Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2830
Peer-review started: August 31, 2014
First decision: September 27, 2014
Revised: November 13, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: March 7, 2015
Processing time: 191 Days and 4.8 Hours
Gastric carcinosarcomas are rare morphologically biphasic tumors, consisting of carcinoma and sarcoma components, with a poor clinical course. Here we report the case of a 70-year-old man with advanced Borrmann type III carcinosarcoma arising from the upper body of the stomach with extensive lymph node metastasis who underwent a total, but palliative, gastrectomy. Histology showed the tumor consisted of a biphasic structure of tubular adenocarcinoma and spindle cell sarcoma. Immunohistochemistry revealed sarcoma cells expressing c-kit (CD117) and CD34, which are criteria for gastrointestinal stromal tumors. Nine months after the surgical operation, tumor metastases had extended to the hepatohilar, retroperitoneal and mediastinal lymph nodes. Radiation therapy of 50 Gy markedly decreased the size of each of these nodes and reduced the risk of respiratory complications and jaundice. However, the patient died of respiratory failure due to bronchopneumonia with multiple lung metastases 22 mo after resection. Autopsy revealed severe necrosis in most of the lymph nodes with tumor metastases. Radiation therapy combined with gastrectomy should be considered to improve survival in patients with gastric carcinosarcomas that express c-kit.
Core tip: Gastric carcinosarcoma is a rare tumor for which there is no established definitive treatment. We present the first report of gastric carcinosarcoma with c-kit expression treated with neoadjuvant chemotherapy, surgical resection and radiation therapy. Radiation therapy to the metastatic tumors dramatically reduced their size and the patient survived 22 mo after surgery with a good quality of life. Although the relation between c-kit expression and radiation susceptibility remains unknown, this report provides new insight into the treatment of gastric carcinosarcoma for improved prognosis.
- Citation: Gohongi T, Iida H, Gunji N, Orii K, Ogata T. Postsurgical radiation therapy for gastric carcinosarcoma with c-kit expression: A case report. World J Gastroenterol 2015; 21(9): 2830-2835
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2830.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2830
Carcinosarcoma is a morphologically biphasic tumor comprised of carcinoma and sarcoma components[1]. Gastric carcinosarcoma is rare, and only 56 cases have been reported, mostly in Japan, since the first report by Queckenstadt in 1904[2]. The sarcoma component was considered to be myogenic, rhabdomyoblastic, chondroblastic or osteoblastic in each case reported[3]. Most patients with gastric carcinosarcoma generally have an unfavorable outcome, due to the advanced stage at the time of diagnosis. The mean survival period is 10-15 mo[4].
Gastrointestinal mesenchymal tumors expressing c-kit are classified as gastrointestinal stromal tumors (GISTs)[5], as they are derived from intestinal pacemaker Cajal cells[6-8]. There have been no reports of carcinosarcoma in which the sarcoma component was classified as a malignant GIST, but a few recent cases of synchronous adenocarcinoma and GIST in the stomach were reported[9,10]. Tyrosine kinase inhibitors (imatinib), which can selectively inhibit the enzymatic activity of several tyrosine kinase receptors including c-kit, are now considered adjuvant therapy for GISTs after complete resection in high-risk patients[11]. Recently, radiation therapy has also attracted considerable attention as a treatment option for malignant GISTs[12-14].
Here, we report an advanced case of gastric carcinosarcoma with c-kit expression. Radiation therapy after surgical resection led to an excellent response regarding recurrent tumors as observed by computed tomography (CT).
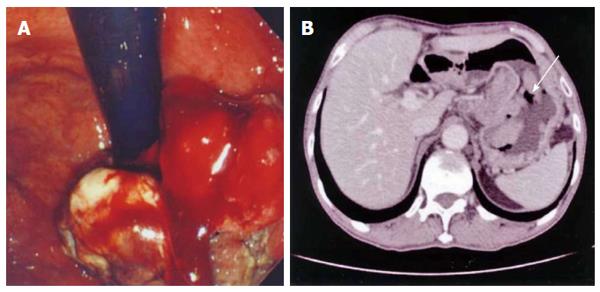
A 70-year-old man was referred to our hospital for dysphagia. He did not drink alcohol, though he had smoked heavily 15 years prior to admission. Laboratory tests showed a low hemoglobin level (12.3 g/dL; normal range: 13.6-16.7 g/dL) and markedly elevated serum alpha-fetoprotein (3019 ng/mL; normal range: < 13.4 ng/mL). Gastroscopy revealed a large exophytic tumor occupying most of the upper anterior wall of the stomach (Figure 1A). CT demonstrated many enlarged lymph nodes (maximum diameter: 25 mm) in the para-aortic and hepatohilar regions (Figure 1B). There was no sign of hematogenic metastasis in any organ.
To increase the chance of a curative operation by tumor downstaging, we applied a neoadjuvant concurrent chemotherapy consisting of tegafur, gimestat, and otastat potassium (80 mg/d, oral for three weeks), and cisplatin (60 mg/m2 body surface area, drip infusion on day 8). A CT scan showed no reduction of tumor size after one course of the adjuvant chemotherapy. We decided to perform surgical resection of the tumor because the symptoms of appetite loss and malnutrition caused by the primary tumor were potential risks for discontinuation of treatment thereafter. A total gastrectomy with dissection of regional lymph nodes including hepatohilar, common hepatic artery and para-aortic regions was performed.
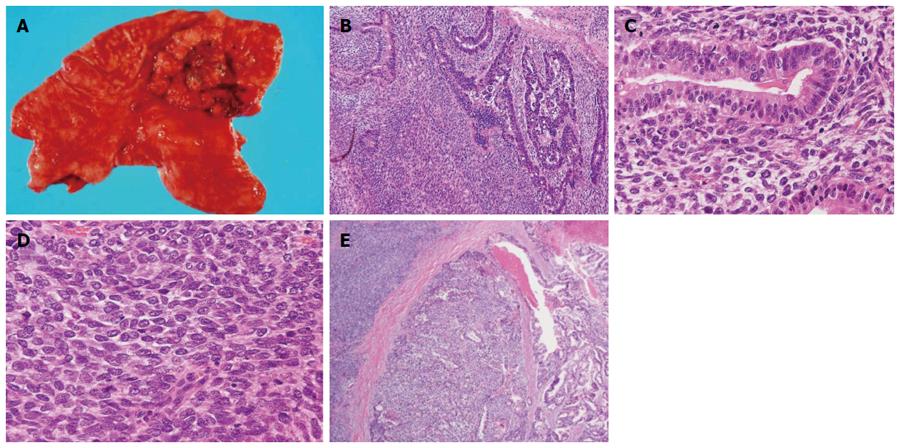
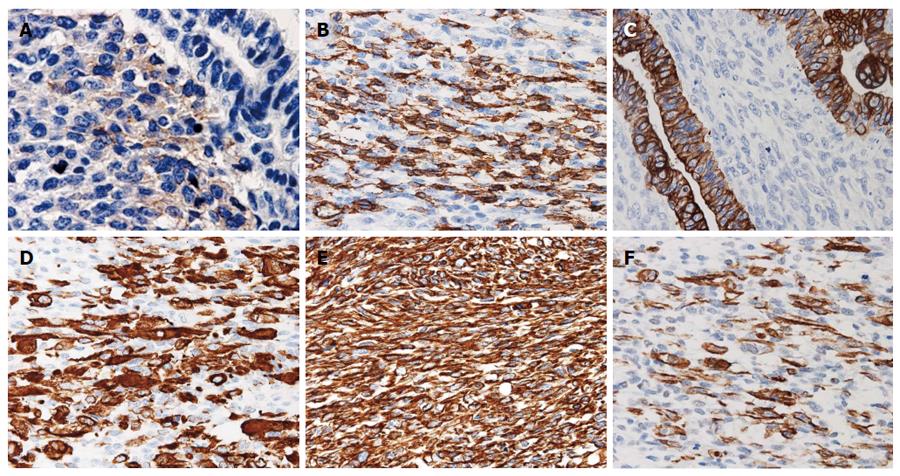
The resected stomach had a 75 mm × 75 mm exophytic and lobulated, Borrmann type III tumor with a central ulceration occupying most of the upper anterior wall and involving all layers of the gastric wall (Figure 2A). Many regional lymph nodes were enlarged and tumor metastasis was histologically revealed in 4 of 11 nodes. Histology also revealed that the gastric tumor consisted of biphasic components of tubular adenocarcinoma and spindle cell sarcoma (Figure 2B), thus the tumor was classified as a carcinosarcoma. Both epithelial and mesenchymal cell elements existed together in each nest that was replaced by sarcoma cells in stromal areas of the adenocarcinoma (Figure 2C and D). The primary tumor included a transitional mixed part of two pure areas of carcinoma and sarcoma components (Figure 2E). Immunohistochemistry revealed the carcinoma component was positive for cytokeratin (AE1/AE3), alpha-fetoprotein and epithelial membrane antigen while the sarcoma component expressed c-kit (CD117) and CD34, in addition to smooth muscle actin, desmin and vimentin (Table 1, Figure 3). Resected lymph nodes also contained biphasic structures consisting of carcinoma and sarcoma. The carcinomatous component was more prominent than the sarcoma component, and a few sarcoma cells were immunopositive for c-kit. The cancer was postoperatively determined as stage IV according to the TNM classification (pT2, N2, M1, P0).
| Tested antigens | Present CS case | Reported GIST cases | Reported CS cases | ||
| Carcinoma | Sarcoma | Carcinoma | Sarcoma | ||
| Cytokeratin (AE1/AE3) | 3+ | - | - | 3+ | - |
| Epithelial membrane antigen | 1+ | - | - | 1+ | - |
| E-cadherin | 3+ | - | - | - | - |
| Alpha-fetoprotein | +/- | - | - | +/- | - |
| c-kit (CD117) | - | 1+ | 3+ (90%-100%) | - | - |
| CD34 | - | 2+ | 3+ (70%) | - | - |
| Smooth muscle actin | - | 2+ | 1+ (20%-30%) | - | 2+ |
| Desmin | - | 2+ | 1+ (2%-4%) | - | 2+ |
| S100 | - | - | - | - | - |
| Vimentin | - | 3+ | - | - | 3+ |
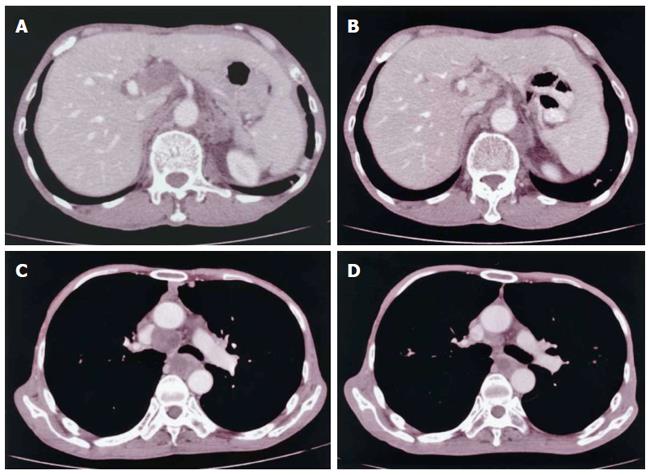
Nine months after the surgical operation, CT revealed enlarged lymph nodes in the hepatohilar and para-aortic regions (Figure 4A) accompanied by no definitive symptoms; there was no evidence of dissemination. Irradiation with 50 Gy (total dose) was performed to these lymph nodes, resulting in marked decreases in size (Figure 4B). Seventeen months after the operation, a CT scan revealed tumor metastasis to the mediastinal lymph nodes at the trachea bifurcation level (Figure 4C), which were markedly reduced in size after 50 Gy irradiation (Figure 4D).
The patient died 22 mo after gastrectomy as a result of respiratory failure due to bronchopneumonia and multiple lung metastases. An autopsy revealed not only lymphogenic metastasis of retroperitoneal, hepatohilar, mediastinal and supraclavicular lymph nodes, but also hematogenic metastases in the liver, lung, kidney, adrenals, and vertebral bone. The majority of the irradiated tumors were necrotic in the surrounding tissues, and the remaining, viable tumor cells of these metastatic lesions displayed adenocarcinoma morphology.
Although the histogenesis of carcinosarcoma still remains uncertain, previous reports have suggested two theories. There is a report of an esophageal carcinosarcoma thought to be derived from a multipotent progenitor or stem cell with the ability to pursue both epithelial and mesenchymal differentiation[15]. Another report of esophageal carcinosarcoma suggests that the tumor originates from the collision of two different types of malignancies with separate epithelial and stromal cell line origins[16]. In contrast, GIST is one of the gastrointestinal non-epithelial neoplasms that likely originates from mesenchymal cells that control gut motility. GIST is mainly composed of spindle shaped cells and/or polygonal epitheloid cells, though the malignant type likely shows more atypical features in nuclear morphology. These tumor cells are immunopositive for stem cell markers such as c-kit and CD34[6]. Miettinen et al[8] stated that c-kit expression and signaling are indicators of mutation, and represent diagnostic criteria for GISTs. The c-kit expression in the sarcoma component in our case suggests a histogenesis similar to that of GIST. Furthermore, the origin of tumor cells in our case may be a common precursor of various intestinal cells, including smooth muscle and Cajal cells. However, it is unclear why epithelial and mesenchymal cell components were coexisting.
The initial clinical presentation of gastric carcinosarcoma resembles that of gastric carcinoma, with signs such as appetite loss, dysphagia, abdominal pain and anemia due to blood loss from the tumor[17]. Moreover, discriminating between these by conventional radiologic examinations such as CT scan and barium gastrography is difficult due to the lack of specific features. Endoscopic findings generally demonstrate polypoid and lobular configuration with or without ulceration in the center[18]. Gastric carcinosarcoma has a lower incidence of metastasis than gastric carcinoma, but a high mortality rate[19]. In fatal cases, however, metastases are found at various sites. Postsurgical survival of patients with gastric carcinosarcoma is less than ten months after diagnosis[20].
Definitive treatment for gastric carcinosarcoma has not been established, and currently, there are no reports describing effective chemotherapy or radiotherapy. Surgical resection is the only curative modality in cases where the tumor remains in the stomach without distant metastasis[21]. The prognosis is worse in cases of advanced clinical stage, and thus a therapeutic approach other than surgery should be considered. In the case presented here, lymph node metastasis was identified at the time of diagnosis. Surgical resection of the primary tumor and extensive lymph node dissection were performed to minimize tumor burden in the body. Although imatinib is widely accepted for the treatment of GIST, its effect would have been limited in this case as the carcinomatous component did not show c-kit expression. There has been no study reported so far regarding imatinib mesylate for the treatment of gastric carcinosarcoma.
Though not often considered for GISTs, some recent publications provide insight into the efficacy of radiation therapy[12-14]. Cuaron et al[14] reported local control in 15/17 treated GISTs. In our case, radiation therapies of 50 Gy each decreased the size of metastases in the hepatohilar, para-aortic, and mediastinal lymph nodes, which appeared as cytoreductive effects upon autopsy, and reduced risks of respiratory trouble and jaundice. However, the relation between c-kit expression and response to radiation is not known, and further studies are needed to examine this.
Although evidence of a survival benefit from combined adjuvant chemotherapy has not been demonstrated, our case indicates a prolonged survival (22 mo) with radiotherapy after tumor resection. Therefore, to improve survival and prognosis of gastric carcinosarcoma, intensive surgery and additional radiation therapy to metastatic foci should be considered, especially in cases of tumors with c-kit expression.
A 70-year-old man presented with dysphagia.
Dysphagia with no abdominal pain and normal bowel movements.
Malignant or benign tumors in the stomach; liver tumor.
Laboratory tests showed a hemoglobin level of 12.3 g/dL (normal range: 13.6-16.7 g/dL) and an elevated serum alpha-fetoprotein level of 3019 ng/mL (normal range: < 3.4 ng/mL).
Gastroscopy revealed a large exophytic tumor occupying most of the upper anterior wall of the stomach; Computed tomography enlarged lymph nodes (25 mm in diameter) in the para-aortic and hepatohilar regions.
Histology revealed the gastric tumor consisted of biphasic components of tubular adenocarcinoma and spindle cell sarcoma, and the tumor was classified as a carcinosarcoma.
The patient underwent total gastrectomy and radiation therapy to enlarged lymph nodes in the hepatic portal and mediastinal regions.
Carcinosarcoma is a rare tumor and the treatment modality is not yet been established. There are no reports of the efficacy of radiation therapy to metastatic sites of gastric carcinosarcoma.
Carcinosarcoma is a rare tumor with a biphasic morphology of carcinoma and sarcoma. C-kit is a proto-oncogene that encodes a transmembrane tyrosine kinase receptor. C-kit is thought to play an important role in hematopoiesis, spermatogenesis, melanogenesis and carcinogenesis.
To improve survival and prognosis of gastric carcinosarcoma, a combination of intensive surgery and additional radiation therapy to its metastatic foci should be considered.
The case report provides clinical details regarding gastric carcinosarcoma with c-kit expression. Palliative radiation therapy was effective in improving the patient’s quality of life. Further study is needed to clarify the relation between c-kit expression and response to radiation.
P- Reviewer: Aoyagi K, Chen L, Chen MJ, Huang L, Lasithiotakis K, Morgagni P, Sung J, Yu B S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Tanimura H, Furuta M. Carcinosarcoma of the stomach. Am J Surg. 1967;113:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Queckenstadt HHG. Ueber Karzinosarkome. Germany: Leipzig 1904; 1-52. |
| 3. | Randjelovic T, Filipovic B, Babic D, Cemerikic V, Filipovic B. Carcinosarcoma of the stomach: a case report and review of the literature. World J Gastroenterol. 2007;13:5533-5536. [PubMed] |
| 4. | Teramachi K, Kanomata N, Hasebe T, Ishii G, Sugito M, Ochiai A. Carcinosarcoma (pure endocrine cell carcinoma with sarcoma components) of the stomach. Pathol Int. 2003;53:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 6. | Koelz M, Wick N, Winkler T, Laengle F, Wrba F. The impact of c-kit mutations on histomorphological risk assessment of gastrointestinal stromal tumors. Eur Surg. 2007;39:45-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1178] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 8. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Uchiyama S, Nagano M, Takahashi N, Hidaka H, Matsuda H, Nagaike K, Maehara N, Hotokezaka M, Chijiiwa K. Synchronous adenocarcinoma and gastrointestinal stromal tumors of the stomach treated laparoscopically. Int J Clin Oncol. 2007;12:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Yamamoto D, Hamada Y, Tsubota Y, Kawakami K, Yamamoto C, Yamamoto M. Simultaneous development of adenocarcinoma and gastrointestinal stromal tumor (GIST) in the stomach: case report. World J Surg Oncol. 2012;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3111] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 12. | Knowlton CA, Brady LW, Heintzelman RC. Radiotherapy in the treatment of gastrointestinal stromal tumor. Rare Tumors. 2011;3:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ulsenheimer K. [Legal liability problems in outpatient operations. View from an anesthesiological perspective]. Anaesthesist. 2012;61:156-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Cuaron JJ, Goodman KA, Lee N, Wu AJ. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat Oncol. 2013;8:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Corbin KS, Kindler HL, Liauw SL. Considering the role of radiation therapy for gastrointestinal stromal tumor. Onco Targets Ther. 2014;7:713-718. [PubMed] |
| 16. | Ota S, Kato A, Kobayashi H, Yonezumi M, Yamaguchi J, Musashi M, Imamura M, Asaka M. Monoclonal origin of an esophageal carcinosarcoma producing granulocyte-colony stimulating factor: a case report. Cancer. 1998;82:2102-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ikeda Y, Kosugi S, Nishikura K, Ohashi M, Kanda T, Kobayashi T, Hatakeyama K. Gastric carcinosarcoma presenting as a huge epigastric mass. Gastric Cancer. 2007;10:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Choi KW, Lee WY, Hong SW, Chang YG, Lee B, Lee HK. Carcinosarcoma of the stomach: a case report. J Gastric Cancer. 2013;13:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bansal M, Kaneko M, Gordon RE. Carcinosarcoma and separate carcinoid tumor of the stomach. A case report with light and electron microscopic studies. Cancer. 1982;50:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Sato Y, Shimozono T, Kawano S, Toyoda K, Onoe K, Asada Y, Hayashi T. Gastric carcinosarcoma, coexistence of adenosquamous carcinoma and rhabdomyosarcoma: a case report. Histopathology. 2001;39:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Kiśluk J, Gryko M, Guzińska-Ustymowicz K, Kemona A, Kędra B. Immunohistochemical diagnosis of gastrointestinal stromal tumors - an analysis of 80 cases from 2004 to 2010. Adv Clin Exp Med. 2013;22:33-39. [PubMed] |