Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2668
Peer-review started: August 20, 2014
First decision: September 27, 2014
Revised: October 16, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: March 7, 2015
Processing time: 202 Days and 8.2 Hours
AIM: To measure the prognostic significance of absolute monocyte count/absolute lymphocyte count prognostic score (AMLPS) in patients with gastric cancer.
METHODS: We retrospectively examined the combination of absolute monocyte count (AMC) and absolute lymphocyte count (ALC) as prognostic variables in a cohort of 299 gastric cancer patients who underwent surgical resection between 2006 and 2013 and were followed at a single institution. Both AMC and ALC were dichotomized into two groups using cut-off points determined by receiving operator characteristic curve analysis. An AMLPS was generated, which stratified patients into three risk groups: low risk (both low AMC and high ALC), intermediate risk (either high AMC or low ALC), and high risk (both high AMC and low ALC). The primary objective of the study was to validate the impact of AMLPS on both disease-free survival (DFS) and overall survival (OS), and the second objective was to assess the AMLPS as an independent prognostic factor for survival in comparison with known prognostic factors.
RESULTS: Using data from the entire cohort, the most discriminative cut-off values of AMC and ALC selected on the receiver operating characteristic curve were 672.4/μL and 1734/μL for DFS and OS. AMLPS risk groups included 158 (52.8%) patients in the low-risk, 128 (42.8%) in the intermediate-risk, and 13 (4.3%) in the high-risk group. With a median follow-up of 37.2 mo (range: 1.7-91.4 mo), five-year DFS rates in the low-, intermediate-, and high-risk groups were 83.4%, 78.7%, and 19.8%, respectively. And five-year OS rates in the low-, intermediate-, and high-risk groups were 89.3%, 81.1%, and 14.4%, respectively. On multivariate analysis performed with patient- and tumor-related factors, we identified AMLPS, age, and pathologic tumor-node-metastasis stage as the most valuable prognostic factors impacting DFS and OS.
CONCLUSION: AMLPS identified patients with a poor DFS and OS, and it was independent of age, pathologic stage, and various inflammatory markers.
Core tip: Our findings suggest that the absolute monocyte count (AMC) and absolute lymphocyte count (ALC) prognostic score combined by AMC and ALC can predicts survival and identify gastric cancer patients with a poor overall survival, and this prognostic score is independent of age, pathologic stage, and various inflammatory markers.
- Citation: Eo WK, Jeong DW, Chang HJ, Won KY, Choi SI, Kim SH, Chun SW, Oh YL, Lee TH, Kim YO, Kim KH, Ji YI, Kim A, Kim HY. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol 2015; 21(9): 2668-2676
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2668.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2668
Gastric cancer is a major public health problem, because it represents one of the major causes of cancer mortality worldwide. Despite a result of advances in surgical treatment, the role of surgery as mainstay treatment is limited to around a quarter of all patients[1], and overall survival (OS) of patients who undergo surgery progressively diminishes as stage increases, ranging from 75% for stage I to 35% or less for stage II and beyond[2]; accurately predicting patients’ prognoses is needed to improve patient survival and to provide important information to the patients.
After curative resection for gastric cancer, pathologic analysis of tumor-related factors guides prognosis and treatment. A variety of high-risk features, including tumor stage, resection margin, and nodal status, are considered to be important in determining cancer recurrence and survival[3]. In addition, a few serum tumor markers have been found to be associated with poor prognosis and are therefore useful for monitoring and predicting early recurrence and poor prognosis[4]. The outcomes of patients with cancer are determined not only by tumor-related factors but also by host-related factors, particularly the systemic inflammatory response[5,6]. Laboratory markers of systemic inflammation have been investigated as both prognostic and predictive biomarkers in several cancer populations. With respect to gastric cancer, the modified Glasgow Prognostic Score[7], mean platelet volume (MPV)[8], absolute neutrophil count (ANC)[9], absolute monocyte counts (AMC)[9], absolute lymphocyte count (ALC)[9], neutrophil-lymphocyte ratio (NLR)[10-13], and platelet-lymphocyte ratio (PLR)[12] have been reported as independent prognostic factors in gastric cancer. Assessment of the inflammatory response to the tumor may be easier and more-cost effective in clinical practice, and the addition of inflammatory factors to tumor-associated factors would be expected to help in disease management.
Recently, the role of monocytes in combination with peripheral lymphocytes has been assessed as a biomarker in lymphomas. In some of those reports, each AMC and ALC was divided into two to create a prognostic index, and it was shown to be an independent prognostic factor for survival in diffuse large B-cell lymphoma (DLBCL)[14-18]. However, to our knowledge, there is limited data available on whether such a prognostic index at diagnosis has prognostic value in other malignancies, including gastric cancer. Therefore, the aim of the present study is to measure the prognostic significance of a preoperative combination of AMC and ALC by using this prognostic index in a cohort of patients with resectable primary gastric cancer.
We retrospectively evaluated 299 patients undergoing potentially curative resection of gastric cancer in a single institution between June 2006 and April 2013. No patient refused authorization to use his or her medical records for research. No patients were lost to follow-up. Approval for the retrospective review of these records was obtained from the Kyung Hee University Hospital at Gangdong Institutional Review Board (IRB file number 2014-02-027), and it was performed in accordance with Korean regulations and the Declaration of Helsinki.
Information regarding patient demographics was collected for analysis. Laboratory measurements, including complete blood counts (CBCs) and biochemical profiles, were performed within seven days before surgery as part of the routine workup. Venous peripheral blood samples for measurement of CBCs were drawn just before the operation to avoid any inflammatory effects of preoperative sequential evaluation, such as gastroscopy, colonoscopy, or esophagogastrography. If several preoperative CBCs were obtainable, the one which was examined on the nearest date before the operation was taken. Two millimeters of venous blood were collected into tubes containing dipotassium ethylenedinitrotetra-acetic acid (EDTA), and all measurements were performed within 30 min after blood collection on a standard Coulter counter model LH 750 (Beckman Coulter Inc., CA, United States).
The tumors were staged according to the tumor-node-metastasis (TNM) criteria from the 7th edition of the International Union Against Cancer’s classification of malignant tumors[19]. Patients were treated with curative surgical resection with D2 lymphadenectomy. All resections were conducted by a specialized gastric cancer surgeon who routinely operates on more than 50 new cases per year and who has two or more consecutive years of surgical practice. Those who had concurrent second malignancies or prior malignancies within the previous five years (other than in situ or non-melanoma skin cancers) were excluded. Those who had received neoadjuvant chemotherapy or who received a blood product transfusion within one month before resection were also excluded to avoid possible effects of such treatments on preoperative laboratory profiles. Patients were excluded if they were human immunodeficiency virus-positive, had evidence of infection, or had concomitant autoimmune disease treated with immunosuppressive therapies affecting their ALC and AMC values.
AMC and ALC were obtained from a standard complete blood count and a differential count was performed manually. Each AMC and ALC was divided into two groups (high and low) by using cut-off points determined by receiver operating characteristic (ROC) curve analysis for survival, and the prognostic index, AMC/ALC prognostic score (AMLPS), was generated. The AMLPS stratified patients into three risk groups: low risk (both low AMC and high ALC), intermediate risk (either high AMC or low ALC), and high risk (both high AMC and low ALC).
The primary objective of the study was to validate the impact of AMLPS on both disease-free survival (DFS) and OS. The second objective was to assess the AMLPS as an independent prognostic factor for survival in comparison with known prognostic factors. The following prognostic factors were evaluated in this study: age, gender, tumor size, TNM stage, lymphatic or vascular invasion, serum albumin concentration, AMC, ALC, hemoglobin concentration, platelet count, MPV, NLR, PLR, and AMLPS.
DFS was defined as the time (in months) from the date of surgery to the date of relapse, death from any cause, or last follow-up. OS was defined as the time (in months) from the date of surgery to the date of death from any cause or last follow-up. Patients without relapse or death were censored at time of the last known follow-up.
P values for the comparison of the mean difference for continuous variables were obtained by using analysis of variance (ANOVA) with post-hoc pairwise comparisons (Scheffe’s test); P values from an independent test for categorical variables were obtained by using a χ2 test. OS was analyzed using the Kaplan-Meier method. Differences between survival curves were tested for statistical significance using a two-tailed log-rank test. The Cox proportional hazards model was used for univariate analysis. Variables with a P value < 0.05 in the univariate analysis were included in the multivariate analysis. The multivariate Cox proportional hazards model was used to identify the most valuable prognostic factors affecting survival. All P values presented are two-sided and statistical significance was declared at P < 0.05. Data were analyzed using SPSS statistical software (version 18.0, SPSS Inc., Chicago, Ill., United States).
Baseline characteristics of the patients are displayed in Tables 1 and 2. The median age at diagnosis was 59 years (range: 25-92 years). Male patients comprised 65.2% of the subjects in this study. Tubular adenocarcinoma was diagnosed in 62.5% of the patients. The median longitudinal tumor diameter was 3.0 cm (range: 0.2-20.0 cm). The most frequent location of the tumor was in the lower third of the stomach (58.9%). Stage I, II, and III disease comprised 59.9%, 20.1%, and 20.1% of the cases, respectively. The median AMC and ALC at diagnosis were 458.2/μL (range: 31.0-1618.5/μL) and 1879.2/μL (range: 341.0-5271.7/μL), respectively. The median hemoglobin concentration was 13.1 g/dL (range: 5.7-17.9 g/dL). The median platelet count and MPV were 235000/μL (range: 54000-577000/μL) and 7.8 fL (range: 5.9-10.9 fL), respectively. The median NLR and PLR were 2.0 (range: 0.4-25.6) and 122.5 (range: 34.3-1190.6), respectively. The median serum albumin concentration was 4.1 g/dL (range: 2.4-5.1 g/dL).
| Variable | |
| Median age (range, yr) | 59 (25-92) |
| Male: female | 195:104 (65.2:34.8) |
| Lauren’s classification | |
| Intestinal | 148 (49.5) |
| Diffuse | 79 (26.4) |
| Mixed | 58 (19.4) |
| Unknown | 14 (4.7) |
| Median tumor size (range), cm | 3.0 (0.2-20.0) |
| Location | |
| Upper1/3 | 28 (9.4) |
| Mid1/3 | 90 (30.1) |
| Lower1/3 | 176 (58.9) |
| Diffuse | 5 (1.7) |
| Lymphatic invasion | |
| Negative | 203 (67.9) |
| Positive | 96 (32.1) |
| Vascular invasion | |
| Negative | 287 (96.0) |
| Positive | 12 (4.0) |
| T category | |
| T1 | 168 (56.2) |
| T2 | 29 (9.7) |
| T3 | 73 (24.4) |
| T4 | 29 (9.7) |
| N category | |
| N0 | 199 (66.6) |
| N1 | 39 (13.0) |
| N2 | 25 (8.4) |
| N3 | 36 (12.0) |
| TNM stage | |
| I | 179 (59.9) |
| II | 60 (20.1) |
| III | 60 (20.1) |
| Variable | Mean ± SD | Median (range) |
| WBC (/μL) | 6857.5 ± 2358.8 | 6500.0 (1900.0-19500.0) |
| ANC (/μL) | 4180.8 ± 2054.4 | 3718.4 (1064.0-17100.0) |
| AMC (/μL) | 480.6 ± 180.1 | 458.2 (31.0-1618.5) |
| ALC (/μL) | 1980.8 ± 633.9 | 1879.2 (341.0-5271.7) |
| Hemoglobin (g/dL) | 12.8 ± 2.2 | 13.1 (5.7-17.9) |
| MCV (fL) | 91.6 ± 7.5 | 92.8 (56.7-109.4) |
| Platelet (× 103/μL) | 245.4 ± 71.8 | 235 (54.0-577.0) |
| MPV (fL) | 7.9 ± 0.8 | 7.8 (5.9-10.9) |
| NLR | 2.4 ± 1.9 | 2.0 (0.4-25.6) |
| PLR | 138.0 ± 85.8 | 122.5 (34.3-1190.6) |
| Albumin (g/dL) | 4.1 ± 0.4 | 4.1 (2.4-5.1) |
Using data from the entire cohort, we selected cut-off points for the AMC and ALC to predict the survival outcomes from the ROC curve analysis. The most discriminative cut-off values of AMC and ALC on the ROC curve were 672.4/μL (sensitivity 23.08, specificity 89.88, AUC 0.560, P = 0.1791) and 1734/μL (sensitivity 59.62, specificity 65.18, AUC 0.602, P = 0.0253), respectively, for DFS. In terms of OS, the most discriminative cut-off values of AMC and ALC on the ROC curve were 672.4/μL (sensitivity 26.19, specificity 89.88, AUC 0.577, P = 0.1189) and 1734/μL (sensitivity 64.29, specificity 64.98, AUC 0.625, P = 0.0129), respectively.
Either AMC or ALC as a single parameter appeared to have a limited ability to identify patients in the poor-risk category. Therefore, we combined the baseline AMC with the baseline ALC as dichotomized variables to obtain a host immunity-related prognostic index of survival, AMLPS, in patients with gastric cancer. We stratified patients into three risk groups depending on the AMLPS: low-risk (both AMC ≤ 672.4/μL and ALC > 1734/μL), intermediate-risk (either AMC > 672.4/μL or ALC ≤ 1734/μL) and high-risk (both AMC > 672.4/μL and ALC ≤ 1734/μL). AMLPS risk groups included 158 (52.8%) patients in the low-, 128 (42.8%) in the intermediate-, and 13 (4.3%) in the high-risk groups.
In order to evaluate the relevance of the AMLPS, we compared the different categories of AMLPS with the baseline characteristics. The variables used for the ANOVA analysis showed normal distribution. Significant mean differences between the low- and high-risk groups were obtained for the following continuous variables: tumor size, serum albumin concentration, AMC, ALC, hemoglobin concentration, NLR, and PLR. Whereas, significant mean differences for categorical variables were obtained for pathologic T category, pathologic TNM stage, and vascular invasion (Table 3).
| Variable | Low risk (n = 158)4 | Intermediate risk (n = 128)4 | High risk (n = 13)4 | P value | |||
| n (%) | Mean ± SD | n (%) | Mean ± SD | n (%) | Mean ± SD | ||
| Age (yr) | 56.4 ± 10.71 | 61.2 ± 12.22 | 64.0 ± 10.11,2 | 0.0006 | |||
| Gender | 0.2602 | ||||||
| Male | 99 (62.7) | 85 (66.4) | 11 (84.6) | ||||
| Female | 59 (37.3) | 43 (33.6) | 2 (15.4) | ||||
| Tumor size (cm) | 3.7 ± 2.91 | 4.3 ± 3.21 | 7.1 ± 5.12 | 0.0005 | |||
| T category | 0.0008 | ||||||
| T1-2 | 117 (74.1) | 76 (59.4) | 4 (30.8) | ||||
| T3-4 | 41 (25.9) | 52 (40.6) | 9 (69.2) | ||||
| N category | 0.0565 | ||||||
| N0 | 111 (70.3) | 83 (64.8) | 5 (38.5) | ||||
| N1-3 | 47 (29.7) | 45 (35.2) | 8 (61.5) | ||||
| TNM stage | 0.0200 | ||||||
| I-II | 133 (84.2) | 99 (77.3) | 7 (53.8) | ||||
| III | 25 (15.8) | 29 (22.7) | 6 (46.2) | ||||
| Lymphatic invasion | 0.0102 | ||||||
| Negative | 117 (74.1) | 81 (63.3) | 5 (38.5) | ||||
| Positive | 41 (25.9) | 47 (36.7) | 8 (61.5) | ||||
| Vascular invasion | 0.0105 | ||||||
| Negative | 156 (98.7) | 120 (93.8) | 11 (84.6) | ||||
| Positive | 2 (1.3) | 8 (6.3) | 2 (15.4) | ||||
| Albumin (g/dL) | 4.2 ± 0.31 | 4.0 ± 0.42 | 3.7 ± 0.53 | < 0.0001 | |||
| AMC (/μL) | 459.1 ± 110.01 | 478.3 ± 226.91 | 765.5 ± 93.92 | < 0.0001 | |||
| ALC (/μL) | 2283.8 ± 423.11 | 1661.2 ± 685.92 | 1444.9 ± 219.52 | < 0.0001 | |||
| Hemoglobin (g/dL) | 13.4 ± 1.91 | 12.4 ± 2.32 | 10.2 ± 2.53 | < 0.0001 | |||
| Platelet (× 103/μL) | 248.8 ± 66.61 | 238.7 ± 78.01 | 270.1 ± 67.01 | 0.2219 | |||
| MPV (fL) | 8.0 ± 0.91 | 7.9 ± 0.81 | 7.6 ± 0.91 | 0.2373 | |||
| NLR | 1.7 ± 0.71 | 2.9 ± 2.62 | 4.2 ± 2.62 | < 0.0001 | |||
| PLR | 111.8 ± 35.31 | 165.0 ± 116.32 | 191.6 ± 58.32 | < 0.0001 | |||
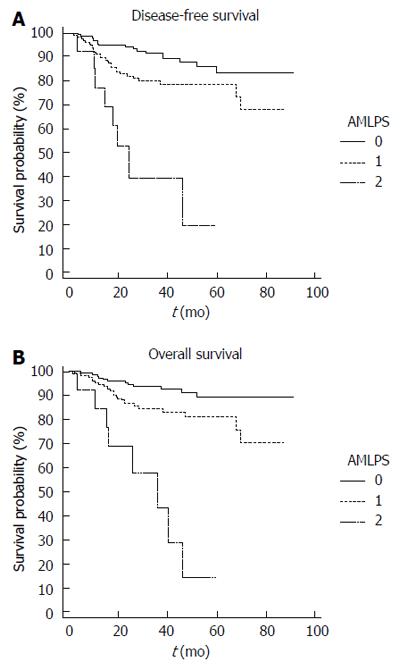
With a median follow-up of 37.2 mo (range: 1.7-91.4 mo), AMLPS had significant effects on survival rates: five-year DFS rates in the low-, intermediate-, and high-risk groups were 83.4%, 78.7%, and 19.8%, respectively, and five-year OS rates in the low-, intermediate-, and high-risk groups were 89.3%, 81.1%, and 14.4%, respectively (Figure 1).
Univariate analysis for DFS identified a significant difference for both continuous and categorical variables, including age, tumor size, T-category, N-category, TNM stage, lymphatic invasion, vascular invasion, serum albumin concentration, AMC, ALC, hemoglobin concentration, platelet count, MPV, NLR, PLR, and AMLPS (high- vs intermediate-risk groups, and high- vs low-risk groups) (Table 4). Using the multivariate Cox proportional hazards model, the only predictors for DFS were age (HR = 3.33; 95%CI: 1.50-7.40; P = 0.0032), TNM staging system (HR = 4.69; 95%CI: 2.15-10.24; P = 0.0001), AMLPS (high- vs intermediate-risk groups; HR = 0.23; 95%CI: 0.10-0.56; P = 0.0011), and AMLPS (high- vs low-risk groups; HR = 0.40; 95%CI: 0.18-0.90; P = 0.0274; Table 4).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 3.32 (1.50-7.35) | 0.0032 | 3.33 (1.50-7.40) | 0.0032 |
| Tumor size (cm) | 4.92 (2.79-8.69) | < 0.0001 | ||
| T category (T1-2 vs T3-4) | 7.26 (3.82-13.81) | < 0.0001 | ||
| N category (N0 vs N1-3) | 5.16 (2.87-9.27) | < 0.0001 | ||
| TNM Stage (I-II vs III) | 8.93 (5.08-15.69) | < 0.0001 | 4.69 (2.15-10.24) | 0.0001 |
| Lymphatic invasion | 3.63 (2.08-6.32) | < 0.0001 | ||
| Vascular invasion | 2.96 (1.17-7.47) | 0.0214 | ||
| Albumin (g/dL) | 0.29 (0.16-0.51) | < 0.0001 | ||
| AMC (/μL) | 2.06 (1.08-3.92) | 0.0285 | ||
| ALC (/μL) | 0.37 (0.21-0.64) | 0.0004 | ||
| Hemoglobin (g/dL) | 0.32 (0.19-0.56) | < 0.0001 | ||
| Platelet (× 103/μL) | 2.45 (1.41-4.26) | 0.0015 | ||
| MPV (fL) | 0.38 (0.18-0.77) | 0.0080 | ||
| NLR | 2.18 (1.22-3.88) | 0.0085 | ||
| PLR | 2.94 (1.68-5.13) | 0.0002 | ||
| AMLPS1 | ||||
| High- vs intermediate-risk2 | 0.11 (0.05-0.26) | < 0.0001 | 0.23 (0.10-0.56) | 0.0011 |
| High- vs low-risk3 | 0.24 (0.11-0.52) | 0.0004 | 0.40 (0.18-0.90) | 0.0274 |
Using univariate analysis for OS, significant differences for both continuous and categorical variables were obtained in the same variables as in DFS (Table 5). In the multivariate analysis, the only predictors for OS were age (HR = 2.34; 95%CI: 1.25-4.37; P = 0.0083), TNM staging system (HR = 5.53; 95%CI: 2.96-10.34; P < 0.0001), AMLPS (high- vs intermediate-risk groups; HR = 0.17; 95%CI: 0.06-0.45; P = 0.0004), and AMLPS (high- vs low-risk groups; HR = 0.30; 95%CI: 0.13-0.71; P = 0.0057; Table 5).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 2.68 (1.46-4.92) | 0.0016 | 2.34 (1.25-4.37) | 0.0083 |
| Tumor size (cm) | 4.47 (2.38-8.37) | < 0.0001 | ||
| T category (T1-2 vs T3-4) | 5.12 (2.63-9.98) | < 0.0001 | ||
| N category (N0 vs N1-3) | 3.57 (1.92-6.64) | 0.0001 | ||
| TNM Stage (I-II vs III) | 6.28 (3.41-11.55) | < 0.0001 | 5.53 (2.96-10.34) | < 0.0001 |
| Lymphatic invasion | 2.88 (1.56-5.28) | 0.0007 | ||
| Vascular invasion | 2.71 (0.97-7.63) | 0.0583 | ||
| Albumin (g/dL) | 0.27 (0.14-0.52) | 0.0001 | ||
| AMC (/μL) | 2.41 (1.21-4.79) | 0.0125 | ||
| ALC (/μL) | 0.31 (0.16-0.58) | 0.0003 | . | . |
| Hemoglobin (g/dL) | 0.40 (0.22-0.73) | 0.0030 | ||
| Platelet (× 103/μL) | 2.11 (1.14-3.93) | 0.0188 | ||
| MPV (fL) | 0.52 (0.28-0.97) | 0.0415 | ||
| NLR | 2.21 (1.17-4.19) | 0.0155 | ||
| PLR | 3.18 (1.70-5.97) | 0.0003 | ||
| AMLPS1 | ||||
| High- vs intermediate-risk2 | 0.08 (0.03-0.21) | < 0.0001 | 0.17 (0.06-0.45) | 0.0004 |
| High- vs low-risk3 | 0.20 (0.09-0.44) | < 0.0001 | 0.30 (0.13-0.71) | 0.0057 |
Approximately 30 years ago, Bruckner et al[9] reported that pretreatment ANC, AMC, and ALC are independent indicators of prognosis for patients with metastatic gastric cancer. In their report, the combination of ANC and ALC also predicted a noticeable difference in OS[9]. Since that report, the use of ANC and ALC as prognostic factors in gastric cancer has been validated, usually by combining the two values to determine the NLR[10-12]. In addition, the role of ALC in combination with platelet count has been reported[20,21]. On the other hand, the prognostic value of AMC in gastric cancer has not been validated to the best of our knowledge.
Recently, the AMC and ALC were combined to generate a score that was shown to be prognostic for survival in DLBCL[14-17]. Contrary to conventional prognostic indices, this scoring system does not incorporate patient and tumor characteristics, which contributes to the simplicity of this score. Instead, it is formed by laboratory values related to a patient’s adaptive immune response. In previous reports, the nomenclature of the scoring system differed between studies: an absolute monocyte and lymphocyte prognostic index[14,18], an absolute monocyte/lymphocyte count prognostic score[15], an absolute monocyte and lymphocyte prognostic score[16], and an immunological index[17]. All of the scoring systems stratified patients into three risk groups: low risk (both low AMC and high ALC), intermediate risk (either high AMC or low ALC), and high risk (both high AMC and low ALC). In most of the studies, both AMC and ALC were dichotomized into high and low groups by using predefined cut-off points[14,15,17] developed by Wilcox et al[16] to enable comparison between studies irrespective of optimal cut-off points.
In the study by Wilcox et al[16], the cut-off point for AMC (630/μL) was determined by ROC curve analysis, whereas the cut-off point for ALC (1000/μL) was determined by the fact that it has been utilized in many previous studies on lymphoma[22-25]. In some of those studies, the choice of 1000/μL as the cut-off point for ALC was supported by the fact that it yielded the greatest differential in survival[23,25]. On the other hand, in a study by Huang et al[18], the cut-off points of both AMC and ALC were determined by ROC curve analysis.
Based on previous reports, we initially evaluated the predefined cut-off point (630/μL for AMC and 1000/μL for ALC) according to the report by Wilcox et al[16] in our cohort, but using this value, no patient belonged to the high-risk group. When comparing low- and intermediate-risk groups with the log-rank test, no statistical significance was obtained for factors predicting DFS (P = 0.3295) or OS (P = 0.1315). In addition, when dichotomizing AMC and ALC by using the predefined value for gastric cancer described by Bruckner et al[9] (300 to 900/μL vs others for AMC, and < 1500/μL vs≥ 1500/μL for ALC) and combining them to produce three risk groups of AMLPS, the log-rank test revealed no statistical significance in predicting DFS (P = 0.1725) or OS (P = 0.0640). In our study, both AMC and ALC were dichotomized into two groups by using cut-off points that were determined by ROC curve analysis for survival (672.4/μL for AMC and 1734/μL for ALC), and an AMLPS with three risk groups were generated, revealing statistical significance for predicting DFS and OS.
In our study, we also intended to analyze the role of host-related factors together, including the serum albumin concentration, platelet count, MPV, NLR, and PLR, to avoid confounding effects. In gastric cancer, serum albumin is reportedly a significant factor for survival[13]. The platelet count is a convenient parameter within the blood cell count that can help to predict patients’ survival. MPV is a laboratory marker associated with platelet function, and a role for MPV as possible biomarker in the early diagnosis and monitoring of gastric cancer has been suggested[8]. The NLR also reflects inflammatory status. An elevated NLR has been reported to be a convenient biomarker to identify patients with a poor prognosis in primary gastric cancer[10-12]. The PLR has been introduced as a prognostic scoring system in various cancers, including gastrointestinal cancer[20,21]. In a recent report, PLR was an independent prognostic factor for OS rates in patients with advanced gastric cancer treated with chemotherapy[12]. In our study, the relationship between DFS and the serum albumin concentration, platelet count, MPV, NLR, or PLR showed statistical significance in univariate analysis, but none reached statistical significance in multivariate analysis. In addition, the relationship between OS and the various inflammatory markers mentioned above also showed statistical significance in univariate analysis, but they also did not reach statistical significance in multivariate analysis. Therefore, the AMLPS was independent of previously evaluated inflammatory markers.
Though the detailed mechanism still remains unclear, monocytes are known to promote tumorigenesis and angiogenesis[26] and suppress the host immune response to cancer, which may explain why elevated monocyte counts in solid tumors confer a negative prognosis[27]. Monocytes in the circulation are an important source of soluble mediators, which may help support the evolution of malignant cells[16,28]. Lymphocytes are markers of host immune competence[29], and they also act as mediators of antibody-dependent cell-mediated cytotoxicity[30]; as a result, lymphopenia may be is an adverse prognostic feature for gastric cancer. In our study, higher percentages of cases for T3-4, N1-3, and stage III were observed from low-risk to high-risk. In similar fashion a decreased percentages for T1-2, N0, and stage I-II were observed from low-risk to high-risk, supporting the balance between immune surveillance (ALC) versus tumor growth (AMC).
The results of this study should be interpreted cautiously, as they have several limitations. It was a retrospective study with a relatively small sample size and a relatively short median follow-up period. As we mentioned before, different investigators have used different cut-off values for the evaluation of ALC and AMC, and their unification for clinical application may require further exploration[31]. In addition, the AMC and ALC levels may vary in the same patient from day-to-day, not static[31]. To overcome this limitation, we evaluated AMLPS together with well-known predictors like serum albumin concentration, platelet count, MPV, NLR, and PLR[32]. In addition, data of CBCs just before the operation was collected to avoid any inflammatory effects of preoperative serial evaluation following diagnosis. Finally, we could not reach a conclusion whether three risk groups can be changed if venous blood is taken for AMC and ALC several times before operation. The reason is that only one preoperative data of CBC was available in most of the patients, and even though several preoperative CBCs were available, the result may have been affected by preoperative procedures.
In conclusion, the AMC and ALC were combined to generate a prognostic score, the AMLPS, which is a simple tool that could be used as a prognostic model for patients with gastric cancer. This prognostic score was independent of various inflammatory factors, age, and pathologic stage. These results should be validated in prospective trials.
Gastric cancer represents one of the major causes of cancer mortality worldwide. Despite a result of advances in surgical treatment, the role of surgery as mainstay treatment is limited to around a quarter of all patients, and overall survival of patients who undergo surgery progressively diminishes as stage increases, ranging from 75% for stage I to 35% or less for stage II and beyond; accurately predicting patients’ prognoses is needed to improve patient survival and to provide important information to the patients.
Assessment of the inflammatory response to the tumor may be easier and more-cost effective in clinical practice, and the addition of inflammatory factors to tumor-associated factors would be expected to help in disease management.
This study aims to measure the prognostic significance of absolute monocyte count/absolute lymphocyte count prognostic score (AMLPS) in patients with gastric cancer. The prognostic value of absolute monocyte count (AMC) in gastric cancer has not been validated ever before.
AMC and absolute lymphocyte count were combined to generate a prognostic score, the AMLPS, which is a simple tool that could be used as a prognostic model for patients with gastric cancer. This prognostic score was independent of various inflammatory factors, age, and pathologic stage. These results should be validated in prospective trials.
The paper is to measure the prognostic significance of absolute monocyte count/ AMLPS in patients with gastric cancer and to assess the AMLPS as an independent prognostic factor for survival in comparison with known prognostic factors. Although some published paper have showed the same change in hematological tumor, the finding in solid cancer in this paper should actually be interpreted cautiously.
P- Reviewer: Porrata LF, Qi F S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, Pohl A, Winder T, Ning Y, Groshen S. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (2)] |
| 2. | Agboola O. Adjuvant treatment in gastric cancer. Cancer Treat Rev. 1994;20:217-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 799] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 4. | Nakajima K, Ochiai T, Suzuki T, Shimada H, Hayashi H, Yasumoto A, Takeda A, Hishikawa E, Isono K. Impact of preoperative serum carcinoembryonic antigen, CA 19-9 and alpha fetoprotein levels in gastric cancer patients. Tumour Biol. 1998;19:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 754] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 6. | Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 734] [Article Influence: 48.9] [Reference Citation Analysis (1)] |
| 7. | Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am J Surg. 2012;204:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Kılınçalp S, Ekiz F, Başar O, Ayte MR, Coban S, Yılmaz B, Altınbaş A, Başar N, Aktaş B, Tuna Y. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25:592-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Bruckner HW, Lavin PT, Plaxe SC, Storch JA, Livstone EM. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA. 1982;247:1004-1006. [PubMed] [DOI] [Full Text] |
| 10. | Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 297] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 11. | Lee DY, Hong SW, Chang YG, Lee WY, Lee B. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer. 2013;13:111-116. [PubMed] |
| 12. | Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Batty N, Ghonimi E, Feng L, Fayad L, Younes A, Rodriguez MA, Romaguera JE, McLaughlin P, Samaniego F, Kwak LW. The absolute monocyte and lymphocyte prognostic index for patients with diffuse large B-cell lymphoma who receive R-CHOP. Clin Lymphoma Myeloma Leuk. 2013;13:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Porrata LF, Ristow K, Habermann TM, Ozsan N, Dogan A, Macon W, Colgan JP, Witzig TE, Inwards DJ, Ansell SM. Absolute monocyte/lymphocyte count prognostic score is independent of immunohistochemically determined cell of origin in predicting survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53:2159-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, Colgan JP, Nowakowski GS, Ansell SM, Witzig TE. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Aoki K, Tabata S, Yonetani N, Matsushita A, Ishikawa T. The prognostic impact of absolute lymphocyte and monocyte counts at diagnosis of diffuse large B-cell lymphoma in the rituximab era. Acta Haematol. 2013;130:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Huang JJ, Li YJ, Xia Y, Wang Y, Wei WX, Zhu YJ, Lin TY, Huang HQ, Jiang WQ, Li ZM. Prognostic significance of peripheral monocyte count in patients with extranodal natural killer/T-cell lymphoma. BMC Cancer. 2013;13:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [PubMed] |
| 20. | Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 22. | Kim DH, Baek JH, Chae YS, Kim YK, Kim HJ, Park YH, Song HS, Chung JS, Hyun MS, Sohn SK. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia. 2007;21:2227-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Song MK, Chung JS, Seol YM, Kim SG, Shin HJ, Choi YJ, Cho GJ, Shin DH. Influence of low absolute lymphocyte count of patients with nongerminal center type diffuse large B-cell lymphoma with R-CHOP therapy. Ann Oncol. 2010;21:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Hogan WJ, Markovic SN. New-onset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant. 2010;16:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Siddiqui M, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, Inwards DJ, White WL, Ansell SM, Micallef IN. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol. 2006;134:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5331] [Article Influence: 333.2] [Reference Citation Analysis (0)] |
| 27. | Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF, Comfere NI, Dietz AB, Novak AJ, Witzig TE, Feldman AL, Pittelkow MR. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Shivakumar L, Ansell S. Targeting B-lymphocyte stimulator/B-cell activating factor and a proliferation-inducing ligand in hematologic malignancies. Clin Lymphoma Myeloma. 2006;7:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 754] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 31. | Li YL, Pan YY, Jiao Y, Ning J, Fan YG, Zhai ZM. Peripheral blood lymphocyte/monocyte ratio predicts outcome for patients with diffuse large B cell lymphoma after standard first-line regimens. Ann Hematol. 2014;93:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Balta S, Unlu M, Arslan Z, Demırkol S. Neutrophil-to-Lymphocyte Ratio in Prognosis of Gastric Cancer. J Gastric Cancer. 2013;13:196-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |