Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2683
Peer-review started: July 1, 2014
First decision: August 6, 2014
Revised: August 25, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: March 7, 2015
Processing time: 251 Days and 20.2 Hours
AIM: To study how lymph node metastasis (LNM) risk is stratified in undifferentiated-type early gastric cancer (undiff-EGC) dependent on combinations of risk factors.
METHODS: Five hundred and sixty-seven cases with undiff-EGC undergoing gastrectomy with lymphadenectomy were examined retrospectively. Using clinicopathological factors of patient age, location, size, an endoscopic macroscopic tumor form, ulceration, depth, histology, lymphatic involvement (LI) and venous involvement (VI), LNM risk was examined and stratified by conventional statistical analysis and data-mining analysis.
RESULTS: LNM was positive in 44 of 567 cases (7.8%). Univariate analysis revealed > 2 cm, protrusion, submucosal (sm), mixed type, LI and VI as significant prognostic factors and > 2 cm and LI-positive were independent factors by multivariate analysis. In preoperatively evaluable factors excluding LVI, sm and > 2 cm were independent factors. According to the depth and size, cases were categorized into the low-risk group [m and ≤ 2 cm, 0% (LNM incidence)], the moderate-risk group (m and > 2 cm, 5.6%; and sm and ≤ 2 cm, 6.0%), and the high-risk group (sm and > 2 cm, 19.3%). On the other hand, LNM occurred in 1.4% in all LI-negative cases, greatly lower than 28.2% in all LI-positive cases, and LNM incidence was low in LI-negative cases even in the moderate- and high-risk groups.
CONCLUSION: LNM-related factors in undiff-EGC were depth and size preoperatively while those were LI and size postoperatively. Among these factors, LI was the most significantly correlated factor.
Core tip: The lymph node metastasis (LNM) risk in cases with undifferentiated-type early gastric cancer was evaluated and stratified using preoperatively as well as postoperatively evaluable factors. In preoperatively evaluable factors, the risk of LNM was predicted based on the size and depth and categorized into the low-risk group: mucosal cancer (m) and ≤ 2 cm, 0% (LNM incidence) (95%CI: 0-2.3), the moderate-risk group: m and > 2 cm, 5.6% (95%CI: 2.6-11.7); submucosal invasion (sm) and ≤ 2 cm, 6.0% (95%CI: 3.2-11.1), and the high-risk group: sm and > 2 cm, 19.3% (95%CI: 13.8-26.4). However, when the postoperatively evaluable factor of LI was included, cases with further lower or higher risk could be stratified even in the moderate- and high-risk groups. Some high-risk cases for surgery due to old age and concurrent disease could be reasonably followed-up after resection of the gastric lesion by endoscopic submucosal dissection.
- Citation: Asakawa Y, Ohtaka M, Maekawa S, Fukasawa M, Nakayama Y, Yamaguchi T, Inoue T, Uetake T, Sakamoto M, Sato T, Kawaguchi Y, Fujii H, Mochizuki K, Hada M, Oyama T, Yasumura T, Omata K, Nishiyama A, Naito K, Hata H, Haba Y, Miyata K, Saitoh H, Yamadera Y, Miura K, Kawaoi A, Abe T, Tsunoda H, Honda Y, Kurosaki M, Enomoto N. Stratifying the risk of lymph node metastasis in undifferentiated-type early gastric cancer. World J Gastroenterol 2015; 21(9): 2683-2692
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2683.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2683
Recently, technique of endoscopic submucosal dissection (ESD) has advanced and ESD has become prevalent as an excellent treatment modality in terms of low-invasiveness, functional preservation of the stomach, and postoperative quality of life, in particular, as curative treatment for differentiated-type early gastric cancer (EGC). Meanwhile, compared with the differentiated-type, undifferentiated-type EGC (undiff-EGC) is expected to have high incidence of lymph node metastasis (LNM), and it has been thought that surgery with lymphadenectomy is necessary and there is no indication of ESD.
However, Gotoda et al[1] reported in 2000 that undiff-EGC with the conditions of mucosal cancer (m), ulceration (UL)(-), ≤ 2 cm, and lymphatic-vascular involvement (LVI)(-) had a risk of LNM at 0% (95%CI: 0-2.6), which suggested a potential indication of ESD for undiff-EGC. Although reports focusing on LNM risk factors in undiff-EGC have been increasing, it was verified in a number of cases (3843 cases) in 2009 that LNM was markedly rare within the conditions proposed by Hirasawa et al[2]. According to this report, the Japan Gastric Cancer Society constructed Gastric Cancer Treatment Guidelines 2010[3], and the lesions satisfying the above mentioned expanded criteria, which are considered an indication for ESD, have been treated by ESD.
Meanwhile, undiff-EGC with low LNM risk has been the focus of exploration, and it has been poorly elucidated how high the LNM risk is in the cases that fail to satisfy the criteria of the guidelines. Surgery with lymphadenectomy is recommended in cases with potentially positive LNM in undiff-EGC, but the incidence of LNM was 2.8%-10.6% even in undiff-EGC without satisfying the indication for ESD[1,2], and LNM was absent in almost 90% of cases. Therefore, should identification of the 90% of cases with low LNM risk be possible, even without the indication for ESD, unnecessary surgery and lymphadenectomy may be avoided in the elderly and those with a variety of concurrent diseases with a high risk for surgery.
In the present study, we elucidated LNM risk factors in a number of cases with surgically-resected undiff-EGC and analyzed clinicopathological factors by introduction of data mining, in addition to conventional statistical analysis, to classify risk groups by combinations of risk factors.
The subjects comprised 567 cases that underwent gastrectomy with lymphadenectomy for undiff-EGC which was diagnosed by biopsy before an operation between March 1983 and April 2012 at University of Yamanashi Hospital and its collaborating institutes (Fujiyoshida Municipal Medical Center, Social Insurance Yamanashi Hospital, Kofu National Hospital, Kofu Kyoritsu Hospital, Social Insurance Kajikazawa Hospital, Yamanashi Prefectural Central Hospital, Kofu Municipal Hospital, Yamanashi Kosei Hospital, and Kanoiwa General Hospital). No cases received endoscopic therapy such as ESD or endoscopic mucosal resection prior to gastrectomy. Then, clinicopathological characteristics which were considered relevant with local LNM were retrospectively investigated.
Clinicopathological factors were categorized as follows: patient age (≤ 60 years or > 60 years), location [upper third (upper), middle third (middle), or lower third (lower) of the stomach], size (maximum diameter ≤ 2 cm or > 2 cm) (evaluation criterion at 2 cm according to the indication criteria for endoscopic treatment), an endoscopic macroscopic tumor type (protrusion, flat, or depression), UL (positive or negative), depth [mucosal (m) or submucosal (sm)], histology, and lymphatic involvement (LI) (positive or negative).
Cases were categorized into five groups macroscopically: type I (protruded), type IIa (superficially elevated), type IIb (flat), type IIc (superficially depressed), and III (depressed). Protrusion type was subcategorized into type I, IIa, I plus IIa, I plus IIc, and IIa plus IIc. Meanwhile, flat or depressed types were subcategorized into type IIb, III, IIc, IIb plus IIc, IIa plus IIc, IIb plus IIc, IIc plus I, IIc plus IIa, IIc plus IIb, IIc plus III, and III plus IIc.
According to the Japanese Classification of Gastric Carcinoma[4], diagnosis was made in each institute by a certified pathologist. Resected specimens were fixed in formalin and sectioned serially by 3 to 5-mm thickness, and then subjected to histological examination.
Histologically, signet ring cell carcinoma (sig), poorly differentiated adenocarcinoma (por), and mucinous adenocarcinoma (muc) were defined as undifferentiated-types[3]. When two or more histological types were present in the tumor, the predominant histological type accounting for 50% or more was defined as the histological type of the tumor. When an undifferentiated-type was mixed with a differentiated-type, such histology was defined as mixed type and subjected to analysis. Histological examinations including the diagnosis of LI and VI were performed with routine hematoxylin and eosin (H and E) staining in each institution.
These paper-based patho-histological information obtained at surgery (location, size, macroscopic type, UL, depth, histology, existence of mixed-type, LI and VI) were collected at the University of Yamanashi, and were used for the analysis.
The protocol of this retrospective study was approved by the ethical committee of University of Yamanashi Hospital, which waived the requirement for written informed consent since the study was a retrospective data analysis with appropriate consideration given to patient risk, privacy, welfare, and rights. According to the Declaration of Helsinki[5], this study was carried out.
Univariate analysis was performed by the χ2 test or Fisher’s exact test. Multivariate analysis was done according to the multiple logistic analysis with the factors extracted as significant by univariate analysis. A P value less than 0.05 was considered significant. Population rates and their 95% confidence intervals were calculated with JMP® 10 Modeling and Multivariate Methods (SAS Institute Inc. Cary, NC, United States). Decision tree analysis was used for data-mining analysis and a decision tree was constructed based on the LNM-related factors. SPSS Decision Tree Version 18 (IBM, Tokyo, Japan) was used for statistical analysis. According to χ2 Automatic Interaction Detection algorithm, classification tree models predicting LNM were constructed[6,7]. The decision tree was verified by 10-fold cross validation.
In 567 patients with undiff-EGC, local LNM was observed in 7.8% (44/567). The details of the patients were as follows: average age, 59.9 years; males, 55.1% (n = 132); location: middle, 61.2% (n = 347); and the form: flat or depression, 93.1% (n = 528). UL was present in 23.4% (n = 132) and depth limited to mucosa was in 54.7% (n = 310). With regard to the histological type, “sig” and “por” accounted for 55.5% (n = 315) and 43.3% (n = 246), respectively, and the mixed type was observed in 17.8% (n = 101) (Table 1).
| Factor | Value |
| Age, yr, mean ± SD | 59.9 ± 12.3 |
| Gender | |
| Male | 312 (55.1) |
| Female | 255 (44.9) |
| Location | |
| Upper | 72 (12.7) |
| Middle | 347 (61.2) |
| Lower | 148 (26.1) |
| Size, mm, mean ± SD | 26.8 ± 17.6 |
| Form | |
| Protrusion | 39 (6.9) |
| Flat or depression | 528 (93.1) |
| Ulceration | 132 (23.4) |
| Depth | |
| Mucosal (m) | 310 (54.7) |
| Submucosal (sm) | 257 (45.3) |
| Histology | |
| Sig | 315 (55.5) |
| Por | 246 (43.4) |
| Muc | 6 (1.1) |
| Mixed type | 101 (17.8) |
| Lymphatic involvement | 135 (23.8) |
| Venous involvement | 47 (8.3) |
| Lymph node metastasis | 44 (7.8) |
Univariate analysis on LNM risk factors extracted size > 2 cm, protrusion, sm, and the mixed type in histology, LI-positive, and VI-positive (Table 2). Multivariate analyses were performed with the factors extracted by univariate analysis with UL, which included in the guidelines (Table 3), with preoperatively evaluable factors by endoscopic findings except LI and VI and postoperatively evaluable factors including LI and VI.
| Factor | Patients (LNM)n (%) | Lymph node metastasis | P value | |
| Positiven = 44 | Negativen = 523 | |||
| Age | 0.118 | |||
| ≤ 60 yr | 296 (6.1) | 18 | 278 | |
| > 60 yr | 271 (9.6) | 26 | 245 | |
| Gender | 0.573 | |||
| Male | 312 (8.3) | 26 | 286 | |
| Female | 255 (7.1) | 18 | 237 | |
| Location | 0.050 | |||
| Upper | 72 (8.3) | 6 | 66 | |
| Middle | 347 (5.8) | 20 | 327 | |
| Lower | 148 (12.2) | 18 | 130 | |
| Size | < 0.001 | |||
| ≤ 2 cm | 268 (2.2) | 6 | 262 | |
| > 2 cm | 299 (12.7) | 38 | 261 | |
| Form | 0.002 | |||
| Protrusion | 39 (20.5) | 8 | 31 | |
| Flat or depression | 528 (6.8) | 36 | 492 | |
| Ulceration | 0.306 | |||
| Absent | 435 (7.1) | 31 | 404 | |
| Present | 132 (9.9) | 13 | 119 | |
| Depth | < 0.001 | |||
| Mucosal (m) | 310 (2.9) | 9 | 301 | |
| Submucosal (sm) | 257 (13.6) | 35 | 222 | |
| Histology | 0.592 | |||
| Sig | 315 (6.7) | 21 | 294 | |
| Por | 246 (9.3) | 23 | 223 | |
| Muc | 6 (0) | 0 | 6 | |
| Mixed type | 0.003 | |||
| No | 466 (6.2) | 29 | 437 | |
| Yes | 101 (14.9) | 15 | 86 | |
| Lymphatic involvement | < 0.001 | |||
| Negative | 432 (1.4) | 6 | 426 | |
| Positive | 135 (28.1) | 38 | 97 | |
| Venous involvement | < 0.001 | |||
| Negative | 520 (6.5) | 34 | 486 | |
| Positive | 47 (21.3) | 10 | 37 | |
| Factor | Without lymphatic-vascular involvement | With lymphatic-vascular involvement | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Size of tumor (> 2 cm) | 5.00 (2.03-12.30) | < 0.001 | 3.62 (1.39-9.46) | 0.009 |
| Form (protrusion) | 0.58 (0.23-1.46) | 0.245 | 0.72 (0.25-2.06) | 0.534 |
| Depth (submucosal) | 4.37 (2.00-9.55) | < 0.001 | 0.46 (0.14-1.58) | 0.218 |
| Ulceration | 1.38 (0.66-2.84) | 0.394 | 1.40 (0.61-3.22) | 0.426 |
| Mixed type | 1.63 (0.80-3.35) | 0.180 | 2.01 (0.87-4.67) | 0.103 |
| Lymphatic involvement | - | - | 40.7 (11.37-145.69) | < 0.001 |
| Venous involvement | - | - | 0.84 (0.32-2.24) | 0.733 |
Analysis with preoperatively evaluable factors revealed that > 2 cm (OR = 5.00, 95%CI: 2.03-12.30) and sm (OR = 4.37, 95%CI: 2.00-9.55) were independent LNM-related factors. Meanwhile, analysis with postoperatively evaluable factors showed that > 2 cm (OR = 3.62, 95%CI: 1.39-9.46) and LI (OR = 40.70, 95%CI: 11.37-145.69) were independent factors and LI, in particular, showed high OR (Table 3).
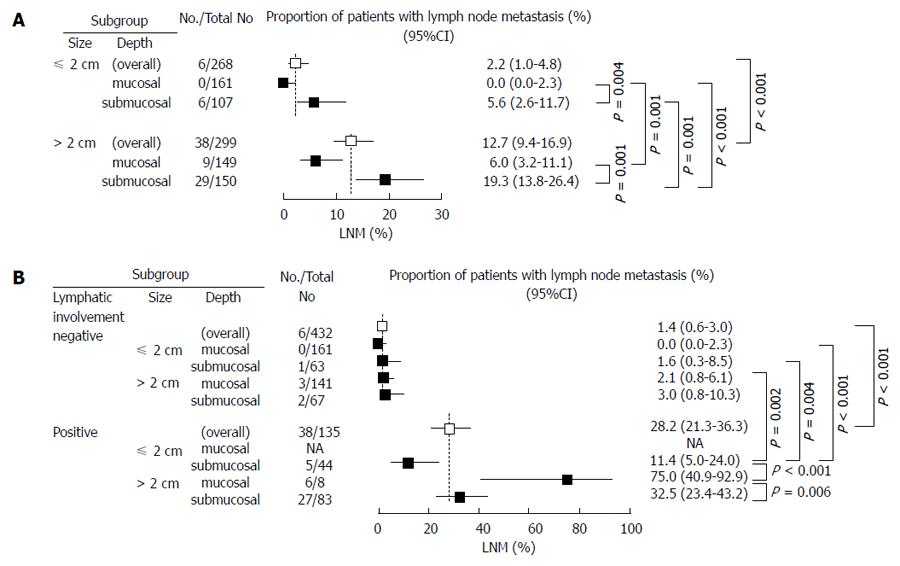
Cases were categorized into three LNM risk groups (high, moderate, or low) by a combination of size and depth, two preoperatively evaluable factors extracted by multivariate analysis (Figure 1A). Cases with ≤ 2 cm and m showed 0% (0/161) (95%CI: 0.0-2.3) as incidence of LNM and were categorized as the low-risk group. LNM was observed in 5.6% (6/107) (95%CI: 2.6-11.7) in cases with ≤ 2 cm and sm and in 6.0% (9/149) (95%CI: 3.2-11.1) in those with > 2 cm and m, and they were categorized as the moderate-risk group. LNM was observed in 19.3% (29/150) (95%CI: 13.8-26.4) in those with > 2 cm and sm, and they were categorized as the high-risk group. There was a significant difference in the incidence of LNM between any two groups. The incidence of LNM was significantly higher in cases > 2 cm than in those ≤ 2 cm.
The LNM rate was compared with three postoperatively evaluable factors of size, depth and LI (Figure 1B). The incidence of LNM was 1.4% (95%CI: 0.6-3.0) in the LI-negative cases and 28.2% (95%CI: 5.0-43.2) in the LI-positive cases, and it was significantly higher in the latter. Taken together, there was no case with LI(+), ≤ 2 cm, and m, and LI was negative in all cases with ≤ 2 cm and m, which had the incidence of LNM at 0% (0/161) (95%CI: 0-2.3).
Although there was no significant difference between subgroups in LI-negative cases, LI-positive cases had a higher incidence of LNM and there was a significant difference between the LI-negative and LI-positive cases. Cases with ≤ 2 cm and sm and those with > 2 cm and m in the moderate-risk group showed the incidence of LNM at 5.6% (95%CI: 2.6-11.7) and 6.0% (95%CI: 3.2-11.1), respectively. Meanwhile, they revealed the incidence of LNM to be as low as 1.6% (95%CI: 0.3-8.5) and 2.1% (95%CI: 0.8-6.1), respectively, when LI was negative, and they were categorized into the low-risk group. Similarly, cases with > 2 cm and sm with LNM in 19.3% (95%CI: 13.8-26.4) in the high-risk group revealed the incidence of LNM at as low as 3.0% (95%CI: 0.8-10.3) when LI was negative (Figure 1A and B).
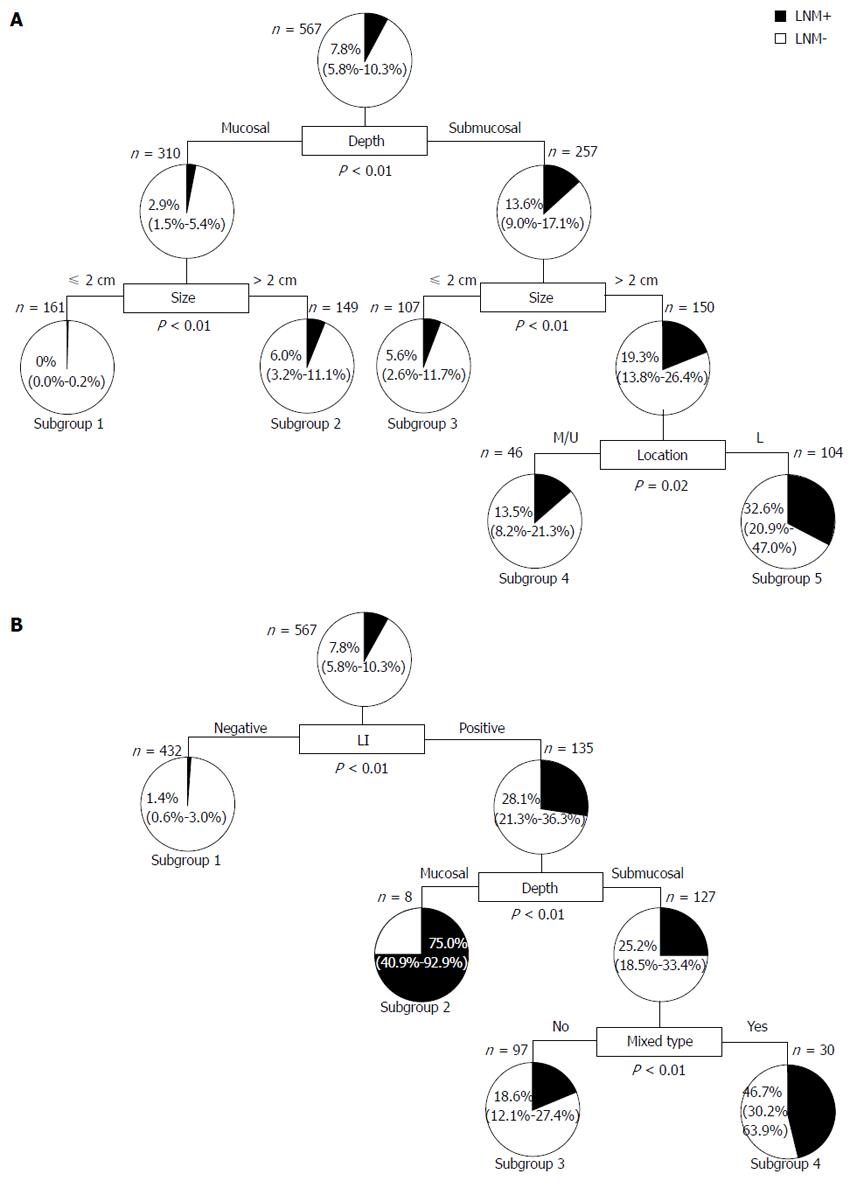
Since data mining is a method for discovering meaningful patterns or information through the analysis of large set of data, decision tree analysis, a method for data mining, was introduced to extract LNM-related risk factors that were not extracted with the conventional statistical analysis. With LNM as the outcome, a decision tree was constructed with nine preoperatively evaluable factors of age, gender, location, size, form, UL, depth, histology, and the mixed type in all 567 cases with undiff-EGC (Figure 2A). According to the decision tree, three factors of depth, size, and location were extracted and cases were categorized into five subgroups with great difference in the incidence of LNM, from 0% to 32.6%, by combinations of the three factors: (Subgroup 1) m and ≤ 2 cm, 0% (0/161); (Subgroup 2) m and > 2 cm, 6.0% (9/149); (Subgroup 3) sm and ≤ 2 cm, 5.6% (6/107); (Subgroup 4) sm, > 2 cm, and upper/middle, 13.5% (14/104); and (Subgroup 5) sm, > 2 cm, and lower, 32.6% (15/46). Interestingly, lower location was related with LNM in cases with sm and > 2 cm.
Next, a decision tree was constructed with 11 factors including postoperatively evaluable factor LI and VI (Figure 2B). Three factors of LI, depth, and the mixed type were extracted and cases were categorized into four subgroups. The incidence of LNM was 1.4% (6/432) in Subgroup 1 with LI(-), 75.0% (6/8) in Subgroup 2 with LI(+) and m, 18.6% (18/97) in Subgroup 3 with LI(+), sm, and the mixed type(-), and 46.7% (14/30) in Subgroup 4 with LI(+), sm, and the mixed type(+). Among the cases with LI(+), sm, and the mixed type(+), those with > 2 cm had LNM in 32.5% (27/83), which was significantly higher than 11.3% (5/44) in cases with ≤ 2 cm.
In this study, LNM risk factors in 567 cases that underwent gastrectomy for undiff-EGC were investigated and the following points were elucidated: (1) Three factors of LI, size, and depth were the most critical factors for LNM; (2) No LNM was observed in 161 cases that satisfied the condition of three factors for low LNM rates as described above [m, ≤ 2 cm, and LI(-)]; and (3) LNM risk other than the condition for low LNM rates, which had been poorly investigated, was stratified, and cases that failed to satisfy the condition of m and ≤ 2 cm had LNM in up to 3.0% (95%CI: 0.8-10.3) as long as LI was negative, which suggested that the risk was not so high.
Previous papers[1,2,8-13] on LNM-related factors in undiff-EGC in a scale of ≥ 500 cases were consistent in that size, depth, VI and LI were related with LNM, although the conditions were slightly different one another (Table 4). In our study, multivariate analysis revealed that size and depth were independent risk factors among preoperatively evaluable factors excluding postoperatively evaluable factors (LI and VI), and LI and size were independent risk factors including postoperatively evaluable factors.
| Ref. | Country | Condition for low LNM incidence | Pts in the condition | Pts with LNM | LNM (95%CI) | |||||
| Size (cm) | Depth | LI | VI | UL | Other | |||||
| Yamao et al[8] | Japan | < 3 | m | LI(-) | VI(-) | UL(-) | 277 | 1 | 0.36% (0.06-2.02) | |
| Gotoda et al[1] | Japan | ≤ 2 | m | LI(-) | VI(-) | UL(-) | 141 | 0 | 0% (0-2.6) | |
| Ye et al[9] | South Korea | < 2.5 | m | LI(-) | NS | NS | 131 | 0 | 0% (0-2.28) | |
| sm1 | ||||||||||
| Li et al[10] | China | ≤ 2 | m | LI(-) | VI(-) | NT | 201 | 1 | 0.5% (0.08-2.76) | |
| Ha et al[11] | South Korea | < 2 | m | LI(-) | NT | NT | Sig | 77 | 0 | 0% (0-4.75) |
| Hirasawa et al[2] | Japan | ≤ 2 | m | LI(-) | VI(-) | UL(-) | 310 | 0 | 0% (0-0.96) | |
| Kunisaki et al[12] | Japan | ≤ 2 | m | LI(-) | VI(-) | Por | 84 | 0 | 0% (0-4.37) | |
| 1Lee et al[13] | South Korea | ≤ 2 | m | LI(-) | NS | NS | Male | 124 | 4 | 3.2% (1.26-8.00) |
| This study | Japan | ≤ 2 | m | LI(-) | NS | NS | 161 | 0 | 0% (0-2.33) | |
UL, currently included in the expanded criteria for ESD in the treatment guidelines for gastric carcinoma, had no evident correlation with LNM by univariate and multivariate analysis. Akamatsu et al[14] reported that intramucosal laminated structure could not be maintained in cases with UL(+) and the risk for submucosal invasion was higher, which suggested the contribution of increased invasion risk of UL(+) to elevated LNM risk. Meanwhile, among the eight papers cited previously, only two reports by Kunisaki et al[12] and Kim et al[15] showed that UL(+) was a risk factor for LNM, making us to speculate that the accurate pathological diagnosis of UL might be difficult. However, UL(+) is not considered important as a risk factor for LNM under the current status of diagnosis. Likewise, though it is speculated that biological characters of undifferentiated gastric cancers are different dependent on histology, histology was not considered as a risk factor for LNM when restricted to undiff-EGC in this analysis. On the other hand, most previous reports evaluated VI as LVI altogether and it was unclear if VI was an independent risk factor. In the present study, VI and LI were strong confounders and univariate analysis revealed that VI was related with LNM, but multivariate analysis showed that VI was not an independent factor.
Previous papers failed to show how much combinations of these risk factors would increase the risk for LNM. In the present study, cases were categorized into the three risk groups according to the combinations of preoperatively evaluable factor size and depth, extracted by multivariate analysis: the low-risk group (LNM, 0%), ≤ 2 cm and m; the moderate-risk group, ≤ 2 cm and sm (LNM, 5.6%) and > 2 cm and m (LNM, 6.0%); and the high risk group, > 2 cm and sm (LNM, 19.3%). Any two groups had a significant difference in the incidence of LNM. The low-risk group had an absolute indication for ESD, while the high-risk group should be treated, in principle, by surgery. The moderate-risk group may have a relative indication for ESD under certain conditions.
On the other hand, when postoperatively evaluable factor LI was added to the combination of size and depth, cases were divided into two groups with a statistical significance in the incidence of LNM by the presence or absence of LI. The LNM rate was 0% in the low-risk group with ≤ 2 cm and m that showed LI(-) in all cases. Meanwhile, the LNM rate was 11.4% in cases with LI(+) and 1.6% in cases with LI(-) in the moderate-risk group with ≤ 2 cm and sm, while it was 75% in cases with LI(+) and 2.1% in cases with LI(-) in the moderate-risk group with > 2 cm and m. The LNM rate was 32.5% in cases with LI(+) and 3.0% in those with LI(-) in the high-risk group with > 2 cm and sm. Taken together, the risk for LNM was divided into two group according to the presence or absence of LI, which clarified the importance of LI.
Although LI was considered the most correlated factor with LNM among the three factors and the importance of LI for the risk of LNM has been shown in other reports, there is a large difference among institutes in the diagnostic rate of LI and the LNM rate in cases with LI(+)[2,8-13,15]. In the present study, LI was positive in 23.8% overall, and LNM was observed in 28.2% of the LI(+) cases, whereas LNM was present only in 1.4% in LI(-) cases. The outstanding correlation of LI with LNM in this study was considered attributable to accurate pathological diagnosis and it was presumed that experienced pathologists thoroughly examined LI. Theoretically, LI is a prerequisite for LNM, and establishment of highly sensitive diagnostic methods, similar to the diagnosis by experienced pathologists, will greatly reduce the necessity of surgery intended for lymphadenectomy. Diagnosis of LI is limited as long as hematoxylin-eosin staining is employed[16], and addition of immunostaining specific for lymphatic vessels, such as immunostaining with D2-40 antibody, increased diagnostic accuracy[17,18]. It is necessary to accurately evaluate LI in the obtained specimens, to develop a new modality, and to establish an evaluation method.
In the present study, clinicopathological factors related with LNM in undiff-EGC were investigated by a decision tree analysis for data mining. Data mining is a technique to extract useful information from massive data. Based on clinicopathological factors in each individual case with different conditions, it was successful to practically present the risk of LNM and categorize cases into subgroups by decision tree analysis using preoperatively (Figure 2A) and postoperatively evaluable factors (Figure 2B). Three factors of LI, size, and depth, among the five extracted factors, were almost similar with Gotoda’s criteria for the condition of low incidence of LNM [m, ≤ 2 cm, and LI(-)], which demonstrated the utility of data mining analysis. Stratification by data mining extracted the mixed type and location other than the independent factors of LI, size, and depth extracted by multivariate analysis (Figure 2A and 2B). Though it has attracted attention that the mixed type has a higher risk for LNM than the pure undifferentiated-type[19-22], our analysis among postoperatively evaluable factors disclosed that the mixed type increases the LNM risk in the limited condition of cases with LI(+) and sm. Among preoperatively evaluable factors, location was extracted and incidence of LNM tended to be higher in cases with > 2 cm and sm at the lower location than in those at the middle/upper location (32.6% vs 13.5%). With regard to the relationship between location and LNM, some reports have demonstrated a correlation but there has been no consensus. Analysis from this point of view will be necessary[13,15,23].
The information of the risk for LNM is important and useful for determining treatment such as ESD and surgery in cases with a high risk for surgery including the elderly. Although a five-year survival after curative resection by surgery was higher than 90% in all ages[24], it was 54%-55% after curative resection of EGC by surgery in the elderly 80 years or older, and other causes of death accounted for a large proportion[25]. On the other hand, a five-year survival in the elderly 80 years or older was 80% by curative resection by ESD and 66.7% by non-curative resection without additional surgery[26], and it was higher by ESD than by surgery. It was estimated that a five-year survival in the general population aged 80 years in Japan was 69%[27]. The five-year survival at 66.7%, obtained by local therapy such as ESD for EGC, even non-curative resection, was comparable to 69% in the general population aged 80 years. In our study, as long as LI was negative, the incidence of LNM was 10% at the highest, and it was likely that local therapy was curative in 90% cases or more. Even in cases that fail to satisfy the criteria in the guidelines for ESD, it may be appropriate to predict incidence of LNM, control lesions locally by ESD, and follow-up cases, when gastrectomy provides shorter life prognosis than average life expectancy in the elderly.
In this study, it is a limitation that the pathological information excluding LI and VI obtained from postoperative resected tissue is assumed to be preoperatively evaluable by endoscopy while preoperative diagnosis of the depth, in particular, is sometimes considered difficult. However, it was reported that the diagnostic accuracy of the depth was as high as 92% with the recently-advanced endoscopic ultrasound (EUS)[28], and we also disclosed that the accuracy to be around 90% with the EUS through the analysis of small number of cases (data not shown). Considering these advances in diagnosis, the present LNM prediction model might be applicable in clinical practice though further prospective study is warranted.
In conclusion, we could stratify the LNM risk in cases with undiff-EGC using preoperatively as well as postoperatively evaluable factors. The treatment modality should be determined after careful estimation of the LNM risk as well as the surgical risk dependent on each different individual.
Undifferentiated-type early gastric cancer (undiff-EGC) fulfilling the criteria of ≤ 2 cm, mucosal, lymphatic-vascular involvement-negative, and ulceration-negative is recently treated by endoscopic submucosal dissection (ESD) since lymph node metastasis (LNM) is rare. On the other hand, it is elusive how LNM risk is increased in undiff-EGCs falling outside the criteria.
Recent researches are focusing on establishing and confirming the condition for ESD procedure that guarantee safety from LNM in the treatment of undiff-EGCs. In particular, a large phase II study is ongoing in Japan to prove long-term safety and effectiveness of ESD in undiff-EGCs. On the other hand, previous studies did not show how LNM risk is increased with ESD in the combinations of risk factors.
LNM risk in undiff-EGC was stratified both in preoperative and postoperative conditions by introduction of data mining, in addition to conventional statistical analysis.
With the information of individual risk for LNM in undiff-EGC, patients with poor physiological conditions due to concurrent diseases or old age could choose therapies considered as most appropriate.
Data mining is a method for discovering meaningful patterns or information through the analysis of large set of data and it is applicable to clinical judgment. ESD is a technique for “en bloc” resection of superficial tumors of the gastrointestinal tract.
The authors demonstrated the risk of LNM in undiff-EGC by well-designed analyses having high statistical power.
P- Reviewer: Declich P, Espinel J, Lee JI, Manner H S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 2. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 5. | World Medical Association Inc. Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107:403-405. [PubMed] |
| 6. | Kurosaki M, Matsunaga K, Hirayama I, Tanaka T, Sato M, Yasui Y, Tamaki N, Hosokawa T, Ueda K, Tsuchiya K. A predictive model of response to peginterferon ribavirin in chronic hepatitis C using classification and regression tree analysis. Hepatol Res. 2010;40:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Lo-Ciganic W, Zgibor JC, Ruppert K, Arena VC, Stone RA. Identifying type 1 and type 2 diabetic cases using administrative data: a tree-structured model. J Diabetes Sci Technol. 2011;5:486-493. [PubMed] |
| 8. | Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, Sasako M, Sano T, Ochiai A, Yoshida S. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602-606. [PubMed] |
| 9. | Ye BD, Kim SG, Lee JY, Kim JS, Yang HK, Kim WH, Jung HC, Lee KU, Song IS. Predictive factors for lymph node metastasis and endoscopic treatment strategies for undifferentiated early gastric cancer. J Gastroenterol Hepatol. 2008;23:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Zhu ZG, Noh SH. Risk factors for lymph node metastasis in undifferentiated early gastric cancer. Ann Surg Oncol. 2008;15:764-769. [PubMed] |
| 11. | Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508-513. [PubMed] |
| 12. | Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Choi MG, Min BH, Noh JH, Sohn TS, Bae JM, Kim S. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg. 2012;99:1688-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Akamatsu T, Katsuyama T. Histochemical demonstration of mucins in the intramucosal laminated structure of human gastric signet ring cell carcinoma and its relation to submucosal invasion. Histochem J. 1990;22:416-425. [PubMed] |
| 15. | Kim KJ, Park SJ, Moon W, Park MI. Analysis of factors related to lymph node metastasis in undifferentiated early gastric cancer. Turk J Gastroenterol. 2011;22:139-144. [PubMed] |
| 16. | Hirasawa T, Fujisaki J, Fukunaga T, Yamamoto Y, Yamaguchi T, Katori M, Yamamoto N. Lymph node metastasis from undifferentiated-type mucosal gastric cancer satisfying the expanded criteria for endoscopic resection based on routine histological examination. Gastric Cancer. 2010;13:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Jeon SR, Cho JY, Bok GH, Lee TH, Kim HG, Cho WY, Jin SY, Kim YS. Does immunohistochemical staining have a clinical impact in early gastric cancer conducted endoscopic submucosal dissection? World J Gastroenterol. 2012;18:4578-4584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Nakata K, Nagai E, Miyasaka Y, Ohuchida K, Ohtsuka T, Toma H, Hirahashi M, Aishima S, Oda Y, Tanaka M. The risk of lymph node metastasis in mucosal gastric carcinoma: especially for a mixture of differentiated and undifferentiated adenocarcinoma. Hepatogastroenterology. 2012;59:1855-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Shimizu H, Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M. The decision criterion of histological mixed type in “T1/T2” gastric carcinoma--comparison between TNM classification and Japanese Classification of Gastric Cancer. J Surg Oncol. 2012;105:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Li H, Lu P, Lu Y, Liu CG, Xu HM, Wang SB, Chen JQ. Predictive factors for lymph node metastasis in poorly differentiated early gastric cancer and their impact on the surgical strategy. World J Gastroenterol. 2008;14:4222-4226. [PubMed] |
| 24. | Saragoni L, Morgagni P, Gardini A, Marfisi C, Vittimberga G, Garcea D, Scarpi E. Early gastric cancer: diagnosis, staging, and clinical impact. Evaluation of 530 patients. New elements for an updated definition and classification. Gastric Cancer. 2013;16:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Bando E, Kojima N, Kawamura T, Takahashi S, Fukushima N, Yonemura Y. Prognostic value of age and sex in early gastric cancer. Br J Surg. 2004;91:1197-1201. [PubMed] |
| 26. | Abe N, Gotoda T, Hirasawa T, Hoteya S, Ishido K, Ida Y, Imaeda H, Ishii E, Kokawa A, Kusano C. Multicenter study of the long-term outcomes of endoscopic submucosal dissection for early gastric cancer in patients 80 years of age or older. Gastric Cancer. 2012;15:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Statistics and Information Department, Minister’s Secretariat, Ministry of Health and Welfare of Japan. Abridged Life Tables for Japan 2012. Available from: http://www.mhlw.go.jp/english/database/db-hw/lifetb12/index.html. |
| 28. | Mandai K, Yasuda K. Accuracy of endoscopic ultrasonography for determining the treatment method for early gastric cancer. Gastroenterol Res Pract. 2012;2012:245390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |