Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2638
Peer-review started: September 21, 2014
First decision: October 14, 2014
Revised: October 29, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: March 7, 2015
Processing time: 169 Days and 7.2 Hours
AIM: To investigate the protective effect of bifidobacterium in endotoxin-induced intestinal injury in preweaning rats.
METHODS: Preweaning rats were randomly divided into three groups (n = 40 for each): a control group (group C), a model group (group E) and a treatment group (group T). Both groups E and T were intraperitoneally injected with lipopolysaccharide (LPS) at a dose of 5 mg/kg (5 mg/L in normal saline), and group T was intragastrically administrated with bifidobacterium suspension (2.0 × 109 CFU/mL, 0.5 mL each time, twice a day, until the end of the experiment) 7 d before LPS administration. Group C was intraperitoneally injected with normal saline. After intraperitoneal injection and intragastric administration, the rats were placed back to the initial cage to receive breast feeding. The rats were killed at 2, 6, 12, 24 or 72 h, respectively, after endotoxin or physiological saline injection to collect serum and ileal tissue samples. Myeloperoxidase (MPO) contents in serum and ileum were detected at different times, and expression of ileal defensin-5 mRNA was evaluated by reverse transcription-polymerase chain reaction.
RESULTS: Serum and ileal MPO contents in group E were significantly higher than those in group C (serum contents: 107.50 ± 17.70 vs 157.14 ± 24.67, P < 0.05; ileal contents: 1.03 ± 0.21 vs 1.57 ± 0.33, P < 0.05), which peaked at 12 h and 6 h, respectively. MPO contents in group T were significantly lower than those in group E (serum contents: 114.38 ± 24.56 vs 145.25 ± 23.62, P < 0.05; ileal contents: 1.25 ± 0.24 vs 1.57 ± 0.33, P < 0.05). The expression of defensin-5 mRNA in group E was significantly higher than that in group C (0.953 ± 0.238 vs 0.631 ± 0.146, P < 0.05), which peaked at 2 h, and then decreased gradually. The expression of defensin-5 mRNA in group T was significantly lower than that in group E (0.487 ± 0.149 vs 0.758 ± 0.160, P < 0.05) apparently in 24 h. The expression of defensin-5 mRNA at 2 h in group T was significantly higher than that in group C (0.824 ± 0.158 vs 0.631 ± 0.146, P < 0.05).
CONCLUSION: MPO and defensin-5 mRNA increase in preweaning rats with LPS-induced intestinal injury. Bifidobacterium protects the gut by inhibiting MPO activity, not by increasing defensin-5 secretion.
Core tip: This study investigated the serum and ileal contents of myeloperoxidase (MPO) and the expression of ileal Defensin-5 mRNA in the preweaning rats with lipopolysaccharide (LPS)-induced intestinal injury. Serum and ileal MPO contents in the model group (group E) were significantly higher than those in the control group (group C) (P < 0.05), which peaked at 12 h and 6 h, respectively. MPO contents in the treatment group (group T) were significantly lower than those in group E (P < 0.05). The expression of defensin-5 mRNA in group E was significantly up-regulated, peaked at 2 h, and then decreased gradually. The expression of defensin-5 mRNA in group T was significantly lower than that in group E (P < 0.05). Serum and ileal MPO contents and ileal expression of defensin-5 mRNA increased in preweaning rats with LPS-induced intestinal injury. Bifidobacterium protects the gut by inhibiting MPO activity, not by increasing defensin-5 secretion.
- Citation: Wang W, Yang SF, Ren LH, Zhang XX, Yu SL. Effect of bifidobacterium on defensin-5 expression in intestinal injury of preweaning rats. World J Gastroenterol 2015; 21(9): 2638-2644
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2638
Severe infection is one of the most common causes of pediatric gastrointestinal dysfunction, and the pathogenesis is closely related to endotoxin and gut barrier dysfunction. Bifidobacterium is one of the main species of beneficial bacteria in the human body, and participates in host digestion, nutrition, metabolism, absorption, immunity and resistance to infection. Especially, bifidobacterium plays an important role in maintaining the integrity of the intestinal mucosa barrier. Defensin-5 is a cationic peptide secreted by Paneth cells in the intestinal mucosa and has an important role in innate immunity in the gut. In this study, we investigated the changes in myeloperoxidase (MPO) contents and defensin-5 in preweaning rats with endotoxin-induced intestinal injury and explored the effect of pretreatment with bifidobacterium on endotoxin-induced intestinal injury.
Bifidobacterium infantis (KLDS2.0002) was purchased from the Key Laboratory of Dairy Science of Northeast Agricultural University. Escherichia coli (E. coli) (O55:B5) lipopolysaccharide (LPS) was purchased from Sigma. Coomassie brilliant blue protein determination kit and MPO determination kit were purchased from Nanjing Jiancheng Biological Engineering Research Institute. Primers for defensin-5 and β-actin were designed and synthesized by Shanghai Shengneng Bocai Biological Technology Co. LTD. Reverse transcription-PCR kit was purchased from Promega (Beijing, China). Wistar rats were provided by the Laboratory Animal Center of the Second Affiliated Hospital of Harbin Medical University.
One hundred and twenty healthy 18-d-old preweaning Wistar rats, weighing 30.36 ± 6.25 g, were used. Except the control group (n = 40), all the other rats were given 5 mg/kg of LPS (5 mg/L in normal saline) by intraperitoneal injection. After injection, the rats were placed back to the initial cage to receive breast feeding.
The animals were randomly divided into three groups (n = 40 for each): a control group (group C), a model group (group E) and a treatment group (group T). Group C received an intraperitoneal injection of 1 mL/kg of normal saline, and group E and group T received an intraperitoneal injection of 5 mg/kg of LPS (5 mg/L). One week before the administration of LPS, group T was intragastrically given mixed bifidobacterium suspension (2.0 × 109 CFU/mL), 0.5 mL each time, twice a day, until the end of the experiment. The rats were killed 2, 6, 12, 24 or 72 h after endotoxin or physiological saline injection to collect serum and ileal tissue samples.
After the animals were killed at different time points, blood samples were collected and centrifuged at 4 °C at 3500 r/min for 15 min. The supernatants were stored at -20 °C for further use. In addition, 4-5 cm of the ileum was taken 3-4 cm away from the ileocecal junction and divided into two parts. One part was stored at -80 °C for total RNA extraction, and the other part was subjected to irrigation of lumen contents with cold normal saline, dried on filter paper, weighed, homogenized and centrifuged at 4 °C at 3000 r/min for 10 min. The supernatant was stored at -20 °C for further use.
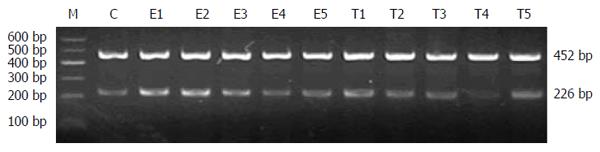
Serum and ileal MPO contents were determined using commercial kits according to the manufacturer’s instructions. To determine the expression of defensin-5 mRNA in the ileum, total RNA was extracted using one-step guanidinium isothiocyanate method and reverse-transcribed into cDNA. PCR primers for defensin-5 were 5′-TGAACCTACCCCAAAAACAGATG-3′ (forward) and 5′-TCAGCGGCAACAGAGTATGG-3′ (reverse). The size of the resulting product was 226 bp. PCR cycling parameters were 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 30 s; and a final extension at 72 °C for 7 min. β-actin was used as an internal standard. PCR primers for β-actin were 5′-CATCTGCTGGAAGGTGGACA-3′ (forward) and 5′-GAGAGGGAAATCGTGCGTGAC-3′ (reverse). The size of the resulting product was 452 bp. PCR cycling parameters were 94 °C for 5 min; 30 cycles of 94 °C for 30 s, 56 °C for 40 s and 72 °C for 22 s; and a final extension at 72 °C for 7 min. PCR products were resolved by 2% agarose electrophoresis and photographed using UV illumination. The results are expressed as the relative level of defensin-5 to β-actin.
Statistical analyses were performed using SPSS 10.0 software. Numerical data are expressed as mean ± SD. Means between two groups were compared using the t-test. P-values < 0.05 were considered statistically significant.
Compared with group C, serum MPO contents at 6, 12 and 24 h increased significantly in group E (145.25 ± 23.62 vs 100.99 ± 14.86; 157.14 ± 24.67 vs 107.50 ± 17.70; 126.24 ± 22.69 vs 103.08 ± 16.48; P < 0.05). Serum MPO contents at 6 and 12 h in group T declined significantly compared with those in group E (114.38 ± 24.56 vs 145.25 ± 23.62; 132.81 ± 18.08 vs 157.14 ± 24.67; P < 0.05) (Table 1).
Compared with group C, ileal MPO contents at 2, 6 and 12 h increased significantly in group E (1.26 ± 0.24 vs 0.93 ± 0.21; 1.57 ± 0.33 vs 1.03 ± 0.21; 1.46 ± 0.28 vs 1.07 ± 0.23; P < 0.05). Ileal MPO contents at 6 and 12 h in group T declined significantly compared with those in group E (1.25 ± 0.24 vs 1.57 ± 0.33; 1.16 ± 0.28 vs 1.46 ± 0.28; P < 0.05) (Table 2).
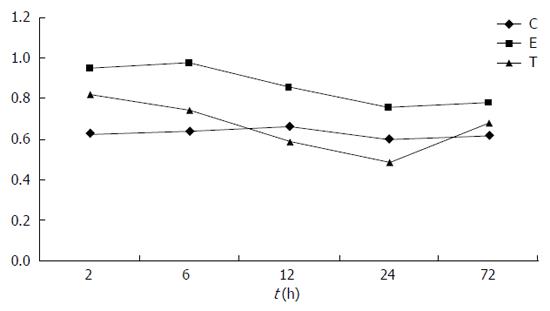
In group C, defensin-5 mRNA was lowly expressed in ileal tissue. In group E, defensin-5 mRNA expression significantly increased 2 h after LPS administration, peaked at 6 h, and gradually declined. Defensin-5 mRNA expression levels at 2, 6 and 12 h were significantly higher in group E than in group C (0.953 ± 0.238 vs 0.631 ± 0.146; 0.972 ± 0.213 vs 0.642 ± 0.154; 0.858 ± 0.198 vs 0.664 ± 0.128; P < 0.05). In group T, defensin-5 mRNA expression decreased at 2 h compared with group E, and the decrease was significant at 6, 12 and 24 h (0.742 ± 0.158 vs 0.972 ± 0.213; 0.590 ± 0.167 vs 0.858 ± 0.198; 0.487 ± 0.149 vs 0.758 ± 0.160; P < 0.05). Compared with group C, defensin-5 mRNA expression was higher at 2 and 6 h in group T (0.824 ± 0.158 vs 0.631 ± 0.146, P < 0.05 at 2 h), but returned to normal later (Figures 1 and 2, Table 3).
Gastrointestinal dysfunction plays an important role in the development and progression of multiple organ dysfunction syndrome (MODS). The intestine is not only one of the organs injured in MODS, but also plays a role in initiating MODS, in which intestinal mucosal barrier damage plays a key role. Bacterial endotoxin translocation is closely related to excessive growth of intestinal opportunistic pathogens, weakened local immunity in the intestine and intestinal mucosal mechanical barrier damage. The biological barrier consisting of intestinal mucosal mechanical barrier and intestinal flora plays a key role in preventing bacterial and endotoxin translocation.
MPO is a marker of polymorphonuclear neutrophils, and its activity reflects the degree of inflammatory cell infiltration in the tissue, namely, the degree of inflammatory activity[1]. This study found that in the normal intestinal tissue, serum MPO content was low. However, MPO activity increased significantly in group E compared with group C, and the increase of MPO content in the ileum occurred earlier than that in serum, suggesting that the accumulation and activation of a large number of neutrophils in ileal tissue participate in the occurrence and development of intestinal tissue injury.
Defensins are an important part of the body’s innate immune system. Because they have broad antibacterial[2] and antiviral[3,4] spectrum and do not generate resistance, defensins are expected to become a new type of antibacterial drug. Defensin-5 is produced mainly by Paneth cells located at the base of crypts in the small intestine[5,6], and it is mainly expressed in the jejunum and ileum. Paneth cell alpha-defensins secreted into the small intestinal lumen persist as intact and functional forms throughout the intestinal tract, suggesting that the peptides may mediate enteric innate immunity in the colonic lumen, far from their upstream point of secretion in small intestinal crypts[7]. Some defensins inhibit the biosynthesis of bacterial cell walls by insulating lipid II, while some others inhibit the biosynthesis of bacterial peptidoglycan by binding to lipid II[8-10]. Defensin expression is altered in IBD, and this suggests their potential role in IBD pathogenesis[11]. Recombinant human β-defensins-5 and -6 have been successfully expressed in E. coli and purified[12]. A previous study constructed a eukaryotic vector expressing human defensin-5, laying a foundation for eukaryotic expression, production and purification of bioactive human defensin-5 protein[13]. Current research shows that, with some modifications, defensin-5 having a purity > 95% can be produced to so as to study its structure, activity and other applications[14].
Many previous studies have proved the antibacterial effects of defensins in vitro or in animal models. A recent study found that human defensin-5 reduced parasite infection in intestinal epithelial cells[15]. Modified E21R-defensin-5 significantly enhanced the antiviral effect of defensin-5 against herpes simplex virus[16]. Human defensin-5 can resist HIV, providing a new avenue for AIDS prevention and treatment[17]. Human defensin-5 could also promote the healing of three-degree burns, reduce the bacteria to colonize, and promote hair growth[18]. In addition, human defensin-5 and β-defensin-2 can promote and coordinate each other in normal conditions, and their expression increases significantly in inflammatory conditions. Their synergistic effect may be one of the reasons for fallopian tube adhesion formation in infertile patients[19]. Different pathologies of the upper gastrointestinal tract may lead to differential expression of defensins in children[20]. In the gastroesophageal junction, defensin-5 secreted by metaplastic Paneth cells reduced the expression of E-cadherin in squamous cells, thus accelerating the formation of Barrett’s esophagus[21]. Studies have found that human defensin-5 promoted intestinal epithelial cell apoptosis[22]. In severely obese individuals, Paneth cell dysfunction resulted in decreased secretion of defensin-5, which was associated with obesity related flora composition changes[23]. Approximately 1/4 of patients undergoing Bricker ileal conduit urinary diversion had symptomatic urinary tract infection and increased secretion of defensin-5 by ureter epithelial cells, suggesting a protective role of innate immunity against symptomatic urinary tract infection[24].
By comparing intestinal tissues between normal children and those with Crohn’s disease (CD), it has been found that when the intestinal tract was exposed to bacteria or their associated antigens, Paneth cells released defensin-5, and the expression pattern of defensin-5 mRNA was different in different parts and in different pathological conditions[25]. In the ileum of CD children, defensin-5 expression was decreased, and this decrease was associated with the degree of inflammation and induced the metaplasia of colonic Paneth cells[26]. Studies have also shown that defensin-5 expression in the terminal ileum showed no significant differences among CD children, colitis children and normal children, but defensin-5 expression in the terminal ileum was significantly higher in ulcerative colitis children than in CD children and normal children[27].
This study showed that defensin-5 mRNA was lowly expressed in the ileum tissue of normal preweaning rats. In group E, defensin-5 expression significantly increased 2 h after intraperitoneal injection of endotoxin, peaked at 6 h, still showed a significant difference compared with the control group at 12 h, and gradually returned to normal at 24 h and 72 h, suggesting the protective role of intestinal defensin-5 in abdominal cavity infection.
The gastrointestinal tract is the body’s largest reservoir of bacteria. In critically ill patients, factors such as no eating and use of antacids and antibiotics can damage the micro-ecological stability in the gut, thus resulting in intestinal flora imbalance, which is the main reason for bacterial translocation and enterogenous infection[28,29]. Bifidobacterium is the main species of the biological barrier in the intestinal innate immune system, and can strengthen various lines of gastrointestinal defense through immune rejection, immune clearance and immune modulation, thereby exerting anti-infection, anti-inflammatory, and anti-tumor effects.
In interleukin 10 gene knockout mice (as an inflammatory bowel disease model), the expression of α-defensins decreased 7 wk after birth; in mice with an age less than 11 wk, the production of α-defensins varied inversely with age. Decreased expression of α-defensins resulted in ineffective modulation of the formation of intestinal flora, thus causing inflammatory changes[30]. In addition, α-defensins play an important role in regulating intestinal flora balance[31]. In induction therapy in organ transplantation, Paneth cells and antimicrobial peptides secreted by Paneth cells have an important role in maintaining the stability of intestinal flora[32]. Rebamipide can regulate intestinal flora by up-regulating the secretion of ileal defensing-5 and thereby alleviate indomethacin induced intestinal injury[33]. Biopsies of adenomatous polyps in the rectum-sigmoid colon had a significant reduction of mucosa adherent bacteria and an increase in α-defensin secretion[34]. α-defensins secreted by Paneth cells can not only mediate gut innate immunity but also determine the composition of the intestinal microbiota[31].
Supplementation of exogenous bifidobacterium can maintain the population of beneficial intestinal bacteria, reduce the load of intestinal pathogenic bacteria, facilitate the restoration of intestinal micro-ecological balance, repair intestinal epithelial barrier, inhibit the excessive growth of exogenous pathogenic bacteria, reduce the production of intestinal endotoxin, promote the secretion of mucins by intestinal epithelial cells and immunoglobulin A by Paneth cells, and regulate the body’s immune function[35]. In a mouse model of inflammatory bowel disease, treatment with probiotics can reduce intestinal inflammation and increase the secretion of intestinal mucin-2, which is beneficial to the recovery of inflammatory bowel disease[36]. Some studies suggest certain species-specificities of the anti-inflammatory properties of bifidobacteria[37], while others found that administration of Bifidobacterium bifidum protects against necrotizing enterocolitis in a neonatal rat model[38]. Bifidobacterium adolescentis BBMN23 and Bifidobacterium longum BBMN68 were orally administrated to specific pathogen-free BALB/c mice at different doses, and the results suggested that BBMN23 and BBMN68 may improve intestinal digestion ability and enhance immune barrier function in the intestine[39].
In this study, compared with rats in the model group, pretreatment with bifidobacterium led to significant reductions of serum and ileal MPO contents, reduced the accumulation and activation of neutrophils, and decreased inflammatory responses, indicating that bifidobacterium can protect the intestinal tissue by inhibiting the activity of MPO. After supplementation of exogenous bifidobacterium, ileal defensin-5 mRNA expression increased compared with the normal group, but significantly decreased compared with the endotoxin group, suggesting that bifidobacterium exerts the intestinal protective effect not via the defensin-5 pathway.
In conclusion, treatment with LPS caused increases in serum and ileal MPO contents and ileal defensin-5 mRNA expression, suggesting that intestinal inflammatory responses and innate immune system were activated. After supplementation of exogenous bifidobacterium, serum and ileal MPO contents decreased, and ileal defensin-5 mRNA expression significantly decreased compared with group E, but still increased compared with group C, suggesting that bifidobacterium could exert a protective effect on the ileum not via the mechanisms associated with defensin-5 up-regulation. Bifidobacterium may activate other protective mechanisms in the body and as a result, Paneth cells do not need secret too much defensin-5 to control inflammation. The exact mechanisms remain to be clarified in future studies.
Severe infection is one of the most common causes of pediatric gastrointestinal dysfunction, and the pathogenesis is closely related to endotoxin and gut barrier dysfunction. However, to date, there are still no effective treatments to protect gastrointestinal gut from dysfunction.
Bifidobacterium is one of the main species of beneficial bacteria in the human body, and participates in host digestion, nutrition, metabolism, absorption, immunity and resistance to infection. Now, bifidobacterium plays an important role in maintaining the integrity of the intestinal mucosa barrier through balancing the intestinal flora. To date, there are still no effective treatments to protect gastrointestinal gut from dysfunction.
In this study, model rats were given 5 mg/kg of lipopolysaccharide (LPS) by intraperitoneal injection, and the treatment group was intragastrically given mixed bifidobacterium suspension (2.0 × 109 CFU/mL), 0.5 mL each time, twice a day, until the end of the experiment. Then we investigated the changes in the serum and ileal myeloperoxidase (MPO) contents and the ileal expression of defensin-5 in preweaning rats with endotoxin-induced intestinal injury and explored the effect of pretreatment with bifidobacterium on endotoxin-induced intestinal injury. In conclusion, serum and ileal MPO contents and ileal expression of defensin-5 mRNA increase in preweaning rats with LPS-induced intestinal injury. Bifidobacterium protects the gut by inhibiting MPO activity, not by increasing defensin-5 secretion.
The study results suggest that pretreatment with bifidobacterium in endotoxin-induced intestinal injury can protect the gut by inhibiting MPO activity, not by increasing defensin-5 secretion, and thus relieve the gastrointestinal dysfunction.
MPO is a marker of polymorphonuclear neutrophils, and its activity reflects the degree of inflammatory cell infiltration in the tissue, namely, the degree of inflammatory activity. After supplementation of exogenous bifidobacterium, serum and ileal MPO contents decreased, and ileal defensin-5 mRNA expression significantly decreased compared with group E, but still increased compared with group C, suggesting that bifidobacterium could exert a protective effect on the ileum not via the mechanisms associated with defensin-5 up-regulation.
This is a good descriptive study in which the authors analyzed the preventive effect of bifidobacterium by inhibiting the activity of MPO, reducing the accumulation and activation of neutrophils, and decreasing inflammatory responses. Meanwhile, bifidobacterium exerts a protective effect on the ileum not via the mechanisms associated with defensin-5 up-regulation.
P- Reviewer: Canan O, Chung SCS, Maja T S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Pang QF, Xu WL, He J, Chen HL. [The protective effect of glutamine on endotoxemic intestinal injury and expression of heme oxygenase-1 in rats]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:95-98. [PubMed] |
| 2. | Zhao L, Lu W. Defensins in innate immunity. Curr Opin Hematol. 2014;21:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol. 2013;425:4965-4980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Il’iasov RA, Gaĭfullina LR, Saltykova ES, Poskriakov AV, Nikolenko AG. [Defensins in the honeybee antinfectious protection]. Zh Evol Biokhim Fiziol. 2012;48:425-432. [PubMed] |
| 5. | Ouellette AJ. Paneth cell α-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68:2215-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Shanahan MT, Vidrich A, Shirafuji Y, Dubois CL, Henschen-Edman A, Hagen SJ, Cohn SM, Ouellette AJ. Elevated expression of Paneth cell CRS4C in ileitis-prone SAMP1/YitFc mice: regional distribution, subcellular localization, and mechanism of action. J Biol Chem. 2010;285:7493-7504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Mastroianni JR, Ouellette AJ. Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. J Biol Chem. 2009;284:27848-27856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Schneider T, Kruse T, Wimmer R, Wiedemann I, Sass V, Pag U, Jansen A, Nielsen AK, Mygind PH, Raventós DS. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328:1168-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 9. | Schmitt P, Wilmes M, Pugnière M, Aumelas A, Bachère E, Sahl HG, Schneider T, Destoumieux-Garzón D. Insight into invertebrate defensin mechanism of action: oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J Biol Chem. 2010;285:29208-29216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Schneider T, Sahl HG. Lipid II and other bactoprenol-bound cell wall precursors as drug targets. Curr Opin Investig Drugs. 2010;11:157-164. [PubMed] |
| 11. | Ramasundara M, Leach ST, Lemberg DA, Day AS. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Huang L, Ching CB, Jiang R, Leong SS. Production of bioactive human beta-defensin 5 and 6 in Escherichia coli by soluble fusion expression. Protein Expr Purif. 2008;61:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Lu H, Li H, Wu XM, Shan Y. Cloning of human defensin-5 gene and construction of eukaryotic expression vector. Progress of Anatomical Sciences. 2010;16:264-266. |
| 14. | Vernieri E, Valle J, Andreu D, de la Torre BG. An optimized Fmoc synthesis of human defensin 5. Amino Acids. 2014;46:395-400. [PubMed] |
| 15. | Leitch GJ, Ceballos C. A role for antimicrobial peptides in intestinal microsporidiosis. Parasitology. 2009;136:175-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Wang A, Chen F, Wang Y, Shen M, Xu Y, Hu J, Wang S, Geng F, Wang C, Ran X. Enhancement of antiviral activity of human alpha-defensin 5 against herpes simplex virus 2 by arginine mutagenesis at adaptive evolution sites. J Virol. 2013;87:2835-2845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Furci L, Tolazzi M, Sironi F, Vassena L, Lusso P. Inhibition of HIV-1 infection by human α-defensin-5, a natural antimicrobial peptide expressed in the genital and intestinal mucosae. PLoS One. 2012;7:e45208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Lough D, Dai H, Yang M, Reichensperger J, Cox L, Harrison C, Neumeister MW. Stimulation of the follicular bulge LGR5+ and LGR6+ stem cells with the gut-derived human alpha defensin 5 results in decreased bacterial presence, enhanced wound healing, and hair growth from tissues devoid of adnexal structures. Plast Reconstr Surg. 2013;132:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Hu SW, Mao C, Zhang D, Lan Z, Wang CF, Lu S. Significance of expression of human α-defensin-5 and beta-defensin-2 in fallopian tube of intertile patients. Sichuandaxue Xuebao. 2010;4:480-482. |
| 20. | Vordenbäumen S, Pilic D, Otte JM, Schmitz F, Schmidt-Choudhury A. Defensin-mRNA expression in the upper gastrointestinal tract is modulated in children with celiac disease and Helicobacter pylori-positive gastritis. J Pediatr Gastroenterol Nutr. 2010;50:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Nomura Y, Tanabe H, Moriichi K, Igawa S, Ando K, Ueno N, Kashima S, Tominaga M, Goto T, Inaba Y. Reduction of E-cadherin by human defensin-5 in esophageal squamous cells. Biochem Biophys Res Commun. 2013;439:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Lu W, de Leeuw E. Pro-inflammatory and pro-apoptotic properties of Human Defensin 5. Biochem Biophys Res Commun. 2013;436:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Hodin CM, Verdam FJ, Grootjans J, Rensen SS, Verheyen FK, Dejong CH, Buurman WA, Greve JW, Lenaerts K. Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J Pathol. 2011;225:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Townes CL, Ali A, Robson W, Pickard R, Hall J. Tolerance of bacteriuria after urinary diversion is linked to antimicrobial peptide activity. Urology. 2011;77:509.e1-509.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Negroni A, Stronati L, Pierdomenico M, Tirindelli D, Di Nardo G, Mancini V, Maiella G, Cucchiara S. Activation of NOD2-mediated intestinal pathway in a pediatric population with Crohn’s disease. Inflamm Bowel Dis. 2009;15:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Perminow G, Beisner J, Koslowski M, Lyckander LG, Stange E, Vatn MH, Wehkamp J. Defective paneth cell-mediated host defense in pediatric ileal Crohn’s disease. Am J Gastroenterol. 2010;105:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Zilbauer M, Jenke A, Wenzel G, Goedde D, Postberg J, Phillips AD, Lucas M, Noble-Jamieson G, Torrente F, Salvestrini C. Intestinal alpha-defensin expression in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2076-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Verhelst X, de Vos M, van Vlierberghe H. Optimal use of corticosteroids in gastroenterology and hepatology. J Translat Intern Med. 2014;2:53-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Sun H, Su X, Shi Y. Current status of in vitro and in vivo antifungal activities of posaconazole. J Translat Intern Med. 2013;1:18-22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Inaba Y, Ashida T, Ito T, Ishikawa C, Tanabe H, Maemoto A, Watari J, Ayabe T, Mizukami Y, Fujiya M. Expression of the antimicrobial peptide alpha-defensin/cryptdins in intestinal crypts decreases at the initial phase of intestinal inflammation in a model of inflammatory bowel disease, IL-10-deficient mice. Inflamm Bowel Dis. 2010;16:1488-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 912] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 32. | Li Q, Zhang Q, Wang C, Tang C, Zhang Y, Jiang S, Li N, Li J. Influence of alemtuzumab on the intestinal Paneth cells and microflora in macaques. Clin Immunol. 2010;136:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Tanigawa T, Watanabe T, Otani K, Nadatani Y, Ohkawa F, Sogawa M, Yamagami H, Shiba M, Watanabe K, Tominaga K. Rebamipide inhibits indomethacin-induced small intestinal injury: possible involvement of intestinal microbiota modulation by upregulation of α-defensin 5. Eur J Pharmacol. 2013;704:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Pagnini C, Corleto VD, Mangoni ML, Pilozzi E, Torre MS, Marchese R, Carnuccio A, Giulio ED, Delle Fave G. Alteration of local microflora and α-defensins hyper-production in colonic adenoma mucosa. J Clin Gastroenterol. 2011;45:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Hu W, Yu FM. Economic evaluation of resonable nutrition support. J Translat Intern Med. 2014;2:3-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Amit-Romach E, Uni Z, Reifen R. Multistep mechanism of probiotic bacterium, the effect on innate immune system. Mol Nutr Food Res. 2010;54:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol. 2012;56:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G940-G949. [PubMed] |
| 39. | Yang H, Liu A, Zhang M, Ibrahim SA, Pang Z, Leng X, Ren F. Oral administration of live Bifidobacterium substrains isolated from centenarians enhances intestinal function in mice. Curr Microbiol. 2009;59:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |