Published online Mar 21, 2016. doi: 10.3748/wjg.v22.i11.3275
Peer-review started: July 4, 2015
First decision: September 29, 2015
Revised: October 20, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: March 21, 2016
Processing time: 257 Days and 7.7 Hours
AIM: To prospectively analyze the impact of increased intestinal permeability (IP) on mortality and the occurrence of infections in patients with cirrhosis.
METHODS: IP was quantified using the lactulose/mannitol (L/M) test in 46 hospitalized patients with cirrhosis (25 Child-Pugh A/B, 21 Child-Pugh C) and in 16 healthy controls. Markers of inflammation [LPS-binding protein, Interleukin-6 (IL-6)] and enterocyte death [intestinal fatty-acid binding protein (I-FABP)] were determined in serum using enzyme-linked immunosorbent assays. Patients were followed for one year and assessed for survival, liver transplantation, the necessity of hospitalization and the occurrence of bacterial infections. The primary endpoint of the study was defined as differences in survival between patients with pathological and without pathological lactulose/mannitol test.
RESULTS: Thirty-nine (85%) patients with cirrhosis had a pathologically increased IP index (L/M ratio > 0.07) compared to 4 (25%) healthy controls (P < 0.0001). The IP index correlated with the Child-Pugh score (r = 0.484, P = 0.001) and with serum IL-6 (r = 0.342, P = 0.02). Within one year, nineteen (41%) patients developed a total of 33 episodes of hospitalization with bacterial or fungal infections. Although patients who developed spontaneous bacterial peritonitis (SBP) (n = 7) had a higher IP index than patients who did not (0.27 vs 0.14, P = 0.018), the baseline IP index did not predict time to infection, infection-free survival or overall survival, neither when assessed as linear variable, as tertiles, nor dichotomized using an established cut-off. In contrast, model for end-stage liver disease score, Child-Pugh score, the presence of ascites, serum IL-6 and I-FABP were univariate predictors of infection-free survival.
CONCLUSION: Although increased IP is a frequent phenomenon in advanced cirrhosis and may predispose to SBP, it failed to predict infection-free and overall survival in this prospective cohort study.
Core tip: Increased intestinal permeability (IP) is a frequent phenomenon in patients with cirrhosis and has been linked to pathological bacterial translocation, bacterial infections and mortality in retrospective studies. In this prospective study on 46 patients with cirrhosis we show that increased small-bowel IP, quantified using the lactulose/mannitol test, is frequently observed and correlates with inflammation and Child-Pugh stage. Although a higher IP index indicated an increased risk for developing spontaneous bacterial peritonitis, it failed to predict the pre-defined endpoints infection-free and overall survival. In contrast, the model for end-stage liver disease and Child-Pugh score, the presence of ascites, and higher serum levels of interleukin-6 or intestinal fatty-acid binding protein were significant univariate predictors of infection-free survival. Thus, the high prevalence of pathological test results in advanced cirrhosis and the lack of association with mortality limit the use of dual-sugar tests as tool for risk stratification in clinical practice.
- Citation: Vogt A, Reuken PA, Stengel S, Stallmach A, Bruns T. Dual-sugar tests of small intestinal permeability are poor predictors of bacterial infections and mortality in cirrhosis: A prospective study. World J Gastroenterol 2016; 22(11): 3275-3284
- URL: https://www.wjgnet.com/1007-9327/full/v22/i11/3275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i11.3275
Bacterial infections are diagnosed in 25% to 47% of hospitalized patients with cirrhosis[1-4] and have been shown to be an important risk factor for acute decompensation[5] and mortality[6]. A relevant mechanism, which contributes to bacterial infections in patients with decompensated cirrhosis, is the phenomenon of pathological bacterial translocation, where bacteria and bacterial products translocate from the lumen of the gastrointestinal tract through the intestinal wall into mesenteric lymph nodes and the systemic circulation[7-9]. Whereas healthy subjects are capable to control bacterial translocation via various immunological barriers, pathological bacterial translocation frequently escalates to systemic inflammation and bacterial infections due to several immunological dysfunctions as reviewed elsewhere[10].
Increased intestinal permeability (IP) is a frequently observed phenomenon in patients with cirrhosis[11-13] and is believed to contribute to pathological bacterial translocation in cirrhosis[14]. It correlates with portal hypertension as well as with systemic inflammation[15]. Catabolic and inflammatory stressors affect the intestinal epithelial barrier and contribute to pathological IP[16-18]. As a result, increased bacterial translocation and induced proinflammatory cytokines leading to alterations of the structure of apical adhesion complexes as well as edema, fibromuscular and vascular proliferation, widening of intercellular space and an enlarged muscularis mucosae augment the destruction of the intestinal integrity[14,19] likely in a self-perpetuating manner. Since it is not known, whether increased IP is the cause or the result of pathological bacterial translocation in cirrhosis, the assessment of IP as a clinical test to predict complications in cirrhosis is controversial.
In some clinical studies, increased IP has been correlated with spontaneous bacterial peritonitis (SBP) and bacteremia mainly in retrospective cohort studies[8,20], which could not be replicated in one prospective study[21]. In a recent report[15] altered IP was associated with variceal bleeding but not with mortality questioning a clinically significant association with bacterial infections. Against that background, we performed a cohort study to prospectively assess the impact of IP on bacterial infections and mortality in cirrhosis and to compare its predictive accuracy with serum surrogate markers of systemic inflammation and a marker of enterocyte damage.
During November 2010 and March 2012, 46 consecutive hospitalized patients with liver cirrhosis, defined by the combination of clinical, analytic and imaging criteria, were included in the study. Exclusion criteria were gastrointestinal bleeding, the use of antibiotics, steroids or immunosuppressive drugs or drinking alcohol within the last two weeks. Written informed consent was given by all patients. The study protocol was approved by the Internal Review Board (Ethics Committee of the Friedrich Schiller University Jena, 2931-09/10). Etiology of the liver cirrhosis, age, gender, grade of ascites and hepatic encephalopathy, concurrent medication, history of SBP and vital parameters of the patients were assessed on the day of inclusion. Components of the model for end-stage liver disease (MELD) score and the Child-Pugh score were assessed using routine laboratory data. To assess intestinal permeability, lactulose/mannitol test was performed as described below. Serum samples were collected at the day of inclusion and stored at -80 °C until further analysis. Patients were followed-up for a maximum of 365 d and assessed for survival, liver transplantation, the necessity of hospitalization and the occurrence of bacterial infections. As a control group, 16 healthy volunteers were enclosed. All of them had an inconspicuous medical history without gastrointestinal abnormalities, infections, liver disease, history of major intestinal surgery or alcohol abuse and were not allowed to have taken antibiotics or anti-inflammatory drugs within the last month.
The primary end-point of the study was defined as differences in 90-d mortality rates between patients with pathological and without pathological lactulose/mannitol test. Owing to few events within 90 d (4 deaths), the observation period was extended to one year and differences in survival between both groups were analyzed. Secondary endpoints were the occurrence of bacterial infections, occurrence of SBP and the number of hospitalizations.
After overnight fasting, the patients and controls were instructed to drink 100 mL of a solution consisting of 2 g mannitol and 5 g lactulose with 40 g glucose as an osmotic filler[21,22]. Concomitant medication with lactulose was stopped two days before the test. Urine was collected over 5 h and the volume was measured. Two aliquots of 9 mL urine each were centrifuged for 5 min (700 rcf, 20 °C) and cell-free supernatants were stored at -20 °C. High performance liquid chromatography analysis (Hitachi High Technology, Schaumburg, IL, United States) was performed by GANZIMMUN Diagnostics AG® (Frankfurt, Germany) to determine the lactulose and mannitol concentration in the 5 h urine collection. The IP index (lactulose/mannitol ratio) was calculated for each study subject using the following formula:
IP index = recoverylac/recoveryman =
clac× V/5 g/cman× V/2g
Pathologically increased IP was defined by an IP index greater than 0.07[23].
From each patient, a maximum of 9 mL of whole blood were taken, allowed to clot at room temperature over 30 min, centrifuged for 10 min at 1000 rcf and serum was stored at -80 °C until further analysis. Enzyme-linked immunoassays (ELISA) were used to asses the concentrations of intestinal fatty acid-binding protein (I-FABP), lipopolysaccharide-binding protein (LBP) and interleukin-6 (IL-6) in serum. I-FABP concentrations were determined in diluted samples using the human I-FABP ELISA Kit (Hycult biotech, Uden, Netherlands) providing a lower limit of detection (LOD) of 47 pg/mL. IL-6 was determined using the human IL-6 ELISA kit (RayBiotec, Norcross, United States) in undiluted samples (LOD 3 pg/mL). LBP was analyzed using the human LBP ELISA Kit (Hycult biotec, Uden, Netherlands) using serially diluted samples (LOD 4.4 ng/mL) as described[24]. Absorbance was measured in 96-well plates on a photospectrometer (Infinite® 200 Pro, TECAN, Männedorf, Switzerland) at 450 nm. Sample volumes were insufficient to determine I-FABP concentration in 10 samples.
The statistical analyzes were performed using SPSS 22 (IBM, Armonk, NY, United States) and Prism 5 (GraphPad, La Jolla, CA, United States). Bivariate nonparametric correlation analysis (Spearman’s rho, rs) was performed to identify correlations between IP index and continuous or ordinal variables. To compare two groups we used Wilcoxon-Mann-Whitney tests for continuous and Fisher’s exact test for nominal variables. Time-to-event variables were displayed by the Kaplan Meier method and groups were contrasted by Log-rank tests and Cox regression analyses. Continuous variables were assessed as linear and dichotomized predictors according to the maximum Youden index in receiver operating characteristics (ROC) analysis or presented as quantiles if not assessable in this way. Multiple stepwise-forward Cox regression analysis was performed using dichotomized variables, which met P < 0.05 in univariate analysis and which were available for the total cohort. We applied a two-sided significance level P = 0.05 and did not correct for multiple testing due to the limited statistical power of this explorative observational study.
Forty-six patients with liver cirrhosis were included. Sixty-one percent (28/46) were male. The median age was 59 years (range, 42-81) and the majority of patients had alcoholic liver disease (72%, 33/46) with advanced liver disease. The median Child-Pugh score was 9 (range, 5-14), the median MELD score 15 (range, 6-27). Frequent comorbidities were diabetes (22%, 10/46) and hepatocellular carcinoma (20%, 9/46) (Table 1). Among the 16 controls included, 44% (7/16) were males. The median age of controls was 33 years (range, 26-59).
| Median (range)/frequency | |
| Age (yr) | 59 (42-81) |
| Male sex | 28 (61) |
| Etiology | |
| Alcoholic liver disease | 33 (72) |
| Viral hepatitis | 6 (13) |
| Other | 7 (15) |
| Comorbidity | |
| Hepatocellular carcinoma | 9 (20) |
| Diabetes | 10 (22) |
| Child-Pugh stage | |
| A | 6 (13) |
| B | 19 (41) |
| C | 21 (46) |
| Ascites | 31 (67) |
| Child-Pugh score | 9 (5-14) |
| MELD score | 15 (6-27) |
| Bilirubin (µmol/L) | 61.4 (4-429) |
| International normalized ratio | 1.4 (0.9-2.6) |
| Creatinine (µmol/L) | 114 (43-533) |
| CrP (mg/L) | 23.1 (2-112.4) |
| Albumin (g/L) | 28 (17-51) |
| Sodium (mmol/L) | 135 (125-144) |
| White blood cells (Gpt/L) | 6.3 (1.2-22.2) |
| Platelets (Gpt/L) | 135 (29-442) |
| Alanine aminotransferase (µmol/L*s) | 1.41 (0.33-20.86) |
| Aspartate aminotransferase (µmol/L*s) | 1.6 (0.4-8.8) |
| Medication | |
| Non-selective beta blockers | 19 (41) |
| Lactulose | 21 (46) |
| Proton pump inhibitors | 36 (78) |
| Terlipressin | 1 (2) |
| Acetylsalicylic acid | 5 (11) |
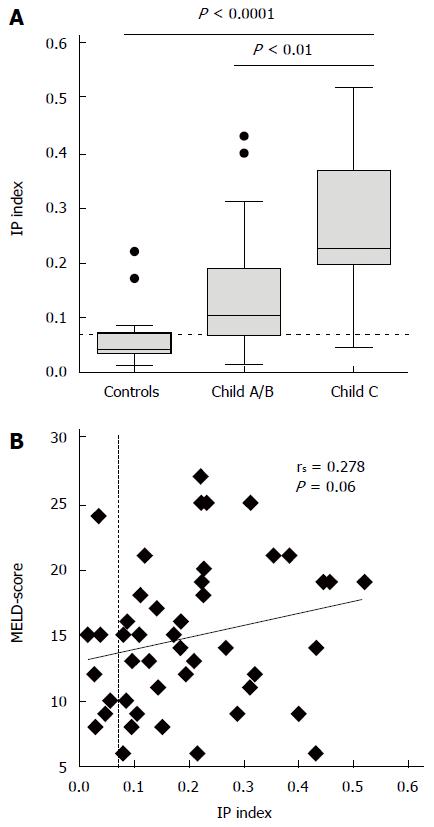
Thirty-nine (85%) patients with cirrhosis had pathologically increased IP in contrast to four (25%) of the healthy controls (P < 0.0001). In comparison to controls patients with cirrhosis had higher lactulose recovery (median 2.0% vs 0.6%, P = 0.005) alongside lower mannitol recovery (11.2% vs 16.1%, P = 0.03). The IP index was highest in patients with Child-Pugh C cirrhosis (Figure 1A) and correlated with Child-Pugh score (rs = 0.484, P = 0.001) and its components bilirubin (rs = 0.381, P = 0.009), albumin (rs = -0.339, P = 0.021) and the international normalized ratio (INR, rs = 0.445, P = 0.002) (Table 2). Because of lack of association of the IP index with creatinine, a correlation of the IP index with the MELD score failed the level of statistical significance (Figure 1B). There was no significant association the IP index with diabetes, serum creatinine or co-medication with proton pump inhibitors (PPI) or non-selective beta blockers (NSBB).
| Spearman’s rho | P value | |
| Age | -0.166 | 0.270 |
| Child-Pugh score | 0.484 | 0.0007 |
| MELD score | 0.282 | 0.060 |
| Bilirubin | 0.381 | 0.009 |
| International normalized ratio | 0.445 | 0.002 |
| Creatinine | -0.214 | 0.154 |
| Serum albumin | -0.339 | 0.021 |
| Sodium | 0.013 | 0.930 |
| White blood cells | 0.174 | 0.247 |
| C-reactive protein | 0.102 | 0.499 |
| Platelets | 0.049 | 0.748 |
| Alanine aminotransferase | -0.063 | 0.686 |
| Aspartate aminotransferase | 0.347 | 0.041 |
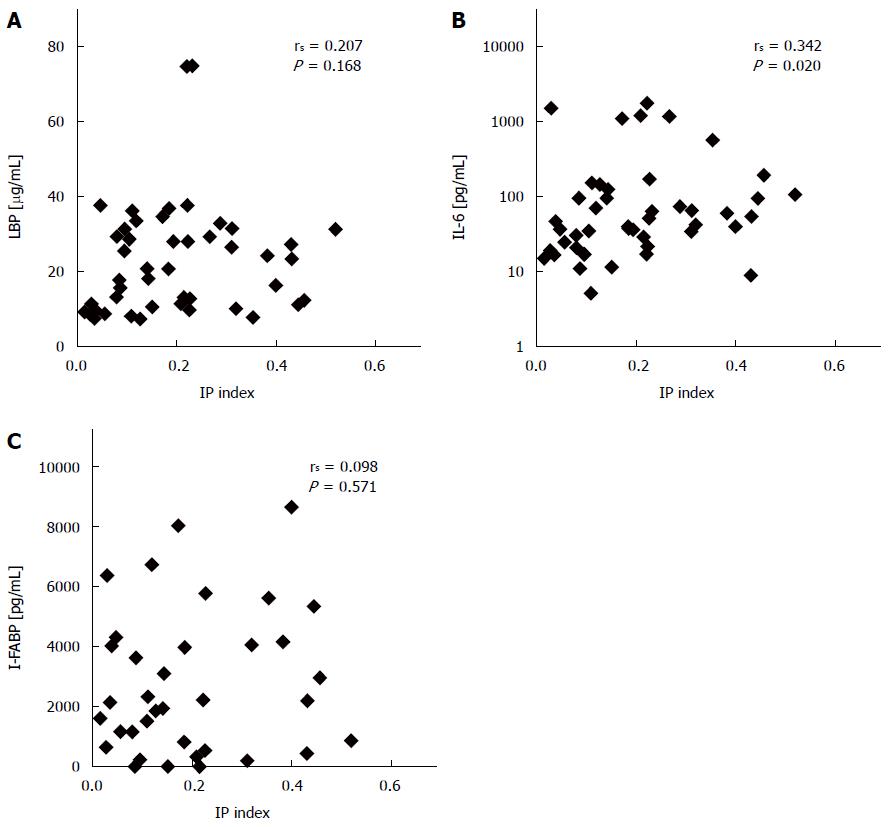
Although there was no overall correlation of the IP index with LBP serum concentrations (Figure 2A), median LBP levels were significantly higher in patients with pathological IP than in patients without (median 25 μg/mL vs 13 μg/mL, P = 0.01). In addition, higher serum concentrations of IL-6 correlated with higher IP index (Figure 2B). When serum was assessed for concentrations of I-FABP, an enterocyte-specific marker of mucosal injury, I-FABP was higher than the expected normal range (210-870 pg/mL) in 72% (26/36) of patients. There was no evidence for a correlation of I-FABP with the IP index (Figure 2C) or with pathological IP (P = 0.61).
Within 365 d follow-up, 78% (36/46) of the patients were hospitalized after a median of 42 d (range, 11-216 d) resulting in 111 hospitalizations with a total duration of 1464 d. On average 2 hospitalizations per patient with a median duration of 20 d were observed. Nineteen (41%) patients had a total of 33 episodes of hospitalization with admission for bacterial or fungal infection or development of infection during hospital stay. The most frequent infections were urinary tract infections in 9 (20%) patients, SBP in 7 patients (15%), spontaneous or secondary bacteremia/fungemia in 6 (13%) patients (4 × Gram-positive cocci, 1 ×E. coli, 1 ×Candida spp.), Clostridium difficile-associated enterocolitis in 5 (11%) patients. Less frequently observed infections were purulent skin and soft tissue infections including wound infections (n = 2), pneumonia (n = 1), campylobacter enteritis (n = 1), spontaneous bacterial empyema (n = 1), monomicrobial bacterascites (n = 1) and severe sepsis with unknown focus (n = 1). The median time to hospitalization for infection was 71 d (range, 17-302). Patients who developed bacterial infection had higher Child-Pugh score and higher MELD scores at baseline, lower platelet count and higher I-FABP serum concentration than patients who did not. The IP index at baseline was not associated with the occurrence of bacterial or fungal infection in general (Table 3). The IP index was not higher in patients who urinary tract infection (median 0.22 vs 0.15, P = 0.41), bacteremia/fungemia (0.16 vs 0.19, P = 0.71) or Clostridium difficile-associated enterocolitis (0.19 vs 0.18, P = 0.49) compared to patients who did not.
| Without subsequent infection | With subsequent infection | P value | |
| (n = 27) | (n = 19) | ||
| Age (yr) | 56 (42-78) | 60 (44-81) | 0.220 |
| Male sex | 17 (63) | 11 (58) | 0.770 |
| Alcoholic liver disease | 22 (81) | 11 (58) | 0.100 |
| Diabetes | 7 (26) | 3 (16) | 0.490 |
| Child-Pugh stage | |||
| A | 6 (22) | 0 | 0.070 |
| B | 11 (41) | 8 (42) | |
| C | 10 (37) | 11 (58) | |
| Ascites | 14 (52) | 17 (89) | 0.010 |
| Child-Pugh score | 9 (5-13) | 10 (7-14) | 0.046 |
| MELD | 12 (6-27) | 17 (9-25) | 0.0008 |
| CrP (mg/L) | 16 (2-91) | 17 (2-112) | 0.720 |
| Sodium (mmol/L) | 136 (125-142) | 136 (131-144) | 0.970 |
| White blood cells (Gpt/L) | 6.1 (2.8-22.2) | 6.5 (1.2-9.0) | 0.860 |
| Platelets (Gpt/L) | 159 (42-442) | 99 (29-163) | 0.020 |
| IP index | 0.14 (0.03-0.52) | 0.22 (0.02-0.045) | 0.250 |
| Pathological IP index | 23 (85) | 16 (84) | 1.000 |
| LBP (µg/L) | 18 (7-75) | 24 (7-75) | 0.720 |
| IL-6 (pg/mL) | 37 (9-1791) | 64 (5-1191) | 0.360 |
| I-FABP1 (pg/mL) | 1161 (nd-8683) | 4036 (1520-8061) | 0.005 |
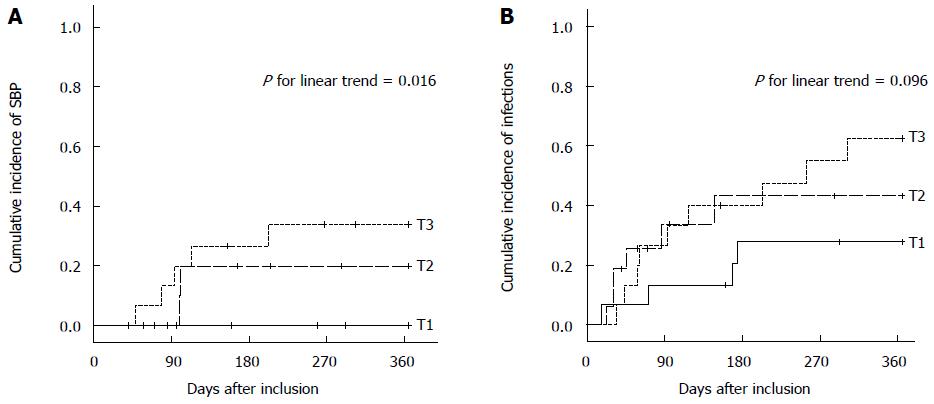
However, patients who developed SBP had a higher IP index (median 0.27) than patients who did not (median 0.14, P = 0.018). The accuracy of the IP index to identify patients with subsequent SBP [area under the ROC curve (AUROC) 0.780, P = 0.02] were comparable to the MELD score (AUROC 0.744, P = 0.04), Child-Pugh score (AUROC 0.736, P = 0.05), serum I-FABP (AUROC 0.879, P = 0.03) or the platelet count (AUROC 0.700, P = 0.10). When IP index was stratified according to tertiles, the cumulative 1-year incidences of SBP were 0%, 20% ± 13%, and 34% ± 12% (estimates ± standard error), in patients in the lowest, median and highest tertile according to Kaplan-Meier analysis (P for linear trend = 0.016) (Figure 3A). The cumulative incidences of hospitalization for infection did not significantly differ between patients in the lowest (28% ± 12%), median (43% ± 13%), and highest (63% ± 13%) tertile (P for linear trend = 0.096) (Figure 3B).
Twenty-eight percent (13/46) of the patients died within 365 d of follow-up after a median of 116 d. Nine percent (4/46) underwent liver transplantation after a median of 180 d. One patient was lost to follow-up after 58 d, and three patients after 267-292 d. Among the 19 patients who developed bacterial infection, 9 (47%) died within the observation period, in contrast to 4 out of 27 (15%) patients who did not develop infection (P = 0.02).
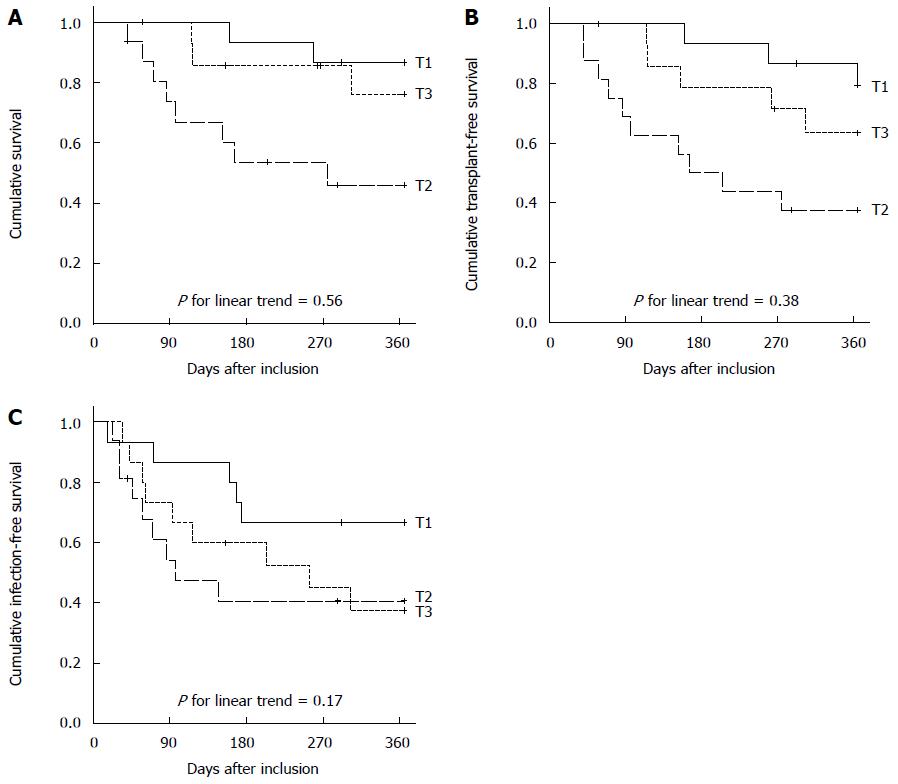
We observed 2 (29%) deaths in patients with normal IP compared to 11 (28%) deaths in patients with pathologically increased IP (P = 1.000). There was no significant correlation between pathological IP and mortality (P = 0.82), transplant-free survival (P = 0.90) or infection-free survival (P = 0.90) according to Kaplan-Meier analysis. Overall survival and transplantation-free survival were lowest in patients in the median tertile group compared to patients in the highest and lowest tertile, and the transplantation-free survival did not differ between the groups (Figure 4).
In contrast to the IP (AUROC 0.550, P = 0.56), higher MELD score (AUROC 0.736, P = 0.006), higher Child-Pugh score (AUROC 0.736, P = 0.006), and higher I-FABP concentration (AUROC 0.774, P = 0.005) were significantly associated with 1-year infection-free survival when linearly assessed in ROC analysis and also in Cox regression after dichotomization. Higher serum IL-6 (AUROC 0.660, P = 0.06) showed a trend for predicting infection-free survival when assessed as linear continuous variable and was significant in a Cox regression model after dichotomization. In multivariate analysis only MELD score > 13 and the presence of ascites at baseline predicted infection-free survival (Table 4).
| Bacterial infection or death within 1 yr | Univariate hazard ratio (95%CI) | P value | Adjusted hazard ratio3 (95%CI) | P value | |
| MELD score1 | |||||
| ≤ 13 | 5/20 (25) | 1.00 (reference) | 0.003 | 1.00 (reference) | 0.02 |
| > 13 | 18/26 (69) | 4.45 (1.64-12.07) | 3.23 (1.18-8.83) | ||
| Child-Pugh | |||||
| Score1 | |||||
| ≤ 7 | 1/13 (8) | 1.00 (reference) | 0.010 | Not included | |
| > 7 | 22/33 (67) | 13.63 (1.83-101.73) | (not significant) | ||
| Ascites1 | |||||
| No | 2/15 (13) | 1.00 (reference) | 0.003 | 1.00 (reference) | 0.01 |
| Yes | 21/31 (68) | 9.01 (2.10-38.70) | 6.95 (1.59-30.28) | ||
| IL-61 | |||||
| ≤ 35 pg/mL | 4/17 (24) | 1.00 (reference) | 0.010 | Not included | |
| > 35 pg/mL | 19/29 (66) | 3.87 (1.31-11.47) | (not significant) | ||
| I-FABP12 | |||||
| ≤ 1300 pg/mL | 1/13 (8) | 1.00 (reference) | 0.010 | Not included | |
| > 1300 pg/mL | 16/23 (70) | 13.85 (1.83-105.04) | (missing data2) |
In this prospective study, increased IP was associated with more advanced liver disease and increased systemic inflammation at baseline but failed to predict adverse clinical outcome in a 1-year follow-up period when focusing on the endpoints mortality, liver transplantation and infections. Because bacterial infections are a common complication during disease progression of cirrhosis and are accompanied by higher mortality rates and prolonged hospital stays[2,6], reliable predictors of infection are needed in order to identify patients at the highest risk. Increased IP is understood as a major contributor to bacterial infections in cirrhosis and acute-on-chronic liver failure[10]. However, prospective studies directly showing a link between the degree of IP in cirrhosis and clinical endpoints are scarce.
To assess the degree of small-bowel IP, we applied the frequently utilized lactulose/mannitol test. As reported[11-13], IP indices in patients with cirrhosis were elevated when compared to healthy controls, and the IP index in patients correlated with the severity of liver cirrhosis as quantified by the Child-Pugh score and its components. The prevalence of pathological IP in our study (85%) was within range reported (62%-100%)[13,15] but exceeded prevalences reported with the 51Cr-EDTA test ranging from 31% in compensated to 60% in decompensated cirrhosis[8].
Although the use of oral sugar tests to assess global intestinal permeability and the risk of pathological bacterial translocation in cirrhosis is debatable[25], the results of the lactulose/mannitol test correlated with degree of portal hypertension and improved after NSBB therapy in a recent study[15]. In our study, IP was associated with surrogates of endotoxemia (LBP) and IL-6 supporting the validity of the lactulose/mannitol to assess pathological bacterial translocation (BT) and its pro-inflammatory consequences in cirrhosis. Particularly, its association with higher LBP levels, which predicted decompensation[26] and bacterial infections[27] in some studies, might therefore suggest a prognostic relevance of increased IP in patients with cirrhosis. However, in our study only surrogates of liver function (MELD score, Child-Pugh score) and consequences of portal hypertension (ascites, low platelet count, high I-FABP) but not the degree of IP was significantly associated with the development of infections.
Because an arbitrary cut-off of the IP index may not be appropriate to assess IP in cirrhosis, we performed analysis for the L/M ratio assessed as linear variable, as quantiles and dichotomized using an established cut-off[15]. In none of these approaches, the IP index was able to predict the predefined clinical endpoints within one-year of follow-up in contrast to the evaluated prognostic indices and serum markers. Our results go in line with the prospective study of Benjamin et al[21], who were not able to demonstrate an association between increased IP and subsequent complications, including bacterial infections, hepatic encephalopathy, hepatorenal syndrome or variceal bleeding in cohort with a lower incidence of pathological IP (35%) using a cut-off of 0.037. Although we did observe an association of higher IP index at baseline with the development of SBP, the diagnostic accuracy of the IP index to predict SBP was comparable to the MELD score providing no additional information over established prognostic indices. In addition, this study was underpowered to assess an association of IP with specific infectious complications but even with mortality. In order to detect differences in outcome events between 5% in patients with normal IP and 20% in patients with pathological IP with a statistical power of 0.85 using Fisher’s exact test, we would have needed to include about 360 patients given the observed prevalence of pathological IP (15%) based on two-sided Fisher’s exact tests at a significance level P = 0.05.
Therefore, in contrast to assessing IP, other biomarkers of enterocyte mass and function, such as I-FABP, seem more appropriate to estimate the clinical outcome of patients suffering from cirrhosis. To our knowledge, this study is the first to demonstrate an association of I-FABP with the development of bacterial infections and with infection-free survival in cirrhosis. I-FABP is specifically stored in enterocytes and released in case of enterocytes death during inflammatory and ischemic processes[28,29]. Similar to the IP index, higher circulating I-FABP levels are associated with the degree of portal hypertension as assessed by the portal vein diameter[30]. In addition, elevated I-FABP levels correlate with serum markers of liver injury and loss of function (ALAT, ASAT, INR, albumin)[29] and have therefore been suggested as a valuable surrogate for the severity of liver injury and dysfunction. Since I-FABP is discussed as a marker for intestinal ischemia in the critically ill[31,32], its interpretation in patients with cirrhosis and circulatory dysfunction has to be examined critically.
Our study has several limitations. First, to address the aforementioned limited power of the study, we did not distinguish spontaneous gram-negative infections other than SBP from bacterial infections, which are unlikely a result of pathological BT. However, SBP is the most common spontaneous infection in cirrhosis, and other spontaneous infections, such as spontaneous bacterial empyema and spontaneous bacteremia are also associated with high mortality[6]. Therefore, a significant increase in spontaneous infections other than SBP should translate into increased mortality if clinically relevant. Second, there is some evidence that in addition to the proximal small bowel, the cecum and colon may significantly contribute to pathological BT[33], which may not be adequately assessed by the use of dual sugar tests. Third, although co-medication with PPI or NSBB as well as renal or diabetic comorbidity was not associated with increased IP in our analysis, we cannot exclude systematic error from these confounders. However, as this study addressed the usefulness of the lactulose/mannitol test to predict end-points in cirrhosis and these putative confounders are frequently present in patients with advanced liver disease, they may limit its role as a prognostic test in clinical practice.
In conclusion, the results of dual sugar tests reflect a few aspects that are associated with the occurrence of complications in cirrhosis, such as portal hypertension, BT-associated inflammation or the development of SBP to some degree. However, the high prevalence of pathological test results in advanced cirrhosis and their failure to predict overall and infection-free survival limit the use of dual-sugar tests as a diagnostic and prognostic tool in clinical practice.
The authors thank the hospital staff for their help with sample collection and patients and volunteers for participation in the study. We would like to express our gratitude to Palak J Trivedi for language editing and to André Scherag for reviewing the statistical methods.
Increased intestinal permeability (IP) is a frequent phenomenon in patients with cirrhosis and has been linked to pathological bacterial translocation, bacterial infections and higher mortality mainly in retrospective studies. However, prospective studies confirming this association are scarce.
To employ pathological IP as an indicator and predictor of complications in cirrhosis in clinical practice, it is important to prospectively assess the association of IP with the development of bacterial infections and mortality in cirrhosis and to compare its predictive accuracy with other biomarkers of complications in cirrhosis.
In this prospective study, increased IP was associated with more advanced liver disease and increased systemic inflammation at baseline but failed to predict adverse clinical outcome in a 1-year follow-up period when focusing on the endpoints mortality, liver transplantation and infections.
Patients with ascites are at the highest risk of developing bacterial infections. For risk stratification, clinicians should apply well-established composite scores, such as Child-Pugh or the model for end-stage liver disease, and pro-inflammatory serum markers but not dual sugar tests of intestinal permeability.
Intestinal permeability refers to the barrier function of the gut, which - if compromised - allows potentially harmful substances to penetrate across this barrier.
This is well performed and interesting prospective clinical study for analyzing the impact of increased intestinal permeability on mortality and the occurrence of infection in patients with liver cirrhosis. The results are described and discussed in a clear way.
P- Reviewer: Vorobjova T, zur Wiesch JS S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM
| 1. | Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [PubMed] |
| 3. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 627] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 4. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 5. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2162] [Article Influence: 180.2] [Reference Citation Analysis (5)] |
| 6. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;1246-1256, 1256.e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 7. | Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991;100:1737-1742. [PubMed] |
| 8. | Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542-2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Liboredo JC, Vilela EG, Ferrari Mde L, Lima AS, Correia MI. Nutrition status and intestinal permeability in patients eligible for liver transplantation. JPEN J Parenter Enteral Nutr. 2015;39:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutiérrez A, Carnices F, Palazón JM. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482-1486. [PubMed] |
| 13. | Norman K, Pirlich M, Schulzke JD, Smoliner C, Lochs H, Valentini L, Bühner S. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 15. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 16. | Katouli M, Nettebladt CG, Muratov V, Ljungqvist O, Bark T, Svenberg T, Möllby R. Selective translocation of coliform bacteria adhering to caecal epithelium of rats during catabolic stress. J Med Microbiol. 1997;46:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Macutkiewicz C, Carlson G, Clark E, Dobrindt U, Roberts I, Warhurst G. Characterisation of Escherichia coli strains involved in transcytosis across gut epithelial cells exposed to metabolic and inflammatory stress. Microbes Infect. 2008;10:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 666] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 20. | Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Benjamin J, Singla V, Arora I, Sood S, Joshi YK. Intestinal permeability and complications in liver cirrhosis: A prospective cohort study. Hepatol Res. 2013;43:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Goto K, Chew F, Torún B, Peerson JM, Brown KH. Epidemiology of altered intestinal permeability to lactulose and mannitol in Guatemalan infants. J Pediatr Gastroenterol Nutr. 1999;28:282-290. [PubMed] |
| 24. | Bruns T, Peter J, Hagel S, Herrmann A, Stallmach A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin Exp Immunol. 2011;164:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 26. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Tang NY, Chen WQ. [Significance of lipopolysaccharide binding protein in serum and ascites of patients with hepatic cirrhosis complicated with spontaneous bacterial peritonitis]. Zhonghua Ganzangbing Zazhi. 2012;20:492-496. [PubMed] |
| 28. | Glatz JF, van der Vusse GJ. Cellular fatty acid-binding proteins: their function and physiological significance. Prog Lipid Res. 1996;35:243-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 371] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220-1230, 1230.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 30. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 534] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 31. | Güzel M, Sözüer EM, Salt Ö, İkizceli İ, Akdur O, Yazıcı C. Value of the serum I-FABP level for diagnosing acute mesenteric ischemia. Surg Today. 2014;44:2072-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Matsumoto S, Sekine K, Funaoka H, Yamazaki M, Shimizu M, Hayashida K, Kitano M. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg. 2014;101:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |