Published online Mar 21, 2016. doi: 10.3748/wjg.v22.i11.3105
Peer-review started: November 4, 2015
First decision: November 27, 2015
Revised: January 5, 2016
Accepted: January 9, 2016
Article in press: January 9, 2016
Published online: March 21, 2016
Processing time: 131 Days and 21.6 Hours
Pancreatic neuroendocrine tumors (PNETs) are a rare and diverse group of tumors; nonfunctional (NF) PNETs account for the majority of cases. Most patients with NF-PNETs have metastatic disease at the time of presentation. A variety of treatment modalities exist, including medical, liver directed, and surgical treatments. Aggressive surgical management is associated with prolonged survival, however available data are limited by selection bias and the frequent combination of PNETs with carcinoid tumors. Although few patients with metastatic disease will be cured, application of currently available therapies in a multidisciplinary setting can lead to excellent outcomes with prolonged patient survival.
Core tip: Treatment options for patients with neuroendocrine tumors of the pancreas have increased in recent years. Surgical management remains an important component of treatment and is associated with prolonged survival, however high level data supporting specific treatment approaches are limited. Although few patients with metastatic disease will be cured, application of available therapies in a multidisciplinary setting can lead to excellent outcomes with prolonged patient survival.
- Citation: Folkert IW, Hernandez P, Roses RE. Multidisciplinary management of nonfunctional neuroendocrine tumor of the pancreas. World J Gastroenterol 2016; 22(11): 3105-3116
- URL: https://www.wjgnet.com/1007-9327/full/v22/i11/3105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i11.3105
Pancreatic neuroendocrine tumors (PNETs) are rare neoplasms accounting for 1% to 2% of all pancreatic tumors[1,2]. PNETs are thought to arise from pluripotent cells in the pancreatic ductal/acinar system rather than from islet cells as the previously favored terminology, islet cell tumors suggested[3]. The unique ability of pancreatic neuroendocrine tumors to synthesize and secrete hormones and neuropeptides results in a variety of clinical syndromes of hormone hypersecretion. Zollinger-Ellison syndrome resulting from excess gastrin production and hyperinsulinemia are the most common of these[4]. However, so-called functional pancreatic neuroendocrine tumors account for a minority of PNETs. Tumors not associated with overt clinical symptoms of hypersecretion, nonfunctional pancreatic neuroendocrine tumors (NF-PNETs), account for 60% to 90% of PNETs[1,2,5-9]. NF-PNETs are biologically diverse and tumor grade is a dominant predictor of outcome[10,11]. While the majority of PNETs occur sporadically, they may also be associated with various inherited disorders including MEN 1, Von Hippel-Lindau syndrome, neurofibromatosis 1, and tuberous sclerosis[4]. While PNETs associated with familial syndromes are typically considered more indolent than sporadic tumors, patients with familial syndromes are at higher risk for multifocal tumors ranging from multiple pancreatic microadenomas to large, malignant tumors[4].
Despite a high rate of hepatic metastases at diagnosis, NF-PNETs are associated with a more favorable prognosis compared to many other gastrointestinal malignancies. Overall, 5-year survival varies from 27% to 33% in large series[1,2]. However, several groups have reported that aggressive management is associated with more favorable outcomes, even in patients with liver metastases. A recent SEER database review of over two thousand patients with NF-PNETs by Franko and colleagues demonstrated that median survival was 4.8 years when patients with metastatic disease underwent aggressive surgical management, compared to 1 year in patients who did not undergo resection[1]. Unfortunately, fewer than half of all patients with metastatic NF-PNETs are candidates for resection of their primary tumors or liver metastases[1]. Furthermore, in patients who do undergo aggressive surgical management, recurrence is frequent. Improved survival in such patients suggests a role for surgical debulking in selected cases, but there is limited high-level evidence supporting such an approach. Moreover, many larger studies of NETs combine data from patients with neuroendocrine tumors from pancreatic and non-pancreatic primary sites, complicating interpretation. The recent introduction of more effective systemic agents, growing experience with liver directed therapies, and the absence of high quality comparative data further cloud efforts to establish a consensus approach.
This review aims to address controversies in the treatment of metastatic NF-PNETs with a critical assessment of the existing literature. A two-part case report from our institution is presented as a means of framing this discussion.
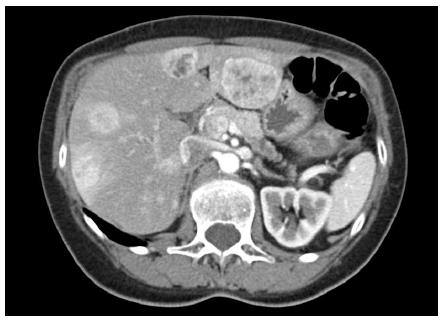
A 55 year-old, previously healthy female presented to her primary care physician complaining of intermittent epigastric pain. Right upper quadrant ultrasound demonstrated liver tumors. A follow-up contrast computed tomography (CT) scan revealed multiple enhancing lesions in both the right and left lobes of her liver, the largest lesion in segment 2 measuring 3 cm × 5 cm. Focal thickening in the mid-body of the pancreas with associated dilation of the distal pancreatic duct and atrophy of the pancreatic tail was also noted.
Laboratory studies revealed mildly elevated transaminases with a normal serum bilirubin. Serum chromogranin A and fasting pancreatic polypeptide levels were significantly elevated at 110 ng/mL and 837 pg/mL respectively, and serum serotonin levels were measured at the upper limits of normal. CT guided core biopsy of a representative hepatic lesion demonstrated pathology consistent with a well-differentiated neuroendocrine tumor. Octreotide scintigraphy demonstrated uptake in the previously mentioned lesions without any evidence of other metastases.
Apart from intermittent abdominal pain, the patient was asymptomatic. She denied flushing or diarrhea, history of peptic ulcer disease or hyper-/hypoglycemia. On initial surgical consultation, the hepatic lesions were deemed unresectable given their distribution throughout all segments of the liver (Figure 1).
Somatostatin is a 14-amino acid regulatory peptide. Somatostatin and its long-acting analogs octreotide and lanreotide act to down-regulate neurotransmission, hormone secretion, and cell proliferation in the myriad cells bearing the G-protein coupled somatostatin receptor, including pancreatic neuroendocrine cells[12]. Somatostatin and its analogs effectively decrease hormone secretion from metastatic carcinoid tumors and functional PNETs, especially glucagonomas and VIPomas, often leading to improved quality of life[13,14]. Until recently, the benefit of somatostatin analogs in the treatment of metastatic NF-PNETs was less clear. The anti-proliferative role of somatostatin in normal physiology led to speculation that somatostatin analog therapy might improve progression-free survival and overall survival in patients with NF-PNETs. Of the studies investigating the anti-tumor effects of somatostatin analogs, only two, the PROMID and the CLARINET trials, were randomized and placebo controlled[15,16].
The PROMID trial studied the use of monthly depot injections of octreotide LAR in 85 treatment naïve patients with unresectable, well-differentiated midgut NETs. Of these 85 patients, 73 had hepatic metastases, 56 had previously undergone resection of the primary tumor, and 33 had carcinoid syndrome at the time of randomization. The investigators were able to confirm the anti-proliferative effect of octreotide, as demonstrated by significantly fewer progressions or tumor-related deaths in the octreotide LAR group (26 vs 40 in the placebo group, HR = 0.34, 95%CI: 0.20-0.59, P = 0.000072). Median time to tumor progression, or progression-free survival as assessed by Response Evaluation Criteria In Solid Tumors (RECIST), was also significantly longer following octreotide therapy as compared to placebo (14.3 mo vs 6.0 mo). Subgroup analysis demonstrated that patients with functionally active and inactive tumors responded similarly, whereas the antiproliferative effect was more pronounced in patients with a resected primary tumor and patients with low (≤ 10%) hepatic tumor burden[17]. Although only patients with midgut neuroendocrine tumors were included in this study, it did provide additional rationale for the application of somatostatin analog therapy in patients with metastatic neuroendocrine tumor from other primary sites.
The CLARINET trial, investigated the use of lanreotide in patients with unresectable or metastatic NETs. A total of 204 patients with sporadic, well-to moderately-differentiated, nonfunctioning tumors from a variety of primary sites, including pancreatic, midgut, and hindgut were randomized to receive either once-monthly placebo or Lanreotide depot injections. Both groups were followed for 24 mo. At the completion of the study, progression-free survival was significantly prolonged with lanreotide as compared to placebo, with 65.1% of patients in the treatment group showing no progression at 24 mo as compared to 33.0% in the placebo group. Subgroup analysis suggested improved progression-free survival among patients whose primary arose in the pancreas, but the difference between the treatment and placebo groups in this case fell short of statistical significance (HR = 0.58, 95%CI: 0.34-1.02). Patients with midgut primaries derived the greatest benefit from lanreotide, with a statistically significant HR of 0.35[16].
As high quality, randomized, placebo-controlled trials, the PROMID and CLARINET studies provided evidence that somatostatin analogs have anti-proliferative effects in non-functional neuroendocrine tumors arising in the midgut and in a group of mixed patients with midgut and pancreatic primary tumors, respectively. Despite the lack of randomized data specifically indicating improved progression-free survival in the NF-PNET subgroup, the use of somatostatin analogs has been widely embraced as a safe and well-tolerated initial systemic therapy for these patients.
Activating mutations in several different protein kinases have been implicated in the tumorigenesis of NF-PNETs. In particular, receptor tyrosine kinases (RTKs) have been implicated in the abnormal signal transduction cascades that contribute to the unchecked proliferation of PNETs. These RTKs include the receptors for platelet-derived growth factor (PDGFRs) and vascular endothelial growth factor receptors (VEGFRs), which play a role in both tumor angiogenesis and tumor cell proliferation[17-20]. It is hypothesized that the simultaneous inhibition of these targets leads to reduced tumor neovascularization, thus inhibiting tumor growth.
This hypothesis was tested using sunitinib, an oral, small-molecule, RTK inhibitor of PDGFR and VEGFR. In a randomized, double-blind, placebo-controlled trial, 171 patients with unresectable and/or metastatic PNETs were assigned to receive either once-daily oral sunitinib or placebo. Disease progression was evaluated using RECIST criteria. A statistically significant improvement in progression-free survival was observed in the treatment group, with a median progression-free survival of 11.4 mo as compared to 5.5 mo with placebo. Subgroup analysis demonstrated that sunitinib’s impact on progression-free survival was even greater for those patients with non-functioning tumors, with a statistically significant HR of 0.26 in the treatment group as compared to placebo. Interestingly, a statistically significant benefit from sunitinib could not be demonstrated for patients with functional tumors (HR = 0.75, 95%CI: 0.3-1.84)[21].
mTOR, a serine-threonine kinase, stimulates cell growth, proliferation, and angiogenesis. In PNETs, the aberrant activation of the mTOR signaling pathway via insulin-like growth factor has been implicated as a driving force for unchecked growth[22]. Yao et al[23] investigated the use of everolimus, an oral mTOR inhibitor, in a randomized, placebo-controlled trial of 410 patients with intermediate-grade, unresectable and/or metastatic pancreatic neuroendocrine tumors. Median RECIST-defined progression-free survival was 11.0 mo in the once-daily everolimus group, as compared with 4.6 in the placebo group, with a HR of 0.35 for disease progression or death with everolimus as compared to placebo. While 76% of study participants had non-functional tumors, there was no subgroup analysis performed to define the efficacy of everolimus specifically in this subset of patients.
The demonstrated efficacy of both everolimus and sunitinib in relatively large, high-quality, randomized, controlled trials suggests a role for these agents in the multimodal treatment of patients with PNETs. Despite proven efficacy in delaying progression, the impact on long-term survival, and specific clinical indications for these agents remain poorly defined.
While somatostatin analogs and targeted protein kinase inhibitors share the benefit of excellent tolerability and improve progression free survival in patients with PNETs, neither class of medication has proven tumoricidal properties or induces treatment response as measured by tumor size[21,23]. In contrast, cytotoxic agents - especially alkylating agents, platinum containing drugs and pyrimidine analogs - have proven efficacy in decreasing tumor burden and lengthening progression-free survival in several retrospective and a few small prospective trials.
Among alkylating agents, streptozotocin is the most studied, and was the first drug approved for the treatment of PNETs. First used to treat PNETs as described by Broder in 1973, streptozotocin has been demonstrated to be effective in a number of prospective trials, especially when combined with other cytotoxic drugs of different classes[24-28]. In a prospective trial by Moertel et al[26], streptozotocin in combination with doxorubicin demonstrated an objective tumor response, defined as radiologic evidence of decreased tumor size or improvement in endocrine abnormalities, in 69% of 38 patients with unresectable or metastatic PNETs. They also reported a median progression-free survival of 18 mo among study participants. The subset of patients with nonfunctional tumors demonstrated a similar response, although this result did not achieve statistical significance.
While effective, the widespread use of streptozotocin has been significantly limited by its difficult dosing schedule and toxicity. More recent prospective studies have investigated the use of the better-tolerated, orally available alkylating agent temozolomide in combination with various other agents including capecitabine, bevacizumab, thalidomide, and everolimus, with promising results[29-32].
The combination of temozolomide and capecitabine (CAPTEM) has received particular interest in the recent literature. In a retrospective study of 30 patients with metastatic PNETs, Strosberg et al[32] demonstrated an objective partial response in 70% of patients treated with CAPTEM. The median progression-free survival was 18 mo among their cohort, which included 22 patients with nonfunctional tumors. This combination was also very well tolerated among patients included in the study, with only 4 patients (12%) requiring dose reductions due to adverse events.
Chan et al[30] also demonstrated the efficacy of temozolomide in combination with everolimus in a prospective trial of 43 patients with unresectable or metastatic PNETs. Using RECIST criteria, these investigators were able to document a partial response in 40% of patients and a median progression-free survival of 15.4 mo. The functional status of each patient’s tumor was not reported, however, and therefore the differential response of functional vs nonfunctional tumors could not be assessed. Study participants experienced toxicities at the rate expected for each drug alone, and there was no evidence of any synergistic toxicity.
Apart from alkylating agents, promising results have also been shown in some retrospective trials for pyrimidine analogs such as 5-FU, capecitabine and gemcitabine, and the platinum-containing agent oxaliplatin[26,28,33,34]. In a recent retrospective study, patients receiving a combination of gemcitabine and oxaliplatin (GEMOX) were compared to patients treated with alkylating agents. Among the 37 patients with unresectable or metastatic PNETs receiving the GEMOX regimen, 38% demonstrated a tumor response, and the median progression-free survival was 7.3 mo[34]. While this retrospective trial demonstrated the GEMOX regimen to be slightly less effective than the previously published trials of alkylating agents, the positive results obtained with GEMOX will likely drive future prospective investigations of both platinum containing agents and pyrimidine analogs in the treatment of PNETs.
Peptide receptor radionuclide therapy (PRRT) targets the somatostatin receptor with radiolabeled somatostatin analogs consisting of three components: a cyclic octapeptide, a chelator, and a radionuclide[35]. PRRT has been widely used in Europe for patients with metastatic neuroendocrine tumors as well as in the neoadjuvant and adjuvant settings[35,36]. [90Y-DOTA0,Tyr3] octreotide and [177Lu-DOTA0,Tyr3] octreotate, the two main compounds currently in use, are roughly equivalent in their complete and partial response rate (10%-30%). However, treatment with [90Y-DOTA0,Tyr3] octreotide is associated with a significantly higher risk of nephrotoxicity, and the kidneys are generally the dose-limiting organ for PRRT[35].
Surgery is the cornerstone of treatment for localized NF-PNETs and has also been advocated in the setting of metastatic disease when all, or most, gross disease can be removed. In both institutional case series and large database analyses, surgery has been correlated with improved survival at every disease stage[1,37-46]; albeit, the potential influence of selection bias on outcomes is substantial in many studies. Conversely, expectant or conservative management strategies in patients with small primary tumors, inherited syndromes at high risk of multifocal disease, or limited stable hepatic metastases has also been advocated by various groups.
Expectant management for small NF-PNETs has long been supported for patients with inherited syndromes such as MEN-1, von Hippel-Lindau, and neurofibromatosis. Low rates of progression or metastasis have been shown in multiple series of patients with MEN-1 and tumors less than 2 cm and von Hippel-Lindau syndrome with primary tumors less than 3 cm without a mutation of exon 3[47-52]. Recent data on the natural history of small primary tumors in patients with NF-PNETs support expectant management in selected sporadic patients as well, although the absolute size threshold for resection remains controversial.
In one observational study of the natural history of sporadic, asymptomatic, well-differentiated NF-PNETs less than 2 cm in size, none of the 41 patients followed with serial imaging developed evidence of distant or nodal metastases during the median follow-up period of 34 mo. Only 13% of patients demonstrated a 20% or greater increase in tumor size during the follow-up period, and 17% of patients ultimately underwent surgery. Of those who had surgery, all lesions were T class 1 (n = 7) or 2 (n = 1), grade 1, node negative, and without evidence of vascular or peripancreatic invasion, suggesting that in selected asymptomatic patients with small primary tumors, expectant management is safe and may spare some patients the morbidity associated with pancreatic resection. Also of note, of those patients who underwent surgery, including five patients who elected to undergo surgery at the initial time of diagnosis, the overall morbidity was 62%, primarily due to pancreatic fistula[40].
Another retrospective study by Lee and colleagues described 133 patients with NF-PNETs less than 4.0 cm in size without evidence of metastatic disease and found no evidence of significant tumor growth on serial imaging or disease-specific morbidity or mortality in patients managed without resection during a mean follow-up period of 48 mo. Of the 56 patients who underwent surgical resection, there was no evidence of recurrence or disease-specific mortality. However, 46% of patients undergoing resection suffered a complication, with pancreatic leak being the cause in over half of cases[53].
There has also been significant interest in parenchyma preserving surgery as an alternative to radical pancreatic resection for small tumors with no preoperative evidence of malignancy. Such approaches may be associated with increased risk of pancreatic fistula but less risk of endocrine and exocrine insufficiency compared to more extensive pancreatic resections[54,55]. This is particularly important in the context of familial syndromes such as MEN-1, von Hippel-Lindau, and neurofibromatosis, as patients with these syndromes are more likely to have multifocal or recurrent disease and may require multiple surgeries throughout their lifetimes. In a retrospective study of 50 patients with small (median 1.4 cm) NF-PNETs treated with either enucleation or central pancreatectomy, Falconi and colleagues confirmed a low rate of exocrine and endocrine insufficiency (8% each). Interestingly, despite the fact that all of the patients included in the study had imaging which demonstrated no evidence of locoregional or distant metastases at the time of surgery, 3 patients (6%) in the study were determined to have lymph node metastases at the time of surgery. A total of 4 patients, 2 of whom had positive lymph nodes at the time of operation, developed hepatic metastases after a mean interval of 68 mo. Despite a low risk of endocrine and exocrine insufficiency, however, these operations were associated with a substantial risk of pancreatic fistula (50%)[56]. Results similar to these were shown in a series of patients who underwent laparoscopic enucleation of small NF-PNETs. In this series 30 consecutive patients were treated with laparoscopic enucleation and local lymph node sampling. Thirteen patients had tumors smaller than 3 cm, with no preoperative evidence of loco-regional metastases on imaging. Three patients with localized tumors had lymph node metastases identified at the time of surgery and underwent a more extensive lymphadenectomy, and a fourth patient developed late hepatic metastases. Five patients developed a pancreatic fistula[57].
While these data support the safety and good long-term functional outcomes associated with pancreatic enucleation, the rates of lymph node metastases and late hepatic metastases among patients with small, benign-appearing tumors was significant in both series. These data raise the concern that simple tumor enucleation, especially in the absence of lymphadenectomy, incurs a risk of understaging and may represent a missed opportunity for more complete resection. In fact, in a series of 177 patients with NF-PNETs published by Bettini et al[58], the rate of lymph node metastases from tumors smaller than 2 cm was 19% after any type of resection with routine lymphadenectomy.
Given the proclivity of NF-PNETs for distant spread, locally advanced disease in the absence of metastatic disease is fairly uncommon. Nonetheless, the occasional patient presents with a bulky primary tumor that invades adjacent organs or surrounding vessels without evidence of metastases. Numerous studies have suggested that surgical resection is associated with prolonged survival in patients with PNETs[1,5,8,11,12,37-39,41,43-46,59-61]. There is a paucity of data, however, to guide the clinician in situations where the primary NF-PNET invades adjacent organs or vasculature. Several series have suggested a role for multi-visceral, en-bloc resection of large PNETs that have invaded adjacent organs[46,62,63]. While such resections are both feasible and often associated with prolonged survival in retrospective series, there is an inherent selection bias in the patients selected for surgery and there are no randomized, controlled trials evaluating aggressive surgical management compared to optimal medical management in patients with locally advanced disease.
Resection of tumors with vascular involvement has been extensively explored in the setting of pancreatic adenocarcinoma. Concomitant vascular reconstructions have entered the surgical mainstream for the management of pancreatic adenocarcinoma in selected patients, with varied results[64-66]. Given the potential for long-term survival following resection, aggressive surgical management of locally advanced PNETs may well be justified. Data, however, are limited to small retrospective case series. Published reports of resection of PNETs with major vascular involvement suggest feasibility and favorable overall and disease-free survival[67,68]. The largest of these series, from Norton and colleagues, includes 44 patients with PNETs either abutting or encasing the IVC, portal vein, SMV, SMA, or splenic vein that underwent surgical resection. Upon operation only 9 of the 42 patients that had resection of their tumor required vascular reconstruction, far fewer than the authors anticipated based on preoperative imaging studies. As the authors note, despite radiologic suggestion of abutment or even invasion of vascular structures, vascular involvement was often not found at the time of surgery, and even in those patients with partial encasement, surgical resection could often be performed without vascular reconstruction. In this series, patients who underwent resection of PNETs had 10 year overall survival of 60% and disease free survival of 30%. For the 14 patients with nonfunctional tumors, however, survival was significantly lower, though there was no difference in disease-free survival[67].
Over half of patients with NF-PNETs have hepatic or distant metastases at the time of diagnosis[1,61,69,70]. Hepatic metastases from pancreatic neuroendocrine tumors are often bilobar and multifocal[41]. Given the sometimes indolent nature of NF-PNETs, the potential for local complications of enlarging pancreatic tumors (e.g., pancreatic duct obstruction or vascular invasion) and the precedent of aggressive surgical management of midgut neuroendocrine tumor primaries, several groups have advocated for resection of PNET primaries even in the face of extensive hepatic disease.
Franko et al[1] published a retrospective review of the SEER database including over 600 patients with metastatic NF-PNETs. Among those patients with unresectable hepatic metastases, those that had only their primary tumors resected experienced significantly longer median survival when compared to those patients that did not have surgery for either their primary or their metastases (1.0 years vs 4.8 years, P < 0.001). Several other series in the literature have also addressed this question, with varied results[71-74]. One of the largest series, from Solorzano and colleagues, included 96 patients with unresectable hepatic metastases from NF-PNETs. Of these 96 patients, 16 underwent resection of their primary tumors while the remaining 80 patients had no surgery at all. Median survival was 3.0 years among the group that had their primary tumors resected and 1.8 years among the group that had no surgery, however this survival difference did not reach statistical significance[61].
In a large retrospective analysis of the impact of surgical resection on patients with PNETs, Hill et al[39] demonstrated a significant survival advantage in patients with distant/metastatic disease undergoing surgical resection of their primary tumor vs those that were recommended surgery but were ultimately not resected (60 mo vs 31 mo, P < 0.001). They demonstrated a survival benefit from resection of the primary tumor in patients with localized, regional, and metastatic disease compared to controls who were also recommended surgery but for various reasons did not undergo resection. Given the available data, resection of the pancreatic primary tumor in selected patients with unresectable hepatic metastases may offer a survival advantage or prevent rare local complications such as pancreatic duct obstruction and vascular or adjacent organ invasion. Other factors such as age, functional status, burden of metastatic disease, and anatomic location of the primary tumor must be taken into consideration.
Complete resection of all visible or imaged liver metastases is often not possible[61]. Moreover, frequent recurrence in the liver suggest the presence of additional subclinical metastases in many patients[75]. Traditionally, surgery for hepatic metastases has been recommended in patients thought to be candidates for curative resection or for palliation in cases where greater than 90% debulking could be performed[76,77]. Although aggressive surgical debulking is thought to prolong survival in patients with hepatic metastases, specific criteria for patient selection remain elusive and several series have suggested that patients with more than 50% liver involvement or bilobar liver involvement have poor outcomes and are less likely to benefit from surgery[41,45,77].
Several groups have investigated the merits of hepatic tumor debulking in patients with neuroendocrine tumor metastases. In an institutional review of 72 patients with hepatic metastases from NF-PNETs by Cusati et al[77], the authors demonstrated that patients who had their primary tumors completely resected and 90% of hepatic metastases debulked had 5-year survival rates of 48.1%. There was a trend toward better 5-year survival (69.6%) in patients that underwent putative R0 resection of both the primary tumor and liver metastases, however this result did not achieve statistical significance. Other institutional case series have suggested a survival benefit for debulking of hepatic metastases from PNETs, and, like the Cusati series, have shown no significant difference between R2/R1 and R0 resection[45,78-82]. A likely explanation for this is the frequent presence of at least microscopic miliary metastatic disease in patients with metastatic NET. This pattern, supported by pathologic studies, belies the concept of an R0 resection and explains the near uniform in-liver recurrence rate documented in other clinical series[75,83].
A large subset of patients with NF-PNETs present with unresectable liver metastases. Liver directed therapies including hepatic trans-arterial embolization (TAE), chemoembolization (TACE), or radioembolization using yttrium-90 microspheres (RE) are increasingly utilized approaches in such patients. Because PNET metastases are highly vascular and derive the vast majority (> 90%) of their oxygen and nutrients from hepatic arterial blood supply, trans-arterial therapies are especially appealing[84]. Additionally, the increasing availability of these approaches has led to their application in resectable cases as well. Advocates for broader application of trans-arterial therapies site safety and efficacy without the need for general anesthesia or an abdominal incision. Moreover, the high rate of recurrence after liver resection for metastatic PNET may favor minimal access approaches in patients for who near complete debulking cannot be achieved surgically. Although there are no randomized, controlled trials comparing TAE/TACE/RE and surgery in the treatment of metastatic pancreatic neuroendocrine tumors consensus guidelines, meta-analyses, cohort studies and retrospective reviews guide the implementation of these therapies.
In a recent meta-analysis comparing surgery to all non-surgical therapies, including trans-arterial as well as medical therapies for patients with both functional and non-functional PNETs, liver resection was demonstrated to be superior to non-surgical treatments, with improved 2, 3, and 5 year survival rates, and no difference in 30-d mortality[85]. In a large, multi-center, retrospective review that examined surgery and/or ablation vs intra-arterial therapies specifically, patients undergoing intra-arterial therapies as primary treatment were significantly more likely to have an unknown primary tumor, extra-hepatic disease, bilateral hepatic disease, and to have > 25% liver involvement than patients treated with surgery. After propensity matching to adjust for these baseline differences, Cox regression analysis demonstrated that surgical management of liver metastases independently improved survival for patients with low-volume (< 25%) disease or those with symptomatic high-volume disease. Asymptomatic patients with a large (> 25%) burden of liver disease who underwent surgical management had no significant difference in long-term outcomes compared with intra-arterial therapies[86].
Recent guidelines developed from the NET-Liver-Metastases Consensus Conference in 2012 and based on available peer-reviewed publications suggest that for those patients unable to undergo resection of liver metastases, there is moderate quality evidence that TAE, TACE, and RE can produce an objective treatment response, decrease tumor markers, and control symptoms in patients with pancreatic NETs and hepatic metastases. The authors noted that there was not enough quality evidence to demonstrate that one modality is superior to another, however RE may cause fewer post-embolization side-effects and require fewer overall treatments[87]. In a study comparing bland embolization with chemoembolization in 67 patients with neuroendocrine liver metastases, chemoembolization trended toward improved time to progression, symptom control, and survival without greater toxicity[88].
In patients with very limited hepatic disease, percutaneous ablative techniques including radiofrequency ablation (RFA) and microwave ablation (MWA) are appealing alternatives to operative approaches. RFA and MWA are frequently used in the treatment of patients with relatively few metastases who are unable to undergo resection or favor a nonoperative approach, in conjunction with surgery at the time of open resection, or as an adjunct to other liver directed therapies[84]. In the largest study to examine RFA and long-term outcomes in a group of 89 patients with mixed types of neuroendocrine tumors that had largely already progressed on other therapies (including 13 patients with NF-PNETs), Akyildiz et al[89] demonstrated that laparoscopic RFA was associated with median disease free survival of 1.3 years and overall survival of 6 years from the time of ablation. Median survival for patients with PNETs was 64 mo. Twenty-two percent of patients went on to develop local recurrence, 63% developed other new liver lesions, and 59% developed extra-hepatic disease during the median follow-up period of 30 mo. In another large, prospective series evaluating laparoscopic RFA in a cohort of 63 patients with mixed neuroendocrine hepatic metastases (including 12 patients with NF-PNETs), Mazzaglia et al[90] demonstrated a median survival of 35 mo from the time of RFA and 54 mo from diagnosis of liver metastases in patients with PNETs, with no perioperative mortality and 5% perioperative morbidity.
Based on available evidence, liver directed therapies should be used for patients deemed to have unresectable hepatic disease or extra-hepatic metastases. In patients with extensive liver disease, trans-arterial approaches may represent a reasonable alternative to subtotal operative debulking. RFA can be a useful adjunct either at the time of surgical resection for discrete foci of unresectable tumor or to treat limited intrahepatic disease in patients with contraindications to surgical resection.
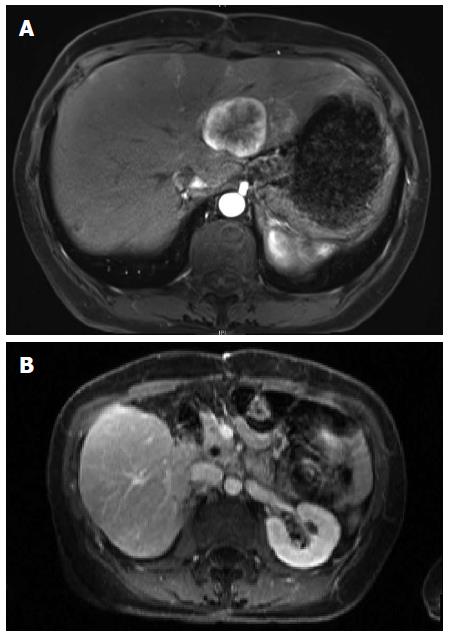
Sandostatin was initiated and the patient was referred for consideration of liver directed therapy. Given an impression of metastatic disease involving approximately 25% of the liver she was offered chemoembolization or radioembolization; she opted for the latter. Radioembolization of right-sided lesions via the right hepatic artery proceeded uneventfully. Angiography of the left hepatic artery demonstrated multiple small branches to the distal stomach. Selective embolization of lesions in segment three was performed. Interval MRI three months later demonstrated excellent response of the right-sided metastases but progression of segments 2 and 4 metastases (Figure 2A). After a detailed discussion of risks and benefits of surgery and alternative treatment options, the patient underwent uneventful distal pancreatectomy and left hepatectomy. Follow up imaging six months later revealed several very small non-enhancing lesions in the right liver consistent with treated metastases and no evidence of recurrent disease (Figure 2B).
PNETs are a rare and diverse group of tumors; NF-PNETs account for the majority of cases. Most patients with NF-PNETs have metastatic disease at the time of presentation. The liver is the most common site of metastatic disease. A variety of medical therapies, including somatostatin analogs, small molecule kinase inhibitors and cytotoxic chemotherapy have efficacy in the treatment of PNETs. Discreet roles for these modalities have not been established.
Aggressive surgical management of primary and metastatic disease is associated with prolonged survival; however, existing studies are limited by selection bias and the frequent combination of PNETs with carcinoid tumors, despite recognition that these entities have distinct biologic behavior. Non-surgical liver directed therapies are increasingly utilized in patients with unresectable liver metastases and have a particular role in patients for who near complete surgical debulking cannot be achieved. The optimal embolic therapy for PNET metastases is not well established. Few patients with liver metastases are curable but excellent outcomes can be achieved through judicious application of existing therapies in a multidisciplinary setting.
P- Reviewer: Kai K, Kin T S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Vortmeyer AO, Huang S, Lubensky I, Zhuang Z. Non-islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab. 2004;89:1934-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 6. | Falconi M, Plockinger U, Kwekkeboom DJ, Manfredi R, Korner M, Kvols L, Pape UF, Ricke J, Goretzki PE, Wildi S. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology. 2006;84:196-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, Di Fonzo M, Tornatore V, Milione M, Angeletti S. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Tomassetti P, Campana D, Piscitelli L, Casadei R, Santini D, Nori F, Morselli-Labate AM, Pezzilli R, Corinaldesi R. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol. 2005;16:1806-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Bilimoria KY, Tomlinson JS, Merkow RP, Stewart AK, Ko CY, Talamonti MS, Bentrem DJ. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J Gastrointest Surg. 2007;11:1460-1467; discussion 1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Kent RB, van Heerden JA, Weiland LH. Nonfunctioning islet cell tumors. Ann Surg. 1981;193:185-190. [PubMed] |
| 11. | Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 13. | Khan MS, El-Khouly F, Davies P, Toumpanakis C, Caplin ME. Long-term results of treatment of malignant carcinoid syndrome with prolonged release Lanreotide (Somatuline Autogel). Aliment Pharmacol Ther. 2011;34:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Öberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124-vii130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 15. | Kulke MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, Choti MA, Clark OH, Doherty GM, Eason J, Emerson L. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10:724-764. [PubMed] |
| 16. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 17. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1742] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 18. | Fjällskog ML, Hessman O, Eriksson B, Janson ET. Upregulated expression of PDGF receptor beta in endocrine pancreatic tumors and metastases compared to normal endocrine pancreas. Acta Oncol. 2007;46:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1267] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 20. | Fjällskog ML, Lejonklou MH, Oberg KE, Eriksson BK, Janson ET. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9:1469-1473. [PubMed] |
| 21. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1829] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 22. | von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E, Wiedenmann B, Boehm BO, Adler G, Seufferlein T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573-4581. [PubMed] |
| 23. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 24. | Broder LE, Carter SK. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med. 1973;79:108-118. [PubMed] |
| 25. | Chernicoff D, Bukowski RM, Groppe CW, Hewlett JS. Combination chemotherapy for islet cell carcinoma and metastatic carcinoid tumors with 5-fluorouracil and streptozotocin. Cancer Treat Rep. 1979;63:795-796. [PubMed] |
| 26. | Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 584] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 27. | Ramanathan RK, Cnaan A, Hahn RG, Carbone PP, Haller DG. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol. 2001;12:1139-1143. [PubMed] |
| 28. | Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762-4771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 29. | Chan JA, Stuart K, Earle CC, Clark JW, Bhargava P, Miksad R, Blaszkowsky L, Enzinger PC, Meyerhardt JA, Zheng H. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30:2963-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Chan JA, Blaszkowsky L, Stuart K, Zhu AX, Allen J, Wadlow R, Ryan DP, Meyerhardt J, Gonzalez M, Regan E. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer. 2013;119:3212-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Kulke MH, Stuart K, Enzinger PC, Ryan DP, Clark JW, Muzikansky A, Vincitore M, Michelini A, Fuchs CS. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 355] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 549] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 33. | Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L, Martinetti A, Platania M, Verzoni E, Formisano B. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol. 2007;59:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Dussol AS, Joly MO, Vercherat C, Forestier J, Hervieu V, Scoazec JY, Lombard-Bohas C, Walter T. Gemcitabine and oxaliplatin or alkylating agents for neuroendocrine tumors: Comparison of efficacy and search for predictive factors guiding treatment choice. Cancer. 2015;121:3428-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | van Vliet EI, Teunissen JJ, Kam BL, de Jong M, Krenning EP, Kwekkeboom DJ. Treatment of gastroenteropancreatic neuroendocrine tumors with peptide receptor radionuclide therapy. Neuroendocrinology. 2013;97:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | van Vliet EI, van Eijck CH, de Krijger RR, Nieveen van Dijkum EJ, Teunissen JJ, Kam BL, de Herder WW, Feelders RA, Bonsing BA, Brabander T. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0,Tyr3]Octreotate. J Nucl Med. 2015;56:1647-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Gomez-Rivera F, Stewart AE, Arnoletti JP, Vickers S, Bland KI, Heslin MJ. Surgical treatment of pancreatic endocrine neoplasms. Am J Surg. 2007;193:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Zerbi A, Capitanio V, Boninsegna L, Pasquali C, Rindi G, Delle Fave G, Del Chiaro M, Casadei R, Falconi M. Surgical treatment of pancreatic endocrine tumours in Italy: results of a prospective multicentre study of 262 cases. Langenbecks Arch Surg. 2011;396:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, Tseng JF. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 40. | Gaujoux S, Partelli S, Maire F, D’Onofrio M, Larroque B, Tamburrino D, Sauvanet A, Falconi M, Ruszniewski P. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98:4784-4789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432-445. [PubMed] |
| 42. | Que FG, Sarmiento JM, Nagorney DM. Hepatic surgery for metastatic gastrointestinal neuroendocrine tumors. Adv Exp Med Biol. 2006;574:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 43. | Hodul PJ, Strosberg JR, Kvols LK. Aggressive surgical resection in the management of pancreatic neuroendocrine tumors: when is it indicated? Cancer Control. 2008;15:314-321. [PubMed] |
| 44. | Fendrich V, Langer P, Celik I, Bartsch DK, Zielke A, Ramaswamy A, Rothmund M. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244:845-851; discussion 852-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, Pitt HA. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776-783; discussion 783-785. [PubMed] |
| 46. | Abu Hilal M, McPhail MJ, Zeidan BA, Jones CE, Johnson CD, Pearce NW. Aggressive multi-visceral pancreatic resections for locally advanced neuroendocrine tumours. Is it worth it? JOP. 2009;10:276-279. [PubMed] |
| 47. | Libutti SK. Evolving paradigm for managing small nonfunctional incidentally discovered pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98:4670-4672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Triponez F, Dosseh D, Goudet P, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Chayvialle JA, Calender A. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Triponez F, Goudet P, Dosseh D, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Calender A, Proye CA. Is surgery beneficial for MEN1 patients with small (& lt; or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30:654-662; discussion 663-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Kann PH, Balakina E, Ivan D, Bartsch DK, Meyer S, Klose KJ, Behr T, Langer P. Natural course of small, asymptomatic neuroendocrine pancreatic tumours in multiple endocrine neoplasia type 1: an endoscopic ultrasound imaging study. Endocr Relat Cancer. 2006;13:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Powell AC, Libutti SK. Multiple endocrine neoplasia type 1: clinical manifestations and management. Cancer Treat Res. 2010;153:287-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, Seidel G, Shutack Y, Yuldasheva N, Eugeni M. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142:814-818; discussion 818.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, Levy MJ, Huebner M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 54. | Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 55. | Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, Capitanio V, Bassi C, Di Carlo V, Pederzoli P. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Fernández-Cruz L, Molina V, Vallejos R, Jiménez Chavarria E, López-Boado MA, Ferrer J. Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB (Oxford). 2012;14:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, Scarpa A, Falconi M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 59. | Jarufe NP, Coldham C, Orug T, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Fendrich V, Habbe N, Celik I, Langer P, Zielke A, Bartsch DK, Rothmund M. [Operative management and long-term survival in patients with neuroendocrine tumors of the pancreas--experience with 144 patients]. Dtsch Med Wochenschr. 2007;132:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Solorzano CC, Lee JE, Pisters PW, Vauthey JN, Ayers GD, Jean ME, Gagel RF, Ajani JA, Wolff RA, Evans DB. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 62. | Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Hellman P, Andersson M, Rastad J, Juhlin C, Karacagil S, Eriksson B, Skogseid B, Akerström G. Surgical strategy for large or malignant endocrine pancreatic tumors. World J Surg. 2000;24:1353-1360. [PubMed] |
| 64. | Martin RC, Scoggins CR, Egnatashvili V, Staley CA, McMasters KM, Kooby DA. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg. 2009;144:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, Weitz J. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2011;254:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 66. | Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93:662-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 67. | Norton JA, Harris EJ, Chen Y, Visser BC, Poultsides GA, Kunz PC, Fisher GA, Jensen RT. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg. 2011;146:724-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Haugvik SP, Labori KJ, Waage A, Line PD, Mathisen Ø, Gladhaug IP. Pancreatic surgery with vascular reconstruction in patients with locally advanced pancreatic neuroendocrine tumors. J Gastrointest Surg. 2013;17:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 70. | Schurr PG, Strate T, Rese K, Kaifi JT, Reichelt U, Petri S, Kleinhans H, Yekebas EF, Izbicki JR. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Birnbaum DJ, Turrini O, Ewald J, Barbier L, Autret A, Hardwigsen J, Brunet C, Moutardier V, Le Treut YP, Delpero JR. Pancreatic neuroendocrine tumor: A multivariate analysis of factors influencing survival. Eur J Surg Oncol. 2014;40:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Bettini R, Mantovani W, Boninsegna L, Crippa S, Capelli P, Bassi C, Scarpa A, Pederzoli P, Falconi M. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis. 2009;41:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Capurso G, Bettini R, Rinzivillo M, Boninsegna L, Delle Fave G, Falconi M. Role of resection of the primary pancreatic neuroendocrine tumour only in patients with unresectable metastatic liver disease: a systematic review. Neuroendocrinology. 2011;93:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Bruzoni M, Parikh P, Celis R, Are C, Ly QP, Meza JL, Sasson AR. Management of the primary tumor in patients with metastatic pancreatic neuroendocrine tumor: a contemporary single-institution review. Am J Surg. 2009;197:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Elias D, Lefevre JH, Duvillard P, Goéré D, Dromain C, Dumont F, Baudin E. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg. 2010;251:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 76. | McEntee GP, Nagorney DM, Kvols LK, Moertel CG, Grant CS. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108:1091-1096. [PubMed] |
| 77. | Cusati D, Zhang L, Harmsen WS, Hu A, Farnell MB, Nagorney DM, Donohue JH, Que FG, Reid-Lombardo KM, Kendrick ML. Metastatic nonfunctioning pancreatic neuroendocrine carcinoma to liver: surgical treatment and outcomes. J Am Coll Surg. 2012;215:117-124; discussion 124-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Sarmiento JM, Que FG, Grant CS, Thompson GB, Farnell MB, Nagorney DM. Concurrent resections of pancreatic islet cell cancers with synchronous hepatic metastases: outcomes of an aggressive approach. Surgery. 2002;132:976-982; discussion 982-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Nguyen SQ, Angel LP, Divino CM, Schluender S, Warner RR. Surgery in malignant pancreatic neuroendocrine tumors. J Surg Oncol. 2007;96:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057-1063; discussion 1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 81. | Elias D, Lasser P, Ducreux M, Duvillard P, Ouellet JF, Dromain C, Schlumberger M, Pocard M, Boige V, Miquel C. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 82. | Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 534] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 83. | Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 84. | Harring TR, Nguyen NT, Goss JA, O’Mahony CA. Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol. 2011;2011:154541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Yuan CH, Wang J, Xiu DR, Tao M, Ma ZL, Jiang B, Li ZF, Li L, Wang L, Wang H. Meta-analysis of Liver Resection Versus Nonsurgical Treatments for Pancreatic Neuroendocrine Tumors with Liver Metastases. Ann Surg Oncol. 2016;23:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Mayo SC, de Jong MC, Bloomston M, Pulitano C, Clary BM, Reddy SK, Clark Gamblin T, Celinski SA, Kooby DA, Staley CA. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18:3657-3665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 87. | Kennedy A, Bester L, Salem R, Sharma RA, Parks RW, Ruszniewski P. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford). 2015;17:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 88. | Ruutiainen AT, Soulen MC, Tuite CM, Clark TW, Mondschein JI, Stavropoulos SW, Trerotola SO. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148:1288-1293; discussion 1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |