was kept inflated in the area of the papilla for 20 min before a DBE; Group DBE (GDBE), DBE was carried out after insuring the balloon’s inflation far from the pancreatic papilla; and Group control (GC). Serum concentrations of amylase, lipase and C-reactive protein (CRP) were evaluated. Pancreases were processed for histopathology examination.
RESULTS: Main changes occurred 24 h after the procedure compared with baseline levels. Amylase levels increased significantly in GP (59.2% higher) and were moderately higher in groups GP + DBE and GDBE (22.7% and 20%, respectively). Lipase increased in GP and GP + DBE, whereas it hardly changed in GDBE and in GC. CRP increased significantly in GP, GP + DBE and GDBE, while no changes were reported for GC. No statistically significant difference between groups GP and GP + DBE was found for the histopathological findings, except for vacuolization and necrosis of the pancreatic parenchyma that was higher in GP than in GP + DBE.
CONCLUSION: The manipulation of the duodenal papilla by the inflated overtube’s balloon during DBE causes pancreatic structural damage and increased biochemical markers associated with pancreatitis.
Core tip: During double balloon enteroscopy (DBE) the manipulation of the duodenal papilla by the inflated balloons around the area of secretion of the pancreas determines structural damage in the organ and increased levels of biochemical markers of pancreatitis. Thus, the widely assumed recommendation of avoiding any contact of the balloons with the duodenal papilla so as to decrease post-DBE pancreatic risk is now supported by empirical results in an animal model.
- Citation: Latorre R, López-Albors O, Soria F, Candanosa E, Pérez-Cuadrado E. Effect of the manipulation of the duodenal papilla during double balloon enteroscopy. World J Gastroenterol 2016; 22(17): 4330-4337
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4330
Double balloon enteroscopy (DBE) is a variety of push and pull endoscopy which allows diagnostic and therapeutic actions deep in the small intestine[1]. Although DBE has been considered a reasonably safe technique[2], articles reviewing complications have been published recently[3]. Among the major complications (0.72%)[4] acute post-procedure pancreatitis is the most severe[5]. DBE can be done by oral or anal approaches but post-DBE pancreatitis is mainly related with the anterograde approach. The average incidence of this complication is not very high (0.3%)[6] however, it may be underestimated in previously published data[7]. With the use of the oral approach increases in the incidence of pancreatitis which may vary from 1%-3%[2,7,8] to 12.5[9].
There is some controversy about the etiology of acute pancreatitis after DBE but most opinions agree that the cause is related with the technique itself[2,10-13]. Ischemia of the pancreas from prolonged mechanical stress due to repeated stretching of the endoscope, reflux of duodenal content into the pancreatic duct consequent to overpressure in the intestinal compartment, and disturbance of the pancreatic secretion due to direct trauma to the papilla of Vater are the most plausible theories[6,7,11-13]. In order to avoid potential trauma in the ampullar zone some endoscopists recommend inflating the balloons after passing the ligament of Treitz[14,15], although no causal relationship has been found with a lower incidence of hyperamylasemia or reduction in pancreatitis rate[16]. However, precise control of this manoeuvre is not always guaranteed[2] and during the learning curve, unintentional shearing of the ampullary area is likely.
Post-DBE hyperamylasemia is the most frequent biochemical marker associated with suspicion of acute pancreatitis[10,11,13], however increased amylase levels after DBE are not always indicatory of pancreatic inflammation since asymptomatic hyperamylasemia is quite common in DBE procedures[2]. Lipase levels are more specific for the pancreatic function but post-DBE hyperlipasemia is not always accompanied by clinical signs of pancreatitis such as increased abdominal pain. Nevertheless, amylase and lipase levels together plus those of the reactive C-protein (CRP) are a valuable tool to diagnose post-DBE acute pancreatitis because they are commonly elevated soon after the procedure[2,9].
One major limitation for research in this field comes from the fact that DBE is mainly restricted to endoscopy procedures in humans, although the use of an appropriate animal model could help to overcome this limitation. The porcine model has been used both ex vivo and in vivo for DBE training and research, helping to improve the technique conditions in humans[17-19]. Pig and human pancreas are partially retroperitoneal, encircle the portal vein and have similar parenchyma firmness. Unlike humans, pigs have a unique pancreatic duct (accessory) which opens at a minor duodenal papilla located 8-10 cm distally from the major duodenal papilla and approximately 12-15 cm from the pylorus[20].
In the present work a pig model was used to test the hypothesis that the length of time the inflated balloons are kept in contact with the duodenal papilla determines changes in the pancreas structure, as well as the biochemical markers of pancreatitis. This study might help to reach a better understanding of the factors involved in the etiology of pancreatitis post-DBE.
The experimental work was carried out at the Minimally Invasive Surgery Center Jesús Usón (CCMIJU) (http://www.ccmijesususon.com). All animals received humane care in compliance with the European Communities Council Directive (86/609/EEC) and protocols were approved by the Ethics Committee for Animal Research of the University of Murcia (Ref.452/2009). An EN-450T5 enteroscope (Fuji Film) for exclusive use in animals was used in this work.
Thirty large white pigs (35-40 kg) were used in this study. Pigs were randomly assigned to one of the following groups: GP, GP + EDB and GC.
GP (Group papilla) (n = 10) aimed at evaluating the effect of shearing the minor duodenal papilla independently of DBE. In this group the overtube’s balloon was inflated in the area of the papilla, kept there for 90 min and then removed without DBE exploration. GP + DBE (Group papilla + DBE) (n = 10) was designed to simulate a potential situation (beginner’s scenario) where papilla compression might occur during the first manoeuvres of DBE exploration. In this group the overtube’s balloon was kept inflated in the area of the papilla for 20 min before proceeding with a standard DBE exploration of 90 min. A control group GC (n = 10) for both papilla compression and DBE was used for comparison with GP, GP+DBE. In the control group a conventional esophagogastroduodenoscopy (EGD) was carried out. A forth group was considered GDBE (Group DBE), it represented the most common situation in conventional oral DBE (clinical situation). DBE was carried out for 90-140 min after insuring the balloon´s inflation occurred distal to the pancreatic papilla. The data from GDBE came from work previously published by our group[19].
Before procedure all pigs were fasted for 24 h and then intramuscularly pre-medicated with diazepam 0.1 mg/kg, ketamine 10 mg/kg and atropine 0.01 mg/kg. General anaesthesia was induced with propofol 2 mg/kg intravenously and maintained with Sevofluorane 1.8%-2% delivered via endotracheal tube. DBE were always performed by two of the authors (Pérez-Cuadrado E or Soria F) who have been routinely performing this technique for at least 9 years. During DBE exploration insertion depth was estimated according to the methodology established by May et al[17], which has also been validated in the pig model[21]. After anesthesia, recovering pigs were checked for 24 h for signs of decreased activity, irritability, vomiting or anorexia. Blood samples were taken before procedure (Basal), at the end of the procedure (End) and just before euthanasia (24 h). The serum concentrations of amylase, lipase and C-reactive protein (CRP) were evaluated. Euthanasia was performed by a pentobarbital overdose.
Immediately after death, pancreases were examined in situ and carefully removed from the abdominal cavity, and the left pancreatic lobe (tail) immersed in 10% buffered formaline. The samples for histopathology were systematically taken, making blocks of 1 cm3 (8-12 blocks per pancreas). Tissues were embedded in paraffin, sectioned at 4 microns, and stained with hematoxylin and eosin. Histology sections were studied under light microscopy. The presence and distribution of lesions were evaluated for edema, vacuolization, necrosis, vascular congestion and inflammation. Histopathological findings were characterized and categorized by a pathologist (Candanosa E, author) as showed in Table 1. GDBE histopathology was not included because in this group euthanasia was performed 7 d after procedure[19].
| Parameter/lesion | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
| Edema | Absent | Diffuse dilation of interlobular septi | Diffuse dilation of interacinous space | Diffuse dilation of intercellular space |
| Vacuolization | Absent | Focal (< 25%) | Diffuse (25%-50%) | Severe (> 50%) |
| Necrosis | Absent | < 50% lobules | 50%-75% lobules | > 75% lobules |
| Vessels congestion | Absent | Focal (< 25%) | Diffuse (25%-50%) | Severe (> 50%) |
| Inflammation | Absent | < 50% lobules | 50%-75% lobules | > 75% lobules |
The statistical analysis was carried out with the SPSS 19.0 (SPPS Inc) package. For the biochemical markers (amylase, lipase and CRP) descriptive statistics were calculated and the analysis of variance (ANOVA, linear model with repeated measures) performed considering the different timing of blood sampling as within-subject factors. To evaluate the significance of the histopathological findings the severity (graded 0-3) was compared between the experimental groups by the non-parametric Mann-Whitney test. In addition, the potential association between the histopathological features and the experimental groups was evaluated by contingency tables and the χ2 test. All statistics were initially performed for a significance level of P < 0.05.
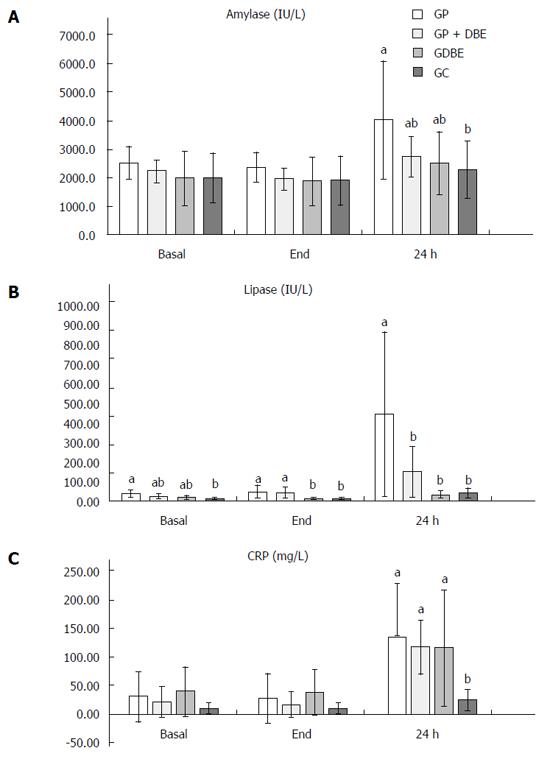
The average serum concentrations of amylase, lipase and CRP at the different sampling periods (Basal, End of experiment and 24 h post-DBE) have been summarized in Table 2 (within groups comparison) and Figure 1 (between groups comparison).
| Group | Time | Amylase (IU/L) | Lipase (IU/L) | CRP (mg/L) |
| GP | Basal | 2507.9 ± 553.61 | 34.3 ± 19.261 | 30.1 ± 43.31 |
| End | 2317 ± 523.52 | 45.4 ± 33.61 | 27.4 ± 41.71 | |
| 24 h | 3993.3 ± 2047.33 | 444.6 ± 417.22,d | 182.3 ± 44.52,d | |
| GP + DBE | Basal | 2214.9 ± 385.91 | 19.6 ± 16.41 | 20.6 ± 26.41 |
| End | 1920.2 ± 360.82 | 37.1 ± 29.52,b | 16.2 ± 21.81 | |
| 24 h | 2717.2 ± 686.62 | 146.8 ± 128.11,2 | 117.3 ± 46.12,d | |
| GDBE | Basal | 1968.1 ± 929.61 | 17.7 ± 7.71 | 39.1 ± 41.21 |
| End | 1850 ± 820.72 | 8.8 ± 3.61 | 37.2 ± 38.31 | |
| 24 h | 2487.2 ± 1093.42 | 26.7 ± 20.91 | 114.8 ± 100.72,d | |
| GC | Basal | 1965.4 ± 856.41 | 10.7 ± 3.11 | 9.8 ± 8.81 |
| End | 1881.9 ± 839.51 | 9.1 ± 2.321 | 10.1 ± 8.31 | |
| 24 h | 2245.8 ± 995.91 | 37.5 ± 23.61 | 24.2 ± 17.41 |
Twenty four hours after the procedure the amylase levels increased quite significantly, such that compared to the basal situation it was 59.2% higher in group GP, and moderately higher in groups GP + DBE and GDBE (22.7% and 20% increases, respectively). However, the amylase levels in GC (control group) hardly changed from the basal levels throughout the experiment (Table 2).
Amylase levels ranged between a maximum in GP and minimum in GC with differences between these two groups. Despite of the fact that the highest amylase levels were found in GP, no significant differences were found between GP, GP + DBE and GDBE at any of the three sampling points (Figure 1A).
The lipase concentration hardly changed throughout the procedure ranging between 8.8 and 45.4 IU/L. However, 24 h after the procedure lipase levels in the papilla-focused groups (GP and GP+DBE) were seen to rise sharply, while hardly changed in those groups where the papilla was not manipulated (GDBE and GC) (Figure 1B). Compared to the basal situation the lipase concentration was 11.4 and 7.5 times higher in GP and GP + DBE, respectively. Thus, inflation and maintenance of the balloons around the papillar area resulted in dramatic changes in the lipase levels one day after the procedure.
None of the procedures resulted in immediate changes in CRP levels (Table 2). The basal CRP range was 11-39.1 mg/L, which included the values observed at the end of the procedures. One day later CRP increased significantly in GP, GP + DBE and GDBE, with values of 5.5 and 6.3 and 3.6 times higher than basal levels, while no significant changes were reported in GC (Table 2).
From a clinical point of view it is important to note that none of the pigs showed significant alterations in the monitored parameters during the procedure (blood pressure, heart and respiratory rates and oxygen saturation). The pigs ate normally and showed no clinical signs of abdominal pain, vomiting, diarrhea or altered sensory behavior in the 24 h following endoscopies.
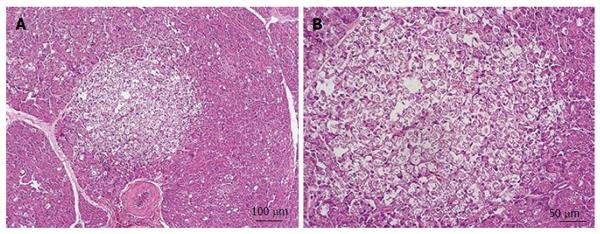
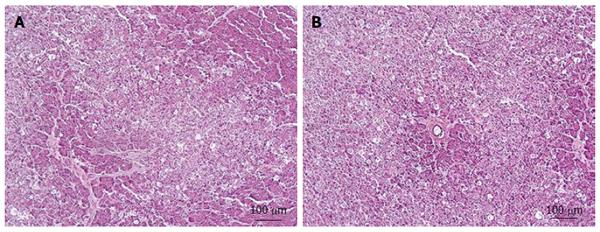
Results from the control group (GC) revealed the absence of edema, vacuolization, necrosis, vascular congestion and inflammation. However, all these pathological features were observed to a certain degree in pancreas samples from groups GP (Figure 2) and GP + EDB (Figure 3). No statistically significant differences between these two groups were found for edema, vessels congestion and inflammation, whereas vacuolization and necrosis of the pancreatic parenchyma was higher in GP than in GP + DBE (Table 3). This result was confirmed by the χ2 test as the frequency of samples with vacuolization and necrosis was significantly different in GP and GP+DBE groups with a 90% of confidence (P < 0.1) (Table 4).
In this study the manipulation of the duodenal papilla by the inflated balloons during DBE was shown to cause pancreatic structural damage and concomitant increase of biochemical markers of pancreatitis. This direct association was experimentally demonstrated in a pig model using two experimental groups (GP and GP+DBE) with different time periods of contact between the balloons and the duodenal papilla. Results were conclusive, the longer the manipulation of the papilla the higher the changes in biochemical markers. Thus, the widely assumed recommendation of avoiding any contact of the balloons with the duodenal papilla so as to decrease post-DBE pancreatic risk[9,14] is now supported by empirical results in an animal model.
Contact between the duodenal papilla and the inflated balloon for 90 min resulted in a very significant increase in amylase (59.2%), lipase (11.4 times) and CRP (5.5 times) levels 24 h after endoscopy (Figure 1). This information gives a clear indication of how the manipulation of the duodenal papilla influences the pancreas independently of DBE. The combined effect of papilla manipulation and DBE was specifically monitored in GP + DBE. Although the results were less dramatic, GP + DBE procedure also resulted in significant increases of amylase (22.7%), lipase (7.5 times) and CRP (6.3 times) levels 24 h after the procedure. In contrast, in GDBE serum levels of amylase and lipase never reached twice the baseline levels (20% for amylase and 1.5 times for lipase), as has been observed in previous studies examining the iatrogenic effects of experimental oral DBE[19]. The differences between groups GP and GP + DBE could be a consequence of the fact that in group GDBE during the oral insertion the balloons were always inflated distally to the duodenal papilla where the pancreatic duct opens into the duodenum, as recommended by some authors[9,22].
The protocol used to monitor post-DBE pancreatitis coincides with the most common protocol in human medicine[2,10,13,22], and was also used in our previous paper[19]. It involves the serological measurement of amylase, lipase and the C-reactive protein (CRP). As a general rule, an increase of amylase and/or lipase higher than twice their basal levels, together with a significant increase of CRP is enough to suspect pancreatitis. There are many potential mechanisms that could hypothetically induce a rise of serum amylase after DBE and some of them have been discussed in previous studies[10,11,13]. For instance, hyperamylasemia has been suggested to occur by the smooth shearing involved while sliding the endoscope and overtube[23] due to its influence in the permeability of the intestine[24]. Thus, in routine DBE in humans amylase levels 24 h after procedure commonly increases up to a bit less than twice baseline levels in a high proportion of patients, 39%[8] 46%[13] and 58%[10]. However, when a relationship between serum amylase and post-endoscopy pancreatitis is suspected, amylase can reach three times the basal levels[25]. Regarding the lipase levels significant increases have also been described in patients at 4 h[9,10,24], 12 h[9] and 24 h[9,10,24] after procedure. It has been suggested that during pancreatic acinar cells injury the sensibility of lipase activity is 82%-100% much better than that amylase activity. Thus, serum lipase activity can increase 2 to 50 times of its upper limit of reference range and remain at such a high level for a longer time than amylase. In previous studies more than 65% of normoamylasemic patients with acute pancreatitis were found to have high lipase activity[26]. Finally, CRP is an indicator sensitive to inflammation but very non-specific regarding the origin of the inflammation. The increased CRP after DBE represents inflammation that could either be mucosal irritation or a clue for suspicion of pancreatitis when combined with hyperamylasemia and hyperlipemia[2,9].
Vacuolization and moderate necrosis in acinar cells was observed in groups GP and GP + DBE, this indicating a relationship between the histological damage and the degree of manipulation of the duodenal papilla during the procedures. As a general rule, the longer the papilla was in direct contact with the inflated balloons the higher the structural changes found in the pancreas (Tables 3 and 4). From an ethiopatological point of view our histology results may resemble those described in experimental models of pancreatic duct obstruction. These studies showed that pancreatic outflow obstruction alone is sufficient to induce necrotizing pancreatitis[27,28] throughout a sequence of events including acinar cell swelling, vacuole formation, swelling and breakdown of mitochondria, nuclear condensation and rupture of the membranes of organelles[29]. As cell vacuolization may be reversible if the cause is removed this may help to explain the lack of clinical symptoms even in pigs of the GP group[29]. On other hand, in previous experimental studies from our group, where the major and minor duodenal papilla were avoided during DBE oral insertion, no vacuolization was observed in acinar cells. The only injures observed in those animals were related to an ischemic process in the vascular supply to the tail of the pancreas[19].
Regarding the experimental model, it might be argued a probably too long contact time between the overtube’s balloon and the papilla in GP and GP + DBE. However, some authors refer to a significant learning curve in acquiring the skills necessary to perform DBE. The results of those authors showed for the first 10 oral-DBE a mean (SD) procedural time of 109 ± 44.6 min[30] or 92.3 ± 38.6 min[31], with a range distance examined between 0 and 665 cm[30] or with a mean speed of less than 1 cm/min for some cases[31]. That indicates that some procedures conducted during the DBE’s learning curve have a very low or even null range of explored distance[30,31] without a clear awareness of the position of the inflated overtube’s balloon. Other authors also correlate the oral-DBE learning curve with the incidence of hyperamylasemia and pancreatitis[10,23]. In DBE, a gripping force by the overtube balloon is used to support endoscopic insertion, and when an untrained endoscopist applies too much insertion force to the endoscope, the forceful insertion causes slippage of the overtube’s balloon and ineffective advancement of the endoscope tip. Finally, in the opinion of several groups DBE should only be performed by an endoscopist with adequate training[15], technical skills and patient volume to maintain their skills[32], a minimum of between 10[31] and 50[33] procedures are required.
In conclusion, during DBE the persistence of the inflated balloon around the area of secretion of the pancreas determines structural damage in the organ and increased levels of biochemical markers (amylase, lipase and CRP). This study in the porcine animal model may help to further understand the potential etiology of post-DBE pancreatitis in humans.
Disturbance of the pancreatic secretion due to direct trauma to the papilla of Vater is one of the most plausible theories about the etiology of acute pancreatitis after double balloon enteroscopy (DBE).
In order to avoid potential trauma in the ampullar zone some endoscopists recommend inflating the balloons after passing the ligament of Treitz, although no causal relationship has been found with a lower incidence of hyperamylasemia or reduction in pancreatitis rate.
This is the first study evaluating the manipulation of the duodenal papilla by the inflated balloons during DBE.
The results of this work support the recommendation of avoiding any contact of the balloons with the duodenal papilla so as to decrease post-DBE pancreatic risk.
DBE is a variety of push and pull endoscopy which allows diagnostic and therapeutic actions deep in the small intestine. Although DBE has been considered a reasonably safe technique, articles reviewing complications have been published recently. Among the major complications acute post-procedure pancreatitis is the most severe. Post-DBE pancreatitis is mainly related with the anterograde approach, it may vary from 1%-3% to 12.5%.
Very interesting paper, well designed, dealing with an important clinical problem.
P- Reviewer: Figueiredo PN, Shah JA, Yen HH S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 2. | Kopacova M, Tacheci I, Rejchrt S, Bartova J, Bures J. Double balloon enteroscopy and acute pancreatitis. World J Gastroenterol. 2010;16:2331-2340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Chavalitdhamrong D, Adler DG, Draganov PV. Complications of enteroscopy: how to avoid them and manage them when they arise. Gastrointest Endosc Clin N Am. 2015;25:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Xin L, Liao Z, Jiang YP, Li ZS. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011;74:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Pohl J, Blancas JM, Cave D, Choi KY, Delvaux M, Ell C, Gay G, Jacobs MA, Marcon N, Matsui T. Consensus report of the 2nd International Conference on double balloon endoscopy. Endoscopy. 2008;40:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Mensink PBF. Complications of Double Balloon Enteroscopy. Techniques in Gastrointestinal Endoscopy. 2008;10:66-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Jarbandhan SV, van Weyenberg SJ, van der Veer WM, Heine DG, Mulder CJ, Jacobs MA. Double balloon endoscopy associated pancreatitis: a description of six cases. World J Gastroenterol. 2008;14:720-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Zepeda-Gómez S, Barreto-Zuñiga R, Ponce-de-León S, Meixueiro-Daza A, Herrera-López JA, Camacho J, Tellez-Avila F, Valdovinos-Andraca F, Vargas-Vorackova F. Risk of hyperamylasemia and acute pancreatitis after double-balloon enteroscopy: a prospective study. Endoscopy. 2011;43:766-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Pata C, Akyüz U, Erzin Y, Mutlu N, Mercan A, Dirican A. Post-procedure elevated amylase and lipase levels after double-balloon enteroscopy: relations with the double-balloon technique. Dig Dis Sci. 2010;55:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kopácová M, Rejchrt S, Tachecí I, Bures J. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest Endosc. 2007;66:1133-1138. [PubMed] |
| 11. | Groenen MJ, Moreels TG, Orlent H, Haringsma J, Kuipers EJ. Acute pancreatitis after double-balloon enteroscopy: an old pathogenetic theory revisited as a result of using a new endoscopic tool. Endoscopy. 2006;38:82-85. [PubMed] |
| 12. | Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 13. | Honda K, Itaba S, Mizutani T, Sumida Y, Kanayama K, Higuchi N, Yoshinaga S, Akiho H, Kawabe K, Arita Y. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: an association with the development of pancreatitis. Endoscopy. 2006;38:1040-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | May A, Ell C. Push-and-pull enteroscopy using the double-balloon technique/double-balloon enteroscopy. Dig Liver Dis. 2006;38:932-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Pérez-Cuadrado E, Latorre R, Carballo F, Pérez-Miranda M, Martín AL, Shanabo J, Esteban P, Torrella E, Mas P, Hallal H. Training and new indications for double balloon endoscopy (with videos). Gastrointest Endosc. 2007;66:S39-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Möschler O, May A, Müller MK, Ell C. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | May A, Nachbar L, Schneider M, Neumann M, Ell C. Push-and-pull enteroscopy using the double-balloon technique: method of assessing depth of insertion and training of the enteroscopy technique using the Erlangen Endo-Trainer. Endoscopy. 2005;37:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Soria F, Lopez-Albors O, Morcillo E, Sarria R, Carballo F, Perez-Cuadrado E, Sanchez F, Latorre R. Experimental laparoscopic evaluation of double balloon versus spiral enteroscopy in an animal model. Dig Endosc. 2011;23:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Latorre R, Soria F, López-Albors O, Sarriá R, Sánchez-Margallo F, Esteban P, Carballo F, Pérez-Cuadrado E. Effect of double-balloon enteroscopy on pancreas: an experimental porcine model. World J Gastroenterol. 2012;18:5181-5187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | López Albors O, Soria F, Pérez Cuadrado E, Morcillo E, Martín C, Carballo LF, Latorre R. Validity of insertion depth measurement in double-balloon endoscopy. Endoscopy. 2012;44:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Aktas H, Mensink PB, Haringsma J, Kuipers EJ. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: has the insertion technique improved? Endoscopy. 2009;41:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Lo SK, Simpson PW. Pancreatitis associated with double-balloon enteroscopy: how common is it? Gastrointest Endosc. 2007;66:1139-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Feng N, Dai J, Lu H, Li XB, Gao YJ, Ge ZZ. Hyperamylasemia is associated with increased intestinal permeability in patients undergoing diagnostic oral double-balloon enteroscopy. World J Gastroenterol. 2014;20:539-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Mallery JS, Baron TH, Dominitz JA, Goldstein JL, Hirota WK, Jacobson BC, Leighton JA, Raddawi HM, Varg JJ, Waring JP. Complications of ERCP. Gastrointest Endosc. 2003;57:633-638. [PubMed] |
| 26. | Yang RW, Shao ZX, Chen YY, Yin Z, Wang WJ. Lipase and pancreatic amylase activities in diagnosis of acute pancreatitis in patients with hyperamylasemia. Hepatobiliary Pancreat Dis Int. 2005;4:600-603. [PubMed] |
| 27. | Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 28. | Lerch MM, Saluja AK, Dawra R, Ramaraò P, Saluja M, Steer ML. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992;103:205-213. [PubMed] |
| 29. | Mareninova OA, Hermann K, French SW, O’Konski MS, Pandol SJ, Webster P, Erickson AH, Katunuma N, Gorelick FS, Gukovsky I. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Fry LC, Mönkemüller K, Neumann H, Weigt J, Bellutti M, Malfertheiner P. Learning Curve of Double Balloon Enteroscopy. Tech Gastrointest Endosc. 2008;10:59-61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Kamal A. Double-balloon enteroscopy: ready for prime time? Gastrointest Endosc. 2008;67:898-901. [PubMed] |
| 33. | Rondonotti E, Sunada K, Yano T, Paggi S, Yamamoto H. Double-balloon endoscopy in clinical practice: where are we now? Dig Endosc. 2012;24:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |