Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2820
Peer-review started: August 6, 2014
First decision: September 15, 2014
Revised: October 18, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: March 7, 2015
Processing time: 215 Days and 17.5 Hours
Intraductal papillary mucinous neoplasm (IPMN) is a mucin-producing epithelial neoplasm that carries a risk of progression to invasive pancreatic ductal adenocarcinoma. Lynch syndrome is an autosomal dominant condition caused by germline mutations in mismatch repair genes such as MSH2 that lead to microsatellite instability and increased risk of tumor formation. Although families with Lynch syndrome have an increased risk of pancreatic cancer, a clear connection between Lynch syndrome and IPMN has not been drawn. We present a report of a 58 year-old Caucasian woman with multiple cancers and a germline mutation of MSH2 consistent with Lynch syndrome. A screening abdominal computed tomography scan revealed a dilated main pancreatic duct and cystic ductular structure in the uncinate process that were consistent with IPMN of the main pancreatic duct on excision. Immunohistochemistry and polymerase chain reaction of the patient’s pancreas specimen did not reveal microsatellite instability or mismatch repair gene loss of expression or function. Our findings may be explained by the fact that loss of mismatch repair function and microsatellite instability is a late event in neoplastic transformation. Given the relative rarity of main duct IPMN, its appearance in the setting of somatic MSH2 mutation suggests that IPMN may fit into the constellation of Lynch syndrome related malignancies.
Core tip: Intraductal papillary mucinous neoplasms (IPMN) are now recognized as important precursor lesions to pancreatic cancer. Although there have been reports linking pancreatic cancer and familial cancer syndromes, only one previous case report has described IPMN in a patient with Lynch syndrome. Our case is a main duct IPMN that contained only low-grade dysplasia and no microsatellite instability despite the presence of a germline MSH2 mutation. Mismatch repair (MMR) gene mutations may be involved in the neoplastic changes that drive the development of IPMN; however, changes in MMR function may not be detectable in the setting of low-grade neoplasia.
- Citation: Flanagan MR, Jayaraj A, Xiong W, Yeh MM, Raskind WH, Pillarisetty VG. Pancreatic intraductal papillary mucinous neoplasm in a patient with Lynch syndrome. World J Gastroenterol 2015; 21(9): 2820-2825
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2820.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2820
Lynch syndrome, formerly referred to as Hereditary Nonpolyposis Colorectal Cancer (HNPCC), is an autosomal dominant condition caused by germline mutations in mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PSM2, and deletions in EPCAM via epigenetic silencing of MSH2[1-3]. Proteins encoded by MMR genes recognize and repair insertion and deletion errors, and single nucleotide mismatches that are produced as cells replicate. Inactivation of the normal alleles of MMR genes may result in loss of their expression in tumors of Lynch syndrome patients[4-6]. Impairment of the MMR system results in accumulated somatic mutations in error prone replication regions containing sequence repeats known as microsatellites. Tumors that develop in patients with Lynch syndrome often show marked variation in the numbers of repeat sequences, a feature that is referred to as microsatellite instability (MSI).
Lynch syndrome is suspected on the basis of patient and family history, MMR protein expression pattern and MSI phenotype. Confirmation of the diagnosis is by molecular analysis of the MMR genes. The main clinical features are early development of colorectal and endometrial cancers, but carriers of MMR gene mutations appear to have increased risk for developing pancreatic, ovarian, small bowel, gastric, urothelial and possibly breast and prostate cancers[7,8]. Cancer risk varies depending upon which MMR gene is mutated, and heterozygosity for an MSH2 mutation is associated with the greatest risk for extracolonic cancers.
Recent evidence suggests a possible association between familial cancer syndromes with extrapancreatic malignancies and intraductal papillary mucinous neoplasm (IPMN), one of the main precursor lesions to pancreatic cancer[9-11]. IPMN are radiographically detectable, mucin-producing epithelial neoplasms affecting main and/or side branch pancreatic ducts[12-15]. Both main-duct and branch-duct IPMN carry a risk of cancer development; however, there is a significantly higher risk in the setting of main duct involvement[12]. Patients with IPMN have a reported risk of 19%-52% of extra-pancreatic malignancies (EPM), suggesting that patients with IPMN may have an increased susceptibility for tumor formation[16-20]. Cases of IPMN with familial adenomatous polyposis (FAP), Peutz-Jeghers syndrome (PJS), and BRCA2 mutations have been reported[21-24]. There is only one prior case report of branch-duct IPMN that developed in a patient with Lynch syndrome, and this involving an MSH2 germline mutation[25]. We herein report a case of main-duct IPMN in a patient with Lynch syndrome.
Our patient is a Caucasian woman who was first seen in our clinic at the age of 58 with a familial and personal history of multiple malignancies. She presented with melena at age 31 and underwent extended right hemicolectomy for a right-sided colon cancer. The margins of resection were negative and there was no nodal involvement. She did not undergo adjuvant treatment. At age 38, she underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy for uterine cancer that was diagnosed during a workup for fibroids and menorrhagia. At age 53, she underwent lumpectomy and sentinel lymph node biopsy for invasive breast cancer detected on screening mammography. Pathology revealed a node-negative, high grade, T1a ER/PR/Her2 negative invasive adenocarcinoma.
In addition to her personal history of multiple cancers, she has an extensive family history of cancer. Multiple maternal and paternal family members had colon cancer, including a brother diagnosed at age 45, mother diagnosed at 54, maternal aunt diagnosed with metachronous lesions at ages 55 and 75, and paternal grandfather diagnosed at 80. Her mother had a brain tumor at 69, and there was a question of uterine or breast cancer in her maternal grandmother. Our patient’s father had both melanoma and prostate cancer, and his mother had uterine cancer in her 50s. Our patient has two sisters aged 45 and 47 without cancer, and three sons aged 27, 31 and 34 who have all had negative screening colonoscopies. Her personal and family history was felt to be consistent with Lynch syndrome, and genetic testing revealed an exon 1 deletion in MSH2 that was classified as a suspected deleterious change.
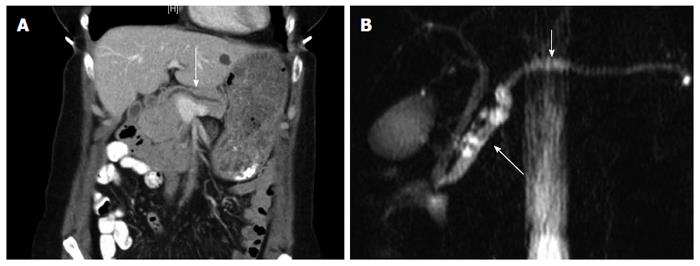
Due to her high risk for new or recurrent malignancies, yearly screening with a computed tomography (CT) scan of the chest, abdomen and pelvis was performed. The patient was referred to our clinic after an annual CT identified an 8 mm dilation of the main pancreatic duct, and a tubular cystic ductular structure in the uncinate process that directly communicated with the main pancreatic duct (Figure 1A). MRI/MRCP confirmed CT findings (Figure 1B). Esophagogastroduodenoscopy with endoscopic ultrasound showed dilation of the main pancreatic duct to 9 mm, with wall irregularity at the area of the genu, mucin extruding from the ampulla and a gaping orifice consistent with IPMN. CA 19-9 was slightly elevated at 40 U/mL, but all other laboratory values were normal. Cyst fluid was not obtainable for CEA analysis.
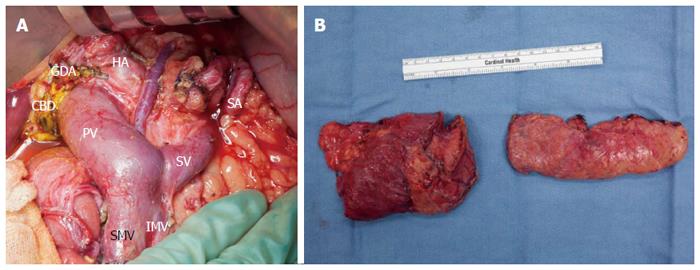
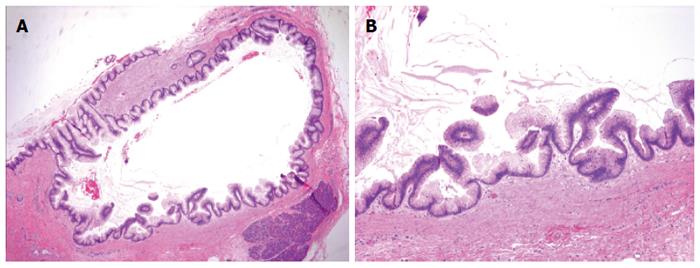
The patient was counseled on the risk of progression of main duct IPMN to invasive PDA, and because of her prior cancer history she elected to undergo a total pancreatectomy. The gross specimen appeared normal, and final pathology revealed low-grade main duct IPMN without high-grade dysplasia or invasive carcinoma (Figures 2 and 3). In addition to main duct IPMN, the distal pancreatectomy specimen contained a focus of low-grade pancreatic intraepithelial neoplasia (PanIN). All sampled lymph nodes were negative for carcinoma.
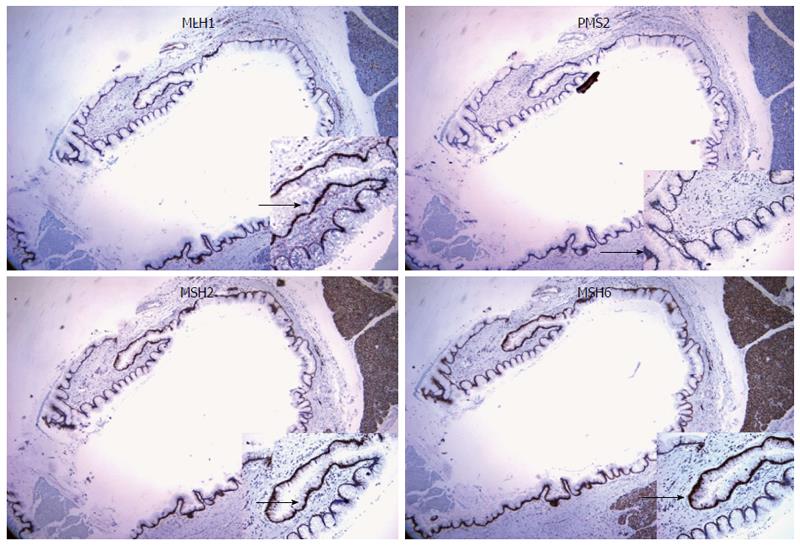
Immunohistochemical stains on the pancreatic specimen were negative for loss of mismatch repair protein expression, including MLH1, PMS2, MSH2, and MSH6 (Figure 4). Testing was performed on Leica Bond III immunostainer using the following monoclonal antibodies: MLH1 (DAKO-ES05); PMS2 (BD-Pharmingen-A16-4); MSH2 (NCL-25D12), MSH6 (BD-44-MSH6). Microsatellite instability was not identified in DNA prepared separately from microdissected normal and tumor cells and analyzed by pentaplex PCR.
To our knowledge, this report is the second case of an IPMN occurring in a patient with an MMR germline mutation, and the first with main duct IPMN. Our patient clinically met Amsterdam I criteria, and was subsequently found to have an MSH2 germline mutation confirming her diagnosis of Lynch syndrome. The patient’s exon 1 deletion in MSH2 was likely involved in the development of her early onset colon and uterine cancers, although pathologic data from her colectomy and hysterectomy specimens from 20 years ago are not available for testing. Interestingly, and in contrast to the previous report of IPMN in a patient with Lynch syndrome and a germline MSH2 mutation[25], our patient’s IPMN specimen was microsatellite stable (MSS) and retained MSH2 expression.
Lynch syndrome is caused by inactivating mutations of DNA MMR genes that impair DNA mismatch repair mechanisms. Loss of function of one of these genes is through a combination of inherited mutation and somatic loss of the normal copy during tumor development. This impairs the ability of the MMR system to recognize and repair DNA mismatches. Although other mechanisms have been reported[26], one hypothesis for the retained MSH2 staining and lack of MSI in our report is that complete loss of MMR function likely requires multiple cell cycle alterations and may be a late event in neoplastic transformation. An analogy can be drawn between low-grade dysplasia in a pre-malignant IPMN and adenomatous colon polyps that regularly form in Lynch syndrome. Adenomatous polyps exhibit a lower rate of MSI than do invasive cancers, and high-grade dysplastic polyps are more likely to exhibit MSI than early polyps[27]. High frequency MSI (MSI-H) is present in most Lynch syndrome tumors, but in a study by Yurgelun et al[28] it was seen in only 41% of Lynch syndrome associated adenomatous polyps. Both MSI-H and loss of MMR expression correlated with increasing polyp size.
Patients with IPMN have increased risk of benign and malignant EPM[20]. Colorectal, breast, lung and gastric cancers are the most commonly reported[20,29]. Increased age and family history of gastric and colorectal cancer are risk factors[17,20], but only a few studies have investigated potential underlying genetic causes. A gene encoding mucin-producing proteins, MUC2, has been implicated[30] along with familial syndrome genes. Case reports have shown cases of IPMN with bilallelic inactivation of the PJS gene in a patient with Peutz-Jeghers syndrome (STK11/LKB1)[22], and loss of the wild-type APC allele in a patient with FAP[23].
The relationship between IPMN and Lynch syndrome is not well described. Lubezky et al[24] reported a patient with IPMN who fulfilled Amsterdam criteria for Lynch syndrome, but whose tumor had no MSI or loss of expression of MMR genes. Sparr et al[25] described a patient with Lynch syndrome whose adenocarcinoma of the colon and IPMN of the pancreas showed identical IHC staining profiles with loss of expression of MSH2 and MSH6 proteins and a high degree of MSI. The loss of expression of both genes reflects the fact that MMR proteins function as heterodimers, and loss of expression of MSH2 often correlates with loss of expression of MSH6[31]. Importantly, the IPMN reported by Sparr et al[25] contained high-grade dysplasia. In contrast, and similar to low-grade colon polyps, we may not have seen loss of MMR expression and MSI in our patient due to the low-grade dysplasia. This suggests that a “second hit” had not yet occurred to drive alterations in MMR expression. Furthermore, mucinous tumors are particularly refractory to MSI testing because of a relatively small amount of tumor DNA[32].
Our report adds to current evidence supporting the inclusion of IPMN in the growing list of extracolonic Lynch syndrome-associated tumors. IPMN is a precancerous condition that, once diagnosed, usually requires either close surveillance or surgical resection due to the possibility of progression to pancreatic cancer. Although the precise nature of the relationship between IPMN and Lynch syndrome is not yet known, a heightened level of suspicion for the risks posed by cystic neoplasms of the pancreas is warranted in patients with Lynch syndrome.
The 58-year-old female patient was asymptomatic; intraductal papillary mucinous neoplasm (IPMN) was diagnosed on annual screening abdominal computed tomography (CT).
On physical examination there was no palpable abdominal mass.
Malignant tumor (pancreatic adenocarcinoma, neuroendocrine tumor, metastasis), IPMN or chronic pancreatitis.
The patient had a slightly elevated CA 19-9 (40 U/mL); all other laboratory values were normal.
CT and magnetic resonance imaging/magnetic resonance cholangiopancreatography showed a dilated main pancreatic duct with a cystic structure in the uncinate process, while esophagogastroduodenoscopy with endoscopic ultrasound showed dilation of the main pancreatic duct with wall irregularity at the area of the genu, mucin extruding from the ampulla and a gaping orifice.
Pathologic examination revealed a grossly normal pancreas, while histologic examination showed a low-grade main duct IPMN without loss of mismatch repair protein expression or microsatellite instability.
The patient underwent a total pancreatectomy.
There is only one other case of IPMN in a patient with Lynch syndrome reported in the literature. The association of IPMN and Lynch syndrome is controversial.
IPMN is a mucin-producing epithelial neoplasm that carries a risk of progression to invasive pancreatic ductal adenocarcinoma.
This report discusses a case of IPMN in a patient with Lynch syndrome, and adds to current evidence supporting the inclusion of IPMN in the list of extracolonic Lynch syndrome-associated tumors.
Authors made a case report about IPMN connected with Lynch syndrome with germline mutation of MSH2 Both are rare diseases, therefore little is known about the connection between Lynch syndrome and IPMN .Though this report is a second one in the literature, this case report adds new information on this topic. The work is well done.
P- Reviewer: Mandi Y, Perini MV, Yalniz M, Yang F S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 588] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 2. | Ligtenberg MJ, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, Lee TY, Bodmer D, Hoenselaar E, Hendriks-Cornelissen SJ. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet. 2009;41:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 579] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 3. | Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat. 2009;30:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169-174. [PubMed] |
| 5. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1548] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 6. | Aaltonen LA, Peltomäki P, Mecklin JP, Järvinen H, Jass JR, Green JS, Lynch HT, Watson P, Tallqvist G, Juhola M. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994;54:1645-1648. [PubMed] |
| 7. | Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 536] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 8. | Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-268. [PubMed] |
| 9. | Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737-7743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766-781; quiz 665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788-97; discussion 797-9. [PubMed] |
| 13. | Bassi C, Sarr MG, Lillemoe KD, Reber HA. Natural history of intraductal papillary mucinous neoplasms (IPMN): current evidence and implications for management. J Gastrointest Surg. 2008;12:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Lévy P, Jouannaud V, O’Toole D, Couvelard A, Vullierme MP, Palazzo L, Aubert A, Ponsot P, Sauvanet A, Maire F. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Lafemina J, Katabi N, Klimstra D, Correa-Gallego C, Gaujoux S, Kingham TP, Dematteo RP, Fong Y, D’Angelica MI, Jarnagin WR. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol. 2013;20:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Riall TS, Stager VM, Nealon WH, Townsend CM, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803-813; discussion 813-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Eguchi H, Ishikawa O, Ohigashi H, Tomimaru Y, Sasaki Y, Yamada T, Tsukuma H, Nakaizumi A, Imaoka S. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006;139:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Choi MG, Kim SW, Han SS, Jang JY, Park YH. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51-56; discussion 56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol. 2005;11:5688-5690. [PubMed] |
| 20. | Reid-Lombardo KM, Mathis KL, Wood CM, Harmsen WS, Sarr MG. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas: implications for management. Ann Surg. 2010;251:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Maire F, Hammel P, Terris B, Olschwang S, O’Toole D, Sauvanet A, Palazzo L, Ponsot P, Laplane B, Lévy P. Intraductal papillary and mucinous pancreatic tumour: a new extracolonic tumour in familial adenomatous polyposis. Gut. 2002;51:446-449. [PubMed] |
| 22. | Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Sudo T, Murakami Y, Uemura K, Hayashidani Y, Takesue Y, Sueda T. Development of an intraductal papillary-mucinous neoplasm of the pancreas in a patient with familial adenomatous polyposis. Pancreas. 2005;31:428-429. [PubMed] |
| 24. | Lubezky N, Ben-Haim M, Lahat G, Marmor S, Solar I, Brazowski E, Nackache R, Klausner JM. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Sparr JA, Bandipalliam P, Redston MS, Syngal S. Intraductal papillary mucinous neoplasm of the pancreas with loss of mismatch repair in a patient with Lynch syndrome. Am J Surg Pathol. 2009;33:309-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Mangold E, Pagenstecher C, Friedl W, Fischer HP, Merkelbach-Bruse S, Ohlendorf M, Friedrichs N, Aretz S, Buettner R, Propping P. Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol. 2005;207:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Iino H, Simms L, Young J, Arnold J, Winship IM, Webb SI, Furlong KL, Leggett B, Jass JR. DNA microsatellite instability and mismatch repair protein loss in adenomas presenting in hereditary non-polyposis colorectal cancer. Gut. 2000;47:37-42. [PubMed] |
| 28. | Yurgelun MB, Goel A, Hornick JL, Sen A, Turgeon DK, Ruffin MT, Marcon NE, Baron JA, Bresalier RS, Syngal S. Microsatellite instability and DNA mismatch repair protein deficiency in Lynch syndrome colorectal polyps. Cancer Prev Res (Phila). 2012;5:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Larghi A, Panic N, Capurso G, Leoncini E, Arzani D, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A. Prevalence and risk factors of extrapancreatic malignancies in a large cohort of patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Oncol. 2013;24:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Lee SY, Choi DW, Jang KT, Lee KT, Choi SH, Heo JS, Lee JK, Paik SW, Rhee JC. High expression of intestinal-type mucin (MUC2) in intraductal papillary mucinous neoplasms coexisting with extrapancreatic gastrointestinal cancers. Pancreas. 2006;32:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Acharya S, Wilson T, Gradia S, Kane MF, Guerrette S, Marsischky GT, Kolodner R, Fishel R. hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci USA. 1996;93:13629-13634. [PubMed] |
| 32. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1020] [Article Influence: 51.0] [Reference Citation Analysis (0)] |