Published online Mar 21, 2016. doi: 10.3748/wjg.v22.i11.3289
Peer-review started: May 25, 2015
First decision: July 19, 2015
Revised: September 5, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: March 21, 2016
Processing time: 294 Days and 14.9 Hours
Post-hepatectomy liver failure (PHLF) is a leading cause of morbidity and mortality following major liver resection. The development of PHLF is dependent on the volume of the remaining liver tissue and hepatocyte function. Without effective pre-operative assessment, patients with undiagnosed liver disease could be at increased risk of PHLF. We report a case of a 60-year-old male patient with PHLF secondary to undiagnosed alpha-1-antitrypsin deficiency (AATD) following major liver resection. He initially presented with acute large bowel obstruction secondary to a colorectal adenocarcinoma, which had metastasized to the liver. There was no significant past medical history apart from mild chronic obstructive pulmonary disease. After colonic surgery and liver directed neo-adjuvant chemotherapy, he underwent a laparoscopic partially extended right hepatectomy and radio-frequency ablation. Post-operatively he developed PHLF. The cause of PHLF remained unknown, prompting re-analysis of the histology, which showed evidence of AATD. He subsequently developed progressive liver dysfunction, portal hypertension, and eventually an extensive parastomal bleed, which led to his death; this was ultimately due to a combination of AATD and chemotherapy. This case highlights that formal testing for AATD in all patients with a known history of chronic obstructive pulmonary disease, heavy smoking, or strong family history could help prevent the development of PHLF in patients undergoing major liver resection.
Core tip: A report of a 60-year-old male who underwent an extended right hepatectomy for metastatic colorectal adenocarcinoma. He subsequently developed post-hepatectomy liver failure secondary to a delayed diagnosis of alpha-1-antitrypsin deficiency. Clinicians should be aware of, and test for, the possibility of undiagnosed alpha-1-antitrypsin deficiency in patients with known chronic obstructive pulmonary disease or a family history, undergoing major liver resection.
- Citation: Norton B, Denson J, Briggs C, Bowles M, Stell D, Aroori S. Delayed diagnosis of alpha-1-antitrypsin deficiency following post-hepatectomy liver failure: A case report. World J Gastroenterol 2016; 22(11): 3289-3295
- URL: https://www.wjgnet.com/1007-9327/full/v22/i11/3289.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i11.3289
Post-hepatectomy liver failure (PHLF) is a leading cause of death following major liver resection with an estimated mortality of 70%[1]. There is no strict definition for PHLF, but it is considered a condition characterised by an inability of the liver to carry out normal excretory, detoxifying and synthetic function after post-operative day 5[2]. There is usually significant biochemical and clinical evidence of liver dysfunction, with persistent hyperbilirubinemia the most sensitive marker, and features of coagulopathy, portal hypertension and ascites[3]. A similar syndrome is seen in liver transplant recipients receiving a donor liver that is too small, resulting in acute liver failure (ALF), termed “small-for-size syndrome”[4]. In both of these scenarios the underlying pathophysiological mechanism of liver dysfunction is complex, and most likely related to liver hepatocyte volume and function. Any factor affecting the ability of the hepatic parenchyma to regenerate following liver resection could significantly affect hepatic flow to the remnant liver, leading to hyperperfusion and cellular damage[5]. Therefore, pre-operative assessment is vital to establish patients with undiagnosed liver disease that are at increased risk of PHLF. We report a case of PHLF secondary to previously undiagnosed alpha-1-antitrypsin deficiency (AATD) after laparoscopic partially extended right hepatectomy and radio-frequency ablation (RFA) for colorectal liver metastases.
A previously well 60-year-old male initially presented to his local hospital with acute large bowel obstruction requiring an emergency Hartmann’s procedure. Intra-operatively, an obstructing tumour was noted in the sigmoid colon with a solitary peritoneal deposit and bi-lobar liver metastasis. Histology confirmed a moderately differentiated adenocarcinoma (pT4 pN2) of the sigmoid colon. Subsequently, he was referred to our centre for further management. There was no significant past medical history apart from mild chronic obstructive pulmonary disease (COPD), managed without inhalers. He was an ex-smoker giving up five years previously, and his alcohol consumption was minimal.
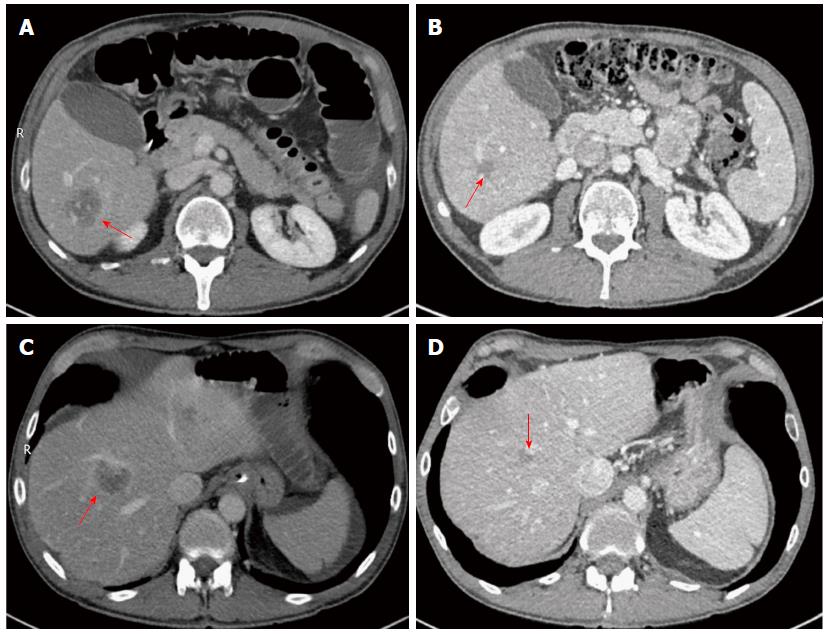
A staging computed tomography (CT) revealed multiple liver metastases located in segments 2/3, 4/8, 5/6, and 6 (Figure 1A and C). He also had a positron emission tomography-CT, which suggested evidence of peritoneal disease in the right side of the abdomen, in addition to the liver metastases. After discussion in the hepatobiliary (HPB) multi-disciplinary team (MDT) he was referred for neo-adjuvant chemotherapy. He received in total 12 cycles of Oxaliplatin and Capecitabine based chemotherapy. After twelve cycles were completed, CT imaging showed evidence of two residual lesions in the right hemi-liver, and a small lesion on the surface of the left hemi-liver with no evidence of peritoneal disease (Figure 1B and D). After discussion in the HPB MDT, he was seen in the clinic and informed about the role of surgery and RFA; he decided to proceed with the operation.
Pre-operative assessment included cardio-pulmonary exercise testing (6.47 min at 20 watt per min ramp, stopping due to knee pain). Anaerobic threshold was 14.2 mL/min per kilogram at 769 mL/min (increased due to low BMI), VO2 max of 21 mL/min per kg and confirmation of moderate COPD with saturations 93% on room air, FEV1/FVC ratio 48%, and significantly elevated VE/VCO2 at 48. He was considered high risk of post-operative respiratory complications. His pre-operative blood results were: alanine transferase 46 (10-41 IU/L), alkaline phosphatase 138 (30-130 U/L), bilirubin 11 (0-20 μmol/L), albumin 47 (35-50 g/L), international normalized ratio 1.1, hemoglobin 168 (130-175 g/L), creatinine 77 (62-106 μmol/L), and urea 4.4 (2.5-7.8 mmol/L).
At diagnostic laparoscopy, there was no evidence of any peritoneal metastasis. On intra-op ultrasound, two lesions were noted in segments 6 and 8/4a, and a solitary lesion in liver segment 2/3. He underwent a laparoscopic partially extended right hepatectomy with cholecystectomy and RFA to the left liver lesion with no intra-operative complications. The liver was transected using a CUSA and Thunderbeat (Olympus®). The total operation time was 390 min with approximately one litre of blood loss recorded and no blood transfusions given.
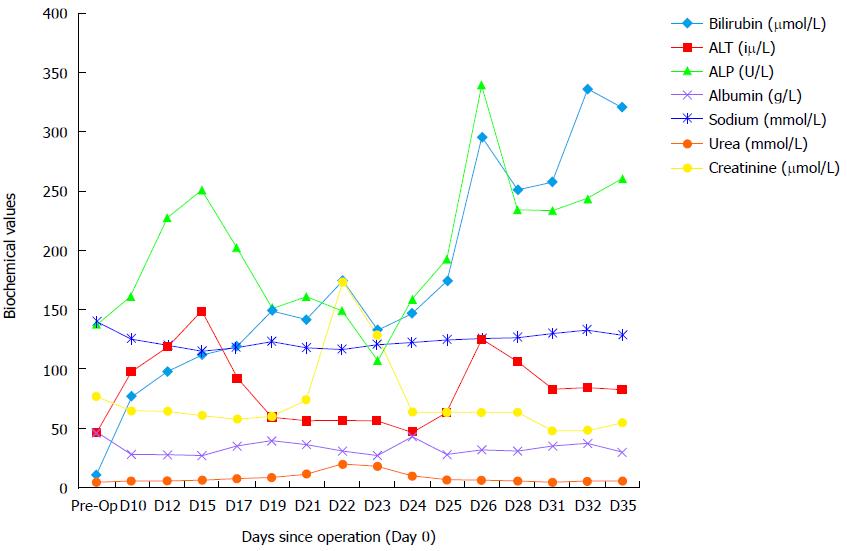
As per our routine practice, post-operatively, he was admitted to the intensive care unit (ICU) and was discharged to the ward four days later. There were no immediate complications while he was on the ICU. On the ward, there was poor progression with reduced oral intake requiring nasogastric feeding, development of ascites with large drain output (> 2.5 L/d) and hyponatremia. Clinical and biochemical markers were suggestive of decompensated liver function (Figure 2). A suspected diagnosis of PHLF was made given his recent major liver resection. He was managed with prophylactic antibiotics, lactulose, albumin replacement, and fluid restriction.
Three weeks after the operation he was readmitted to the ICU because of multi-system dysfunction, including PHLF, hypoxia, hyponatremia, acute kidney injury and suspected sepsis. He had an ultrasound liver scan, magnetic resonance cholangiopancreatography (MRCP), and triple phase CT to identify a source of sepsis and problems with inflow and outflow to the remnant liver. MRCP showed no evidence of biliary obstruction and triple phase CT confirmed patent inflow and outflow with good residual liver volume. CT also showed large volume ascites and bilateral pleural effusions. As no obvious cause could be identified for the development of PHLF, his case for reviewed at the HPB MDT.
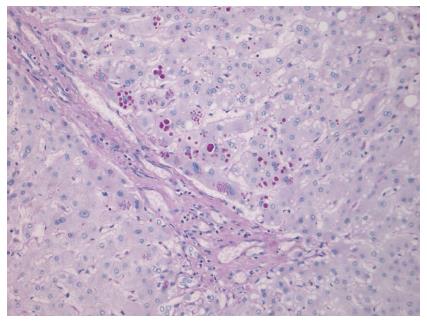
Histology of the resected liver showed four fibrotic nodules, two of which contained residual degenerate moderately differentiated intestinal adenocarcinoma, entirely consistent with colorectal liver metastases. In view of his development of PHLF due to unknown cause, the case was reviewed at the HPB MDT, and it was decided to re-analyse the histology of the liver. This showed features consistent with mild chronic inflammation with a very minor degree of interface activity. Within the liver lobules there was zone three hepatocyte atrophy with patchy inflammation and sinusoidal dilatation; features consistent with chemotherapy effect. Special stains highlighted prominent DPAS positive globules within hepatocytes, which were positive with alpha-1-antitrypsin (AAT) immunohistochemistry (Figure 3). There was no evidence of cirrhosis, and it was concluded that these features would be consistent with AATD. This was confirmed clinically, with blood tests revealing a low serum AAT level of 0.2 (0.9-2.0 g/L), but there was in vivo degradation of the phenotype specimen.
After stabilization in ICU, he was again discharged back to the ward, but had a complicated course over the next month. He experienced several post-operative complications as a result of PHLF. These included severe portal hypertension causing significant ascites requiring frequent paracentesis, malnutrition, recurrent sepsis with a suspected focus either in the chest on ascitic fluid (managed with meropenem and ambisome), parastomal varices with one episode of stomal bleeding requiring a embolisation, persistent hyponatremia, and hypotensive episodes. After several weeks of co-ordinated care between the hepatobiliary surgeons, hepatologists, microbiologists, and intensivists, he developed a second, extensive parastomal bleed, which was decided not for active management. He subsequently died ten weeks following liver resection with his death attributed to PHLF secondary to chemotherapy and previously undiagnosed AATD.
Post-hepatectomy liver failure represents a major cause of morbidity and mortality following liver resection, expected to occur in 10% of cases[6,7]. The success of liver resection, especially for more extensive operations, is dependent on regeneration of hepatic tissue. Hepatic regeneration is a process whereby liver mass is replenished by existing hepatocytes. The mechanism of hepatic regeneration is thought to revolve around three major networks: cytokines, growth factors and metabolic functions. These networks are proposed to interact with one another to promote cellular replication, and provide a driving force through the cell cycle[8]. After liver resection, the remaining hepatic parenchyma needs to accommodate the haemodynamic changes to allow effective regeneration[9]. If the parenchyma is unable to accommodate these changes, which may be due to a severely reduced hepatic volume or poor hepatocyte function, then regeneration is prevented and the liver will become damaged[5]. The decreased hepatocyte volume leads to hyperperfusion causing sinusoid dilatation, kupffer cell activation, and subsequently progressive necrosis of the remnant liver, leading to impairment in its metabolic function and development of PHLF[10].
The volume of the remaining hepatic tissue that must accommodate the haemodynamic changes following liver resection, is termed the functional liver remnant (FLR)[5]. The risk of death in patients undergoing liver resection for malignancy was found to be directly related to the volume of the FLR[11,12]. All patients undergoing liver resection must have a full medical assessment to check for any potential co-morbidity, which could affect regeneration of the FLR. This may include a full blood count, renal function, liver function, albumin, and coagulation studies. Patients with pre-existing liver disease, such as cirrhosis or chemotherapy-associated steatohepatitis (CASH), need to be assessed for suitability of liver resection as this can effect regeneration, putting them at higher risk of developing PHLF[13]. The need to identify high risk patients pre-operatively has led to volumetric analysis using 3D CT reconstructions or magnetic resonance imaging (MRI), as it allows estimation of acceptable FLR percentages[14,15]. Although many authors currently view CT/MRI volumetric analysis as essential prior to major liver resection, there is no standardization for accurately calculating FLR volumes. Imaging tends to over-estimate liver volume, and different formulae have been proposed in an attempt to account for this error[15,16]. Additionally, volumetric analysis does not give any indication as to the underlying hepatic function. For example, there are many predictive patient-factors associated with an increased risk of PHLF not accounted for by imaging. These factors are predominantly related to underlying liver disease, which include: CASH, diabetes and obesity (non-alcoholic fatty liver disease), cirrhosis, hepatic B and C, hyperbilirubinemia, and AATD[13].
In our case, pre-operative assessment did not identify any significant underlying liver disease. The CT images were reviewed at the HPB MDT, which deemed the expected FLR size to be acceptable for a partially extended right hepatectomy, although formal 3D volumetric analysis was not undertaken. Our centre will consider liver resection if we can leave a FLR of 30% in post chemotherapy livers, 40% in patients with established cirrhosis/fibrosis, and 20% in a normal a liver. In this case, more formal testing for underlying liver disease, such as AATD, as part of the pre-operative assessment, could have altered our surgical management, and ultimately, helped to prevent the development of PHLF. We believe that PHLF in our patient was due to several factors; mainly undiagnosed AATD disorder, neo-adjuvant chemotherapy, and extended liver resection. However, it is currently not routine practice to check for AATD in patients requiring liver resection with no evidence of cirrhosis or family history.
AATD is an under-diagnosed condition with a suspected worldwide prevalence of 3.4 million people[17]. It is an autosomal recessive disease, with co-dominant expression, caused by a mutation in the SERPINA1 gene[18,19]. There are approximately 123 single nucleotide polymorphisms affecting the gene[19]. The M allele accounts for over 95% of alleles in the general population, with other alleles including S and Z, accounting for 2%-3% and 1% respectively[18]. The normal phenotype is denoted Pi-MM, while the most severe phenotype is Pi-ZZ, which leads to low concentrations of the AAT protein and highest risk of organ damage[20]. The most significant organs affected are the lungs and liver. AAT is usually produced within hepatocytes, but defective alleles can lead to its polymerization, cellular aggregation, and subsequent liver dysfunction[21,22]. AAT is normally excreted from hepatocytes where it may counteract the action of neutrophil elastase. Failed excretion can lead to pulmonary inflammation and proteolytic damage, which may manifest as COPD[23]. Other associated clinical manifestations of AATD can include panniculitis and vasculitis[19].
Due the effect of AATD on hepatocyte function, left undiagnosed, it could have deleterious effects following liver resection. Using a Pi-Z mouse model, one study showed that proliferation of hepatocytes predominantly occurred in cells without abnormal AAT accumulation[24]. Therefore, it is unsurprising that hepatocyte regeneration was affected in our case of undiagnosed AATD. At present, the ATS/ERS recommends testing for AATD in all patients with COPD[25]. Moreover, it is suggested that living-donor liver transplantations should be screened for underlying metabolic disorders (e.g., AATD) as they could pose safety issues to both donor and recipient[26].
In a situation where AATD is detected pre-operatively, we recommend avoidance of major liver resection as it will affect liver regeneration. We would recommend pre-operative liver biopsy and liver volumetric analysis before deciding whether or not to consider a patient for major liver resection. If the FLR is less than 40% then major liver resection should be avoided and measures should be taken to increase FLR prior to surgery (e.g., portal vein embolization). In our case, we would not have considered major liver resection if we were aware of his diagnosis prior to surgery. Especially since he received several cycles of neo-adjuvant chemotherapy.
Currently, there is no recommendation for AATD testing in patients undergoing major liver resection. To help prevent the development of PHLF as seen in our case, we recommend formal testing for AAT levels and AATD phenotyping, in all patients with a known history of COPD, heavy smoking history, or those with a strong family history undergoing major liver resection. If AATD is identified, we recommend avoidance of major hepatectomy (more than 4 segments), even if patients do not receive neo-adjuvant chemotherapy.
In conclusion, this case demonstrates the need for better screening for AATD, which is an under-diagnosed condition, in susceptible patients undergoing major liver resection. Identification of patients with AATD prior to surgical intervention, would aid pre-operative assessment and surgical planning, and ultimately, help to prevent the development of post-hepatectomy liver failure.
A report of a 60-year-old male who underwent an extended right hepatectomy for metastatic colorectal adenocarcinoma. He subsequently developed post-hepatectomy liver failure secondary to a delayed diagnosis of alpha-1-antitrypsin deficiency (AATD).
Post-hepatectomy liver failure (PHLF) associated with significant ascites, malnutrition, recurrent sepsis and parastomal varices.
AATD, chemotherapy-associated steatohepatitis, non-alcoholic fatty liver disease.
Hyponatremia, acute kidney injury, acute liver failure and alpha-1-antitrypsin (AAT) deficiency: AAT level of 0.2 (0.9-2.0 g/L).
Magnetic resonance cholangiopancreatography showed no evidence of biliary obstruction and triple phase computed tomography confirmed patent inflow and outflow with good residual liver volume. No obvious cause could be identified on imaging for the development of PHLF.
AATD. Prominent DPAS positive globules within hepatocytes, which were positive with AAT immunohistochemistry.
Conservative management failed and unfortunately the patient died.
PHLF is a leading cause of death following major liver resection, which is related to failure in regeneration of the remaining hepatic tissue. This may be due to intrinsic liver disease and/or reduced volume of the functional liver remnant. To date, this is the first report of PHLF secondary to a delayed diagnosis of AATD following major liver resection.
AATD is an autosomal recessive disease that is under recognised. The AAT protein is usually produced within hepatocytes, but defective alleles can lead to cellular aggregation and subsequent liver dysfunction. This causes impaired regeneration post-hepatectomy.
This case demonstrates the need for better screening for AATD, which is an under-diagnosed condition, in susceptible patients (e.g., COPD, family history) undergoing major liver resection.
This is a well written and informative case report.
P- Reviewer: Lykoudis PM, Sanal MG, Wu CC S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 2. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1724] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 3. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862; discussion 862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 4. | Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155. [PubMed] |
| 5. | Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr. 2014;3:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 6. | Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, Belghiti J. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 9. | Asencio JM, Vaquero J, Olmedilla L, García Sabrido JL. “Small-for-flow” syndrome: shifting the “size” paradigm. Med Hypotheses. 2013;80:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 13. | Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 14. | Mise Y, Tani K, Aoki T, Sakamoto Y, Hasegawa K, Sugawara Y, Kokudo N. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat Sci. 2013;20:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | D'Onofrio M, De Robertis R, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J Radiol. 2014;6:62-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Niehues SM, Unger JK, Malinowski M, Neymeyer J, Hamm B, Stockmann M. Liver volume measurement: reason of the difference between in vivo CT-volumetry and intraoperative ex vivo determination and how to cope it. Eur J Med Res. 2010;15:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122:1818-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Fairbanks KD, Tavill AS. Liver disease in alpha 1-antitrypsin deficiency: a review. Am J Gastroenterol. 2008;103:2136-2141; quiz 2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Kelly E, Greene CM, Carroll TP, McElvaney NG, O’Neill SJ. Alpha-1 antitrypsin deficiency. Respir Med. 2010;104:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345-351. [PubMed] |
| 21. | Perlmutter DH, Brodsky JL, Balistreri WF, Trapnell BC. Molecular pathogenesis of alpha-1-antitrypsin deficiency-associated liver disease: a meeting review. Hepatology. 2007;45:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Wu Y, Whitman I, Molmenti E, Moore K, Hippenmeyer P, Perlmutter DH. A lag in intracellular degradation of mutant alpha 1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc Natl Acad Sci USA. 1994;91:9014-9018. [PubMed] |
| 23. | American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 665] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 24. | Llewellyn-Jones CG, Lomas DA, Carrell RW, Stockley RA. The effect of the Z mutation on the ability of alpha 1-antitrypsin to prevent neutrophil mediated tissue damage. Biochim Biophys Acta. 1994;1227:155-160. [PubMed] |
| 25. | Teckman JH, Qu D, Perlmutter DH. Molecular pathogenesis of liver disease in alpha1-antitrypsin deficiency. Hepatology. 1996;24:1504-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Schielke A, Conti F, Goumard C, Perdigao F, Calmus Y, Scatton O. Liver transplantation using grafts with rare metabolic disorders. Dig Liver Dis. 2015;47:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |