INTRODUCTION
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is a chronic idiopathic inflammatory disorder of the gastrointestinal tract. The precise pathogenesis of IBD is not well-defined, various factors, including genetic, environmental, and immune factors, have been documented to contribute to the development of intestinal homeostasis disruption and excessive inflammatory responses in the gut[1]. Peyer’s patches (PPs) are mainly distributed in the human ileum and serve as important antigen entry sites in gut-associated lymphoid tissue (GALT). PPs play a critical role in modulating intestinal inflammatory responses or tolerance, and IBD patients usually had abnormalities in the PPs, as featured uncontrolled innate and adaptive immune responses[2,3].
Regulatory T (Treg) cells are heterogeneous populations of the adaptive immune system enriched in PPs that suppress over-activated immune response as well as maintain tolerance. Forkhead box protein 3 (Foxp3)+Treg cells are one of these populations that constitutively express Foxp3 and the high-affinity α-chain of interleukin (IL)-2 receptors. Animal studies showed that Foxp3+Treg are required to prevent and treat experimental colitis[4-6]. Th17 cells converted from Treg cells due to low expression of transforming growth factor-β (TGF-β) cause IBD by excessive production of pro-inflammatory factors including IL-17, IL-6, and IL-23[7-10]. Therefore, regulating the functions of Treg cells might be a promising strategy for the treatment of IBD[11,12].
Astragalus membranaceus (AM) is a Chinese herb that has been widely used in China for more than 2000 years to strengthen human immunity. Active components of AM include astragalosides I-VII (saponins), polysaccharides, amino acids, flavonoids and trace elements[13,14]. AM and its active components have been demonstrated to exert potent therapeutic effects against a number of diseases such as diabetes mellitus[15] and cardiovascular disorders[13,16,17]. Astragalus polysaccharide (APS) is an important active component extracted from AM and is comprised of the uniform polysaccharide fraction obtained after direct water decoction[18]. APS has received a great deal of attention in view of its antioxidation[19], immunoregulatory[20], antiviral[20], antitumor activities[21] and cardiovascular protective properties[22,23]. Previous studies showed that oral AM and intraperitoneal administration of APS could diminish the overexpression of tumor necrosis factor (TNF)-α and IL-1β during experimental colitis induced by hapten[13,23]. However, it is still not elucidated whether oral administration of APS could also provide a protective effect during colitis and what is the underlying mechanism, especially its role in regulating the function of Treg cells. In the current study, we investigated the curative effect of APS in 2,4,6-trinitrobenzene sulfonic acid (TNBS) induced experimental colitis in rats, and measured the level of Treg cells in PPs and Treg/Th17 associated cytokines to explore its mechanism.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 200 to 220 g were purchased from the Animal Center of Peking University Health Science Center (The animal certificate number was SCXK 2012-0001). All rats were caged at 20 ± 2 °C with a humidity of 50% ± 5% in a 12 h light/dark cycle with food and water ad libitum. The animals were acclimatized for 7 d and the experiment was performed according to the Guidelines of Jiangxi University of Traditional Chinese Medicine (TCM) Animal Research Committee. The experimental protocols were approved by Jiangxi University of TCM Biomedical Ethics Committee Experimental Animal Ethics Branch (JZ2014-78).
Drugs
TNBS (batch number: p2297) was purchased from Sigma (St. Louis, MO, United States). APS (batch number: HQ090312, purity > 98% by HPLC) was provided by Sciphar (Xi’an, Shannxi Province, China). Mesalazine (batch number: 130407) was purchased from Sunflower Pharma (Jiamusi, China)
TNBS induced colitis in rats
TNBS was applied to induce experimental colitis as described previously[24,25]. Briefly, after a 12-h fast, rats were lightly anesthetized with pentobarbital (60 mg/kg, i.p.) and then administrated with TNBS solution (100 mg/kg TNBS was dissolved in 0.3 mL of 50% ethanol) into the colon at the depth of 8 cm from the rectum using a soft polyethylene catheter (the diameter is 2 mm). Normal control animals received rectal administration with the same volume of physiological saline. The rats were maintained in a head-down posture for 15 min to fill with the colon.
Experimental protocol
A total of 32 rats were randomly assigned to 4 groups with 8 mice in each group: a normal group (rats were administrated with physiological saline into the colon and then physiological saline after 24 h by gavage for continuous 7 d), a TNBS group (rats were administrated with TNBS and physiological saline after 24 h by gavage for 7 d), an APS treatment group (TNBS + APS; rats were administrated with TNBS and received APS at 400 mg/kg per day after 24 h by gavage for 7 d), and a mesalazine treatment group (TNBS + MES; rats were administrated with TNBS and received mesalazine at 300 mg/kg per day after 24 h by gavage for 7 d). Two days after the last treatment, all rats were sacrificed after deep anesthesia by intraperitoneal administration of urethane (2.0 g/kg).
Macroscopical evaluation
The distal 8 cm of the colon from the rectum was removed rapidly and measured for its length, opened longitudinally along colonic mesentery with isotonic saline and assessed for macroscopic injuries according to the criteria described by Liu et al[25]. The criteria were as follows: 0: normal appearance; 1: focal hyperaemia, no ulcers; 2: ulceration without hyperaemia or bowel wall thickening; 3: ulceration with inflammation at one site; 4: ≥ two sites of ulceration and inflammation; 5: major sites of damage extending > 1 cm along the length of the colon; and 6-10: damage extended to > 2 cm along the length of the colon, increasing the score by one for each additional centimeter of damage. Colon weight index (colonic weight/body weight × 100%) was calculated (n = 8). The colonic tissues were separated into two parts. One part was fixed and stained for microscopic evaluation, and the other part was prepared into tissue homogenate for enzyme-linked immunosorbent assay (ELISA) and Western blot.
Microscopical evaluation
The colon was fixed in 4% paraformaldehyde solution for at least 7 d, and then processed for paraffin sectioning and hematoxylin-eosin staining (n = 8). The microscopic evaluation including inflammatory cell infiltration and tissue damage was performed as described previously by Liu et al[25] and Schmidt et al[26]. Scores of infiltration are as follows: 0: no infiltration; 1: increased number of inflammatory cells in the lamina propria; 2: inflammatory cells extending into the submucosa; and 3: transmural inflammatory cell infiltration. The scores of tissue damage are as follows: 0: no mucosal damage; 1: discrete epithelial lesions; 2: erosions or focal ulcerations; and 3: severe mucosal damage with extensive ulceration extending into the bowel wall.
Isolation of lymphocyte from intestinal PPs
After the colon was separated, PPs were sheared from the small intestine, triturated in 3% fetal calf serum (FCS)/PBS solution on ice, and filtrated via a 300 section stainless steel cell cribble to obtain lymphocytes. Cells were centrifuged at 5000 rpm at 4 °C for 2 min and suspended at a density of 1 × 106-107/mL.
Measurement of CD4+CD25+Foxp3+ Treg cells by flow cytometry
Cells from intestinal PPs were resuspended in 3% FCS/PBS solution at a final cell concentration of 1 × 106-107/mL. The cell suspension (n = 8) was incubated for 30 min with APC conjugated anti-rat CD4 antibody (RM4-5) and PE conjugated anti-mouse CD25 antibody (PC61.5)at 37 °C in dark. Cells were centrifuged at 5000 rpm at 4 °C for 2 min, fixed in Fix/Perm Buffer (eBioscience, San Diego, CA, United States) for at least 1 h at 37 °C, and then incubated with FITC conjugated anti-mouse Foxp3+ antibody (FJK-16s) for 30 min at 37 °C in the dark. Cells labeled with PE conjugated rat IgG2a were used as the isotype negative control. Rate of CD4+CD25+Foxp3+Treg cells was analyzed using FACS Calibur (BD Biosciences).
ELISA
To quantify colonic tissue cytokines, 50 mg of colonic tissue was extracted using 500 μL of 5 mol/L guanidine-HCl and 50 mmol/LTris-HCl (pH 8.0) with a protease inhibitor. The extracts were centrifuged at 15000 g for 30 min at 4 °C to remove insoluble materials. The supernatant fractions were analyzed with an ELISA kit (n = 8) following the manufacturers’ instructions (eBioscience, San Diego, CA, United States).
Western blot analysis
Colonic tissues were washed twice with cold PBS. Samples from each group were pooled and crushed using a pestle in liquid nitrogen. For each 25 mg of tissue, 100 mL lysis buffer (7 mol/L urea, 2 mol/L thiourea, 4% CHAPS, 30 mmol/LTris, 2 mmol/L DTT was added. The mixture was sonicated on ice and centrifuged at 15000 g at 4 °C for 5 min. The supernatant containing the tissue protein was quantified using a BCA Protein Assay Kit. Intestinal proteins (20-40 μg) were used for Western blot[27,28]. The primary antibodies including rabbit anti-GAPDH (1:2000), anti-IL-23 (1:2000), anti-related orphan receptor-γt (ROR-γt) (1:2000) and anti-STAT-5a (1:1000) were purchased from Abcam (Cambridge, United Kingdom). Bands were quantified using Image-Pro Plus 5.0 software (Media Cybernetic, Bethesda, MD, United States).
Statistical analysis
Data are shown as mean ± standard error of mean (SEM). Statistical analysis was performed using analysis of variance (ANOVA) followed by the Tukey’s test using the Prism 6 software. A P value < 0.05 was considered statistically significant.
RESULTS
APS ameliorates colonic mucosal injuries in TNBS induced colitis in rats
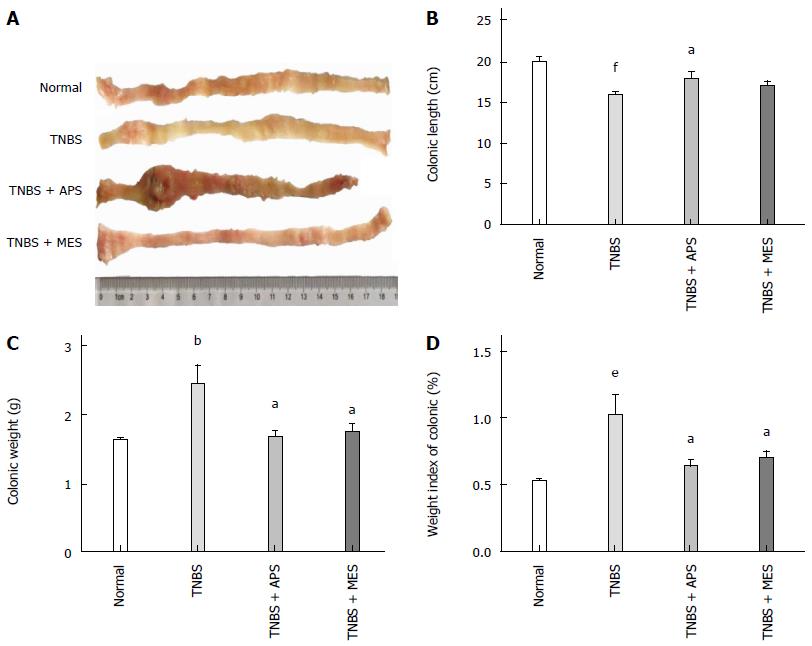
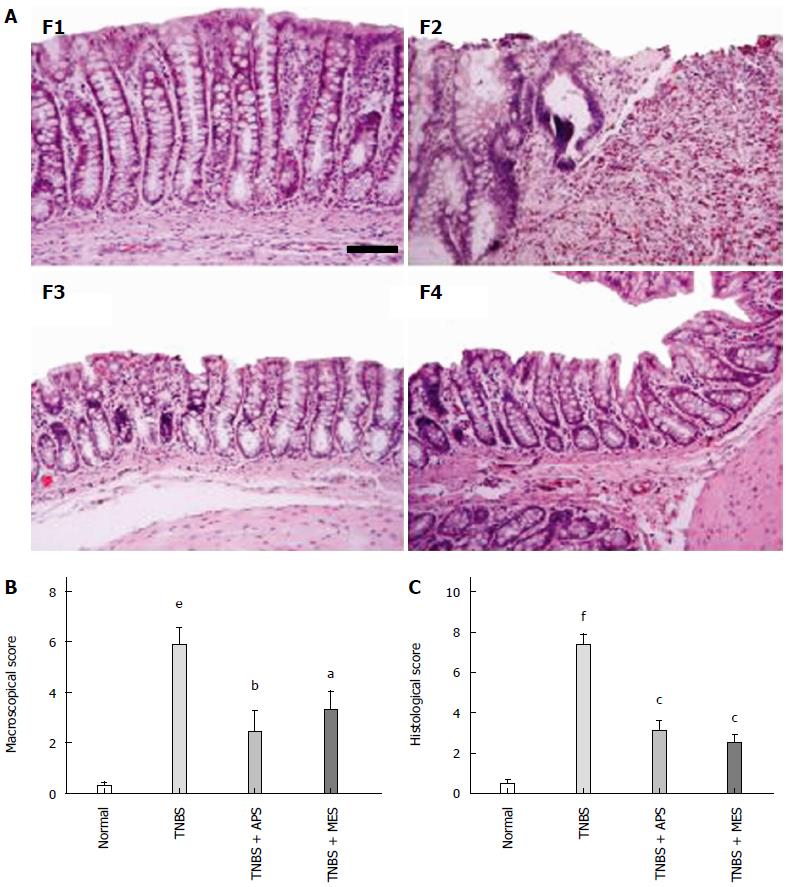
After intracolonic installation of TNBS for 7 d, stool frequency was increased, stool was loose with mucus and hemorrhages, and apparent colonic mucosal injuries including hyperaemia, edema, bleeding and ulcer were found on the surface of the colonic mucosa (Figure 1A).The colonic lengths were significantly shortened, and colonic weight, colonic weight index and macroscopic score were higher in the TNBS group compared with the normal group. Treatment with APS ameliorated TNBS induced colonic injuries as shown by increased colonic length, decreased colonic weight, colonic weight index and macroscopic score compared with the TNBS group, and similar effects were observed in the TNB + MES group (Figure 1B, C and D). As shown in Figure 2A, abundant inflammatory cell infiltration, colonic epithelium loss, ulcerative formation, incrassate intestinal wall and fewer red cells were seen in the colonic mucosa in the TNBS group. Noticeably, those histological changes were attenuated remarkably in the TNBS + APS and TNBS + MES groups (Figure 2A, F3 and F4). Blinded histopathology evaluation was consistent with H&E staining, indicating that APS effectively ameliorates colonic injury induced by TNBS (Figure 2C).
Figure 1 Representative images of colons and colonic character assay.
A: Representative images of colons; B: Colonic length; C: Colonic weight; D: Weight index of the colon. Data are mean ± SEM (n = 8). bP < 0.01, eP < 0.001, fP < 0.0001 vs normal group; aP < 0.05 vs TNBS group. TNBS: 2,4,6-trinitrobenzene sulfonic acid; APS: Astragalus polysaccharide; MES: Mesalazine.
Figure 2 Representative histological images and injury scores.
A: Representative histological images stained with HE. F1: Normal; F2: TNBS; F3: TNBS + APS; F4: TNBS + MES. Bar = 100 μm. B: Macroscopic scores. C: Histological scores. Data are mean ± SEM (n = 8). eP < 0.001, fP < 0.0001 vs Normal group; aP < 0.05, bP < 0.01, cP < 0.0001 vs TNBS group. TNBS: 2,4,6-trinitrobenzene sulfonic acid; APS: Astragalus polysaccharide; MES: Mesalazine.
APS improves the level of Treg cells in intestinal PPs
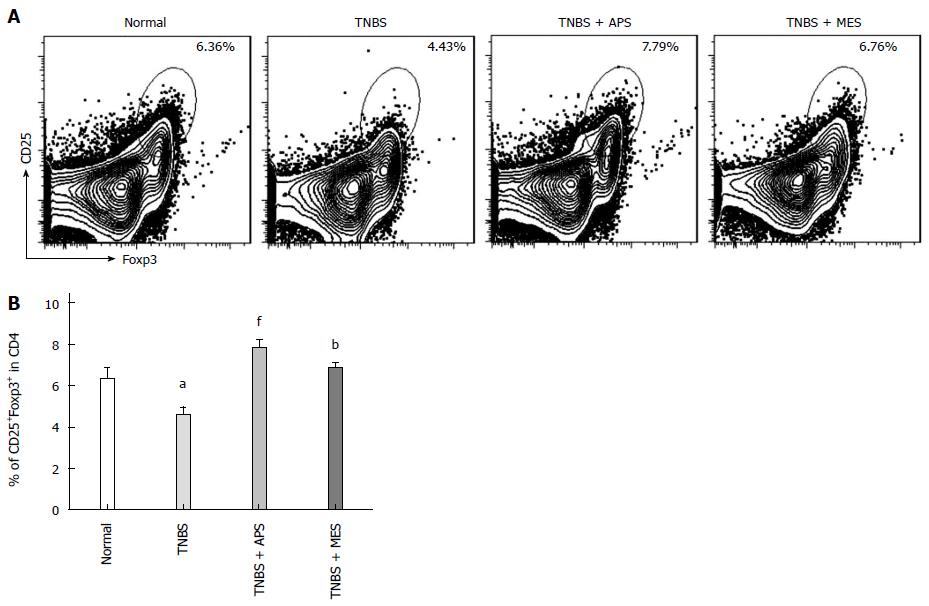
To observe changes of Treg cells in intestinal PPs, the levels of CD4+CD25+ T cells and CD4+CD25+Foxp3+ T cells were analyzed by flow cytometry. As shown in Figure 3, compared with the normal group, reduced CD4+CD25+Foxp3+ T cells were shown in PPs of TNBS-induced colitis rats. Treatment with APS significantly improved the deduction of these Treg cells in TNBS groups, with slightly better effect on increasing Treg cells compared with the MES treated group.
Figure 3 Assay of Treg cells.
A: Representative flow plots of CD4+CD25+Foxp3+ T cells. Histograms show the distribution of immunofluorescence labeling intensity of CD4, CD25 and Foxp3 expression in each group. Ordinate indicates cell counts; abscissa represents fluorescent intensity. B: Levels of CD4+CD25+Foxp3+ T cells. Data are mean ± SEM (n = 8). aP < 0.05 vs normal group; bP < 0.01, fP < 0.0001 vs TNBS group. TNBS: 2,4,6-trinitrobenzene sulfonic acid; APS: Astragalus polysaccharide; MES: Mesalazine.
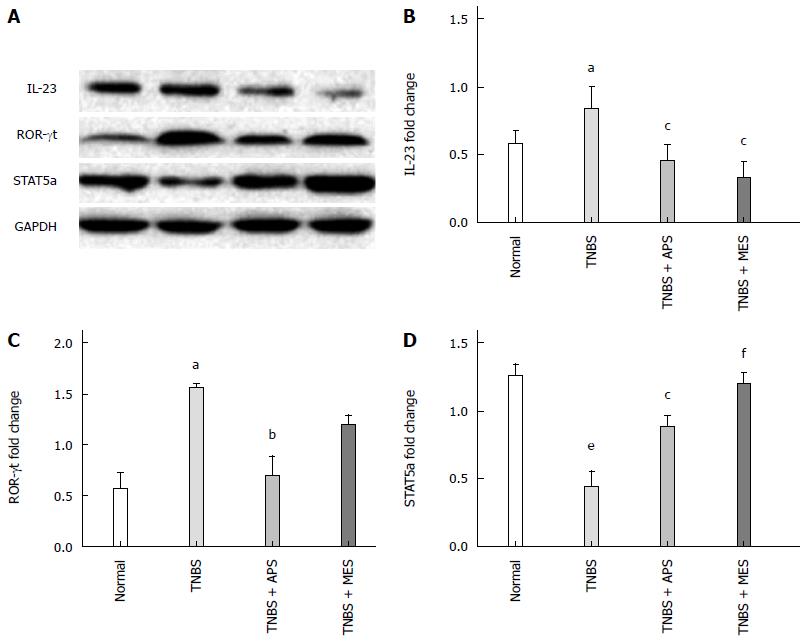
APS regulates cytokine expression in the colonic mucosa
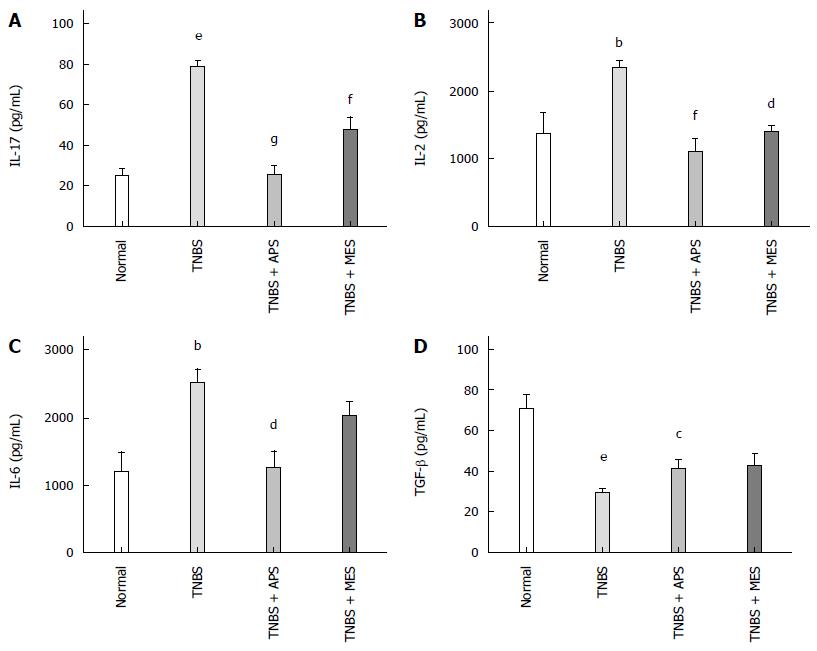
ELISA and Western blot were performed to evaluate the levels of IL-17, IL-2, IL-6, TGF-β and IL-23 in the colonic tissue. As shown in Figures 4 and 5B, the levels of IL-2, IL-6, IL-17 and IL-23 were significantly increased and the level of TGF-β was significantly decreased in colonic tissues in the TNBS group compared with the normal group. Treatment with APS and MES reversed the changes in these cytokines, indicating that the protective effect of APS might be mediated through regulation of pro- and anti-inflammatory cytokines.
Figure 4 Concentrations of cytokines in the colonic mucosa.
A: Concentrations of IL-17 in the colonic mucosa from different groups; B: Concentrations of IL-2 in the colonic mucosa from different groups; C: Concentrations of IL-6 in the colonic mucosa from different groups; D: Concentrations of TGF-β in the colonic mucosa from different groups. Data are mean ± SEM (n = 8). aP < 0.05, bP < 0.01, eP < 0.0001 vs normal group; cP < 0.05, dP < 0.01, fP < 0.001, gP < 0.0001 vs TNBS group. TNBS: 2,4,6-trinitrobenzene sulfonic acid; APS: Astragalus polysaccharide; MES: Mesalazine.
Figure 5 Western blot analysis of IL-23, ROR-γt and STAT-5a expression.
A: Representative Western blots of IL-23, ROR-γt, STAT-5 and GAPDH (n = 6); B: Quantitative analysis of ROR-γt protein (n = 6); C: Quantitative analysis of IL-23 protein (n = 6); D: Quantitative analysis of STAT-5a protein (n = 6). Data are mean ± SEM (n = 8). aP < 0.05, eP <0.000 vs normal group; cP < 0.05, bP < 0.01, fP < 0.001 vs TNBS group. TNBS: 2,4,6-trinitrobenzene sulfonic acid; APS: Astragalus polysaccharide; MES: Mesalazine.
APS increases the expression of retinoid-ROR-γt and STAT-5 in the colonic mucosa
To investigate the mechanism of APS in regulating the expression of Treg cells, we evaluated the expression of ROR-γt and STAT-5 in the colonic mucosa by Western blot. Compared with the TNBS group, the result of ROR-γt was contrary to that of STAT-5 (Figure 5). Compared with the normal group, the TNBS group showed increased ROR-γt expression and decreased STAT-5 expression, and treatment with APS and MES decreased ROR-γt expression and increased STAT-5 expression significantly.
DISCUSSION
IBD is a chronic, relapsing-remitting gastrointestinal disease with unknown etiology[29]. It is characterized by mucosal inflammation and ulcerative formation[30]. We established a murine model of colitis by intracolonic injection of TNBS. Colonic tissue from TNBS administrated mice showed apparent pathological changes including serious hyperemia, edema, and ulcers. APS effectively attenuated mucosal injuries in mouse experimental colitis by shortening colonic length, decreasing weight index of the colon, and reducing macroscopic and histological scores (Figures 1 and 2).
As a bioactive chemical in AM, APS is widely used to treat chronic colitis in clinic for its multi-targeting biological activities, including antioxidant, radical scavenging and anti-inflammatory activities and immune regulation[18]. Although APS has been used widely to treat chronic colitis for centuries in China, its mechanism, especially the anti-inflammatory effect, is ill-defined. It is unknown whether APS could regulate the number and suppressive function of Treg cells in the intestine mucosa during IBD.
Previous studies suggest that abnormal mucosal CD4+ T cell immune responses in the intestinal tract lead to the occurrence and development of IBD[31]. As an uppermost organ induced mucosal immune response in the intestinal tract, PPs establish their importance in the immune surveillance of the intestinal lumen and in the generation of the immune response within the mucosa, and they are covered by special immune cells that contain dendritic cells, B-lymphocytes, and T-lymphocytes.
Treg cells constitute up to 6% of the CD4+ T cells in human peripheral blood and also reside in the human intestine. Treg cells play a crucial role in the maintenance of self-tolerance and the prevention of autoimmune diseases such as multiple sclerosis, systemic lupus erythematosus, rheumatoid arthritis, psoriasis and IBD[32]. In IBD, the suppressive function of Treg cells from the peripheral blood or intestinal mucosal tissue was mostly implemented by maintaining normal cell contact-dependent, cytokine-independent suppressive capacity, and inhibiting proliferation and cytokine production, even restraining pathogenic T-effector cells derived from the inflamed mucosa[33].
Notably, adoptive transfer of Treg cells can boost and restore Treg cells quantity and function, and may effectively prevent IBD, halt the progression of established IBD, and then reverse pathology of IBD. So restoring the suppressive function of Treg cells is an effective strategy to prevent and treat recurrent attacks of IBD[34-37].
The function of Treg cells can be weakened by a high level of IL-17 locally[11]. Many studies have indicated that lower level of Treg cells with excessive IL-17 were important characters in the pathogenesis and prognosis of IBD[38]. IL-17 has a variety of potent pro-inflammatory activities which directly and indirectly cause the destruction of the colonic mucosa[39]. In the present study, APS effectively ameliorated intestinal inflammation during experimental colitis, and APS treatment increased the number of CD4+CD25+ T cells (data not shown) and CD4+CD25+Foxp3+ T cells in colonic tissue (Figure 3), and decreased the level of IL-17 (Figure 4). These results suggest that APS might exert a protective effect toward colitis by inducing Treg cells and inhibiting IL-17 secretion.
Abundant factors including transcription factors (such as STAT-5), cytokines (such as IL-2, IL-6 and IL-23), nuclear receptors (such as ROR-γt) and growth factors are involved in maintaining the quantity and inhibitory function of Treg cells. As a specific mark of Treg cells, Foxp3 is a determinant factor in the development and gain-of-function of Treg cells[40]. TGF-β enhances the expression of Foxp3 and ROR-γt[10]. Foxp3 inhibits the expression of ROR-γt and IL-17 to improve the level of Treg cells[38]. In fact, in patients with IBD, a high level of IL-17 accompanied by ROR-γt overexpression may aggravate inflammatory injury of the colonic mucosa, and lead to IBD recurrence. Fundamental roles of IL-17 and ROR-γt in the pathogenesis of IBD were realized by inhibiting the function of Treg cells[41]. Meanwhile, the balance of Foxp3/ROR-γt determines the level of initial T cells transformed into Treg cells[42,43], and regulates STAT-5 by inhibiting the expression of ROR-γt, and/or improving TGF-β to induce the expression of Foxp3 and activation of Treg cells[44,45]. A previous study has shown that STAT-5 knockdown could lessen the inhibiting effect of IL-2 to up-regulate the expression of ROR-γt[46]. In addition, IL-23 can promote the expression of IL-17 in the meantime[47,48], and TGF-β can promote initial T cells to differentiate into Treg cells in a lower level of IL-6. Interestingly, on the contrary, a high level of IL-6 also promotes the production of IL-17[49,50]. So the balance of Foxp3/ROR-γt is still affected by cytokines such as IL-6 and IL-23. It is known that IBD is characterized by a cytokine-driven mixed inflammatory infiltrate in the intestinal mucosa. Multiple interleukins including IL-2, IL-6, IL-12, IL-17, and IL-23 have been found to be elevated in IBD patients and colitis animals[51,52]. In our mice with experimental colitis, the level of Foxp3+ T cells in PPs was decreased while the ROR-γt was up-regulated in the colonic mucosa (Figures 3 and 5C). It showed that the balance of Foxp3/ROR-γt was interrupted in the forming process of IBD. Meanwhile, in the colonic mucosa, the expression of IL-2, IL-6, IL-17, IL-23 and ROR-γt was increased, and the levels of TGF-β and STAT-5 were decreased. Based on the above results, the expression of IL-17 was increased and the level of Treg cells was decreased in the TNBS induced colitis in colonic tissue. It hints that relations between expression of IL-17 and function of Treg cells were according with the balance of Foxp3/ROR-γt and related above-mentioned factors in this model. In the present study, after 7-d treatment with APS, expression of IL-2, IL-6, IL-17, IL-23 and ROR-γt was notably inhibited, and the levels of TGF-β and STAT-5 were up-regulated in the TNBS + APS groups. These demonstrate that APS might inhibit the expression of ROR-γt to regulate the balance of Foxp3/ROR-γt and decrease the secretion of pathogenic IL-17. On one hand, APS improved STAT-5 to suppress the level of ROR-γt and to potentially promote transformation of Th17 cells into Treg cells. Meanwhile, APS decreased the level of IL-6, increased TGF-β to increase Treg cells, and reduced the expression of IL-23. All results suggest that APS possibly protected the colon possibly by enhancing the level of inhibitory Treg cells and decreasing the production of IL-17. Consequently, the immune suppression of Treg cells was recovered, and the level of IL-17 was down-regulated after treatment with APS.
In summary, APS exerts an effective therapeutic effect against experimental colitis by recovering the functional status of Treg cells, and restraining the expression of IL-17 in PPs.
COMMENTS
Background
Regulatory T (Treg) cells play a crucial role in the maintenance of self-tolerance and the prevention of autoimmune diseases such as multiple sclerosis, systemic lupus erythematosus, rheumatoid arthritis, psoriasis and inflammatory bowel disease (IBD). Peyer’s patches (PPs) play a critical role in modulating intestinal inflammatory responses or tolerance, and IBD patients usually had abnormalities in the PPs, as featured uncontrolled innate and adaptive immune responses. So the level of Treg cells in PPs has a pivotal role in the treatment of IBD.
Research frontiers
Many studies have shown that adoptive transfer of Treg cells can effectively prevent IBD and halt the progression of established IBD. However, there are very few studies on whether effective components of Traditional Chinese medicine regulate the level of Treg in intestinal Peyer’s patches to treat IBD.
Innovations and breakthroughs
This is the first study exploring the mechanism of Astragalus polysaccharide (APS) in treating experimental colitis by regulating Treg cells in intestinal Peyer’s patches.
Applications
APS is used to treat chronic colitis in clinic for its multi-targeting biological activities, however, its mechanism, especially the anti-inflammatory effect, is ill-defined. In the present study, APS effectively treated experimental colitis by recovering the functional status of Treg cells, and restraining the expression of IL-17 in Peyer’s patches. The results are favorable to explore the mechanism of APS in treating chronic colitis, and spread and understand clinical applications of APS.
Terminology
It is known that APS has wide pharmacologic actions including antioxidant, radical scavenging and anti-inflammatory activities and immune regulation. However, it is ambiguous whether APS regulates the level of Treg cells in Peyer’s patches. In the present study, the results had confirmed that APS effectively treated experimental colitis by recovering the functional status of Treg cells. The evidences enriches the immunological mechanisms of ASP in treatment of IBD.
Peer-review
The manuscript addresses an important topic of the inflammatory process, that is, the role of regulatory T cells in the inflammatory process in experimental colitis, similar to Crohn’s disease, using a Chinese herb that has shown anti-inflammatory effect in various diseases.