Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2116
Peer-review started: July 7, 2014
First decision: August 6, 2014
Revised: September 1, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: February 21, 2015
Processing time: 219 Days and 2 Hours
AIM: To investigate the predictors of proximal kidney tubular dysfunction (PKTD) induced by adefovir dipivoxil (ADV) treatment for chronic hepatitis B.
METHODS: Seventy-nine patients (age at the evaluation of PKTD: 56.9 ± 10.7 years) with chronic hepatitis B undergoing long-term oral antiviral nucleos(t)ide analogue treatment were consecutively recruited. PKTD was defined by the presence of at least two of the following five abnormalities: phosphate diabetes, nondiabetic glucosuria, metabolic acidosis, β2-microglobulinuria, or renal hypouricemia. The single-nucleotide polymorphisms (SNPs) in the SLC22A6 gene encoding human organic anion transporter 1 (hOAT1) and ABCC2 encoding multidrug resistance protein 2 (MRP2) were analyzed using the TaqMan Allelic Discrimination Demonstration Kit.
RESULTS: Nine (30.0%) of the 30 ADV-treated patients were diagnosed with PKTD, while no patients without ADV developed PKTD (P < 0.001). Three patients with ADV were diagnosed with symptomatic osteomalacia. Among the patients who took ADV, those with PKTD were of higher age at initiation, had significantly longer treatment duration, and had a significantly lower body mass index than those without PKTD. The incidence of PKTD dramatically increased after 96 mo from the start of ADV administration. In contrast, the SNPs were not correlated with PKTD. Logistic regression analysis extracted older age at initiation (OR = 5.0, 95%CI: 1.1-23.4; P = 0.040) and longer treatment duration (OR = 3.2, 95%CI: 1.2-8.6; P = 0.020) as significant factors associated with PKTD.
CONCLUSION: Our results suggest that the tubular function of the kidney of older patients undergoing long-term ADV treatment should be carefully evaluated.
Core tip: This paper reports that high prevalence rates of proximal kidney tubular dysfunction (30.0%) and symptomatic osteomalacia (10.0%) were found for chronic hepatitis B virus infection patients treated with low-dose adefovir dipivoxil (ADV) and that age at the initiation of ADV and treatment duration of ADV were independently associated with the development of proximal kidney tubular dysfunction.
- Citation: Shimizu M, Furusyo N, Ikezaki H, Ogawa E, Hayashi T, Ihara T, Harada Y, Toyoda K, Murata M, Hayashi J. Predictors of kidney tubular dysfunction induced by adefovir treatment for chronic hepatitis B. World J Gastroenterol 2015; 21(7): 2116-2123
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2116.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2116
Chronic hepatitis B virus (HBV) infection affects more than 350 million people worldwide, with 75% living in the Asia-Pacific region[1,2]. Although Japan was historically endemic for HBV infection, our previous studies have shown that the prevalence of hepatitis B surface antigen carriage in Okinawa, Japan markedly decreased from 12.4% in 1970 to 4.2% in 1996[3,4]. However, chronic HBV infection continues to be a major health problem because it leads to the development of liver cirrhosis, hepatocellular carcinoma, and raises the risk of hepatic disease-related death. Thus, to stop or reduce disease progression and to prevent the development of hepatic decompensation through the sustained suppression of HBV replication[5], interferon or oral antiviral nucleos(t)ide analogues (NA) have been approved for the treatment of many patients with chronic HBV infection[6,7].
Adefovir dipivoxil (ADV), a nucleotide analogue of adenosine monophosphate, is effective in viral suppression for both treatment-naive and lamivudine-resistant chronic HBV infection patients[8,9]. Large prospective studies have shown that the nephrotoxicity of ADV is dose-dependent and that low-dose ADV is relatively safe for the kidney[10]. However, some cases of proximal kidney tubular dysfunction (PKTD) by ADV, including the development of Fanconi syndrome or symptomatic osteomalacia, have been reported[11]. In addition, ADV is known to enter proximal kidney tubule cells through the basolateral human organic anion transporter 1 (hOAT1) and is transported from the cells to the tubular lumen through multidrug resistance protein 2 (MRP2). Extensive intracellular drug accumulation has been reported to be associated with ADV nephrotoxicity[12,13].
This retrospective study was done to evaluate the prevalence of PKTD and its related factors, including the single-nucleotide polymorphisms (SNPs) in genes encoding hOAT1 and MRP2, among chronic HBV infection patients taking low-dose ADV.
Participants in this retrospective study were consecutively recruited at the outpatient clinic of the Department of General Internal Medicine, Kyushu University Hospital from November 2012 to October 2013. The inclusion criteria required patients with chronic HBV infection who had received NA for more than one year at enrollment. The 79 Japanese patients enrolled included 30 treated with a 10-mg daily dose of ADV and 49 treated with an NA other than ADV (19 with lamivudine, 29 with entecavir, and 1 with tenofovir). All patients treated with ADV had been previously treated another NA (29 with lamivudine and 1 with entecavir). Exclusion criteria were as follows: positivity for antibodies to human immunodeficiency virus (HIV) or hepatitis C virus; clinical or biochemical evidence of hepatic decompensation; suspected hepatocellular carcinoma at entry; or chronic renal failure and currently on hemodialysis. The study was registered on the University Hospital Medical Information Network Clinical Trials Registry: No. UMIN000012870.
The study design was approved by the Kyushu University Hospital Ethics Committee, and written informed consent was obtained from all patients who were enrolled. The study was conducted in accordance with the principles of the Helsinki Declaration of 1975, as revised in 2000.
All available medical records were evaluated thoroughly. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Hypertension was defined as clinic systolic blood pressure ≥ 140 mmHg and/or clinic diastolic blood pressure ≥ 90 mmHg or the use of antihypertensive agents. Diabetes mellitus was defined as a fasting plasma glucose level ≥ 7.0 mmol/L, a casual plasma glucose level ≥ 11.1 mmol/L, a 2-h plasma glucose level ≥ 11.1 mmol/L during an oral glucose tolerance test, HbA1c≥ 6.5%, or the use of oral hypoglycemic agents or insulin. Smokers were defined as current smokers. Habitual drinking was defined as drinking alcohol more than three times a week. Blood and urine samples were collected in the morning after an overnight fast. Blood variables included aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase (ALP), plasma glucose, serum creatinine, calcium, phosphorus, sodium, potassium, chloride, uric acid, pH, and bicarbonate. Urine variables included glucose, creatinine, phosphorus, uric acid, and β2-microglobulin. When the urine β2-microglobulin value was below the detectable value (30 μg/L), it was taken as 30 μg/L. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation modified for Japanese subjects: eGFR (mL/min per 1.73 m2) = 194 • age-0.287• serum creatinine (mg/dL)-1.094 (if female • 0.739)[14].
The tubular metabolism of phosphates was assessed using a tubular maximum for phosphate corrected for the glomerular filtration rate (TmP/GFR). Tubular reabsorption of phosphate (TRP) was calculated using the following formula: TRP = (1-urine phosphate/urine creatinine) • (serum creatinine/serum phosphate). The formula used to calculate TmP/GFR is dependent on the value of TRP and can be calculated using the formulas below: if TRP is ≤ 0.86, then TmP/GFR = TRP • serum phosphate; if TRP is > 0.86, then TmP/GFR = 0.3 • TRP/(1 - 0.8 • TRP) • serum phosphate[15]. Fractional excretion of uric acid (FEUA) was calculated as [(urine uric acid • serum creatinine)/(urine creatinine • serum uric acid)] • 100 (%). For the analysis of β2-microglobulinuria, urine β2-microglobulin levels corrected for urine creatinine (urine β2-microglobulin-creatinine ratio; UBCR) were used.
PKTD was defined by the presence of at least two out of five abnormalities[16]: phosphate diabetes (TmP/GFR ≤ 0.77 mmol/L with hypophosphatemia (< 0.8 mmol/L), renal hypouricemia (FEUA > 15%), β2-microglobulinuria (UBCR > 300 μg/gCr), nondiabetic glucosuria (positive urine glucose with plasma glucose < 7.0 mmol/L), or metabolic acidosis (blood pH ≤ 7.34 and serum bicarbonate ≤ 22 mmol/L).
The presence of HBeAg was determined by chemiluminescence enzyme immunoassay (Abbott Japan Co., Tokyo, Japan)[17]. Quantification of serum HBV DNA was performed by real-time polymerase chain reaction (PCR) assay (Abbott Real Time HBV, Abbott Laboratories), within the range 10 to 109 IU/mL[18]. HBV genotype analysis was performed serologically by the PCR-invader method with genotype specific probes[19,20].
Human genomic DNA was extracted from the peripheral blood of each participant. Genotyping of the SNPs in SLC22A6 encoding hOAT1 (AJ249369, Pos: 453) and ABCC2 encoding MRP2 (rs717620) was done using the TaqMan Allelic Discrimination Demonstration Kit (7500 Real-Time PCR System; Applied Biosystems, Foster City, CA).
All data are expressed as mean ± SD or percentages. Because the distribution of the UBCR was highly skewed, it was log-transformed before the statistical analysis and expressed as the geometric mean ± SD. Differences between the groups were analyzed with the unpaired t-test for continuous variables or χ2-test for categorical variables. The odds ratio (OR) and the 95% confidence interval (CI) were calculated using multiple logistic regression analyses after adjustment for known covariates. The statistical calculations were performed using the computer software package SPSS version 19.0 (SPSS Inc., IBM, Somers, New York, United States). A two-tailed P value < 0.05 was considered to be statistically significant.
The age at the evaluation of PKTD of the 79 participants, 51 men (64.6%) and 28 women (35.4%), ranged from 34 to 84 years (mean ± SD: 56.9 ± 10.7 years). For the entire study population, the prevalences of phosphate diabetes, renal hypouricemia, β2-microglobulinuria, nondiabetic glucosuria, and metabolic acidosis were 11.4%, 12.7%, 22.8%, 6.3%, and 2.5%, respectively, and there were 19 patients (24.1%) with eGFR < 60 mL/min per 1.73 m2 at examination.
The clinical characteristics of the study participants are summarized in Table 1. The distribution of genotypes of the SLC22A6 and ABCC2 genes was not significantly different between patients with/without ADV. The treatment duration of NA was longer and the positive rate of HBV DNA was lower for the patients with ADV. The prevalences of phosphate diabetes, renal hypouricemia, β2-microglobulinuria, and nondiabetic glucosuria were significantly higher for the patients treated with ADV. The ALP level was higher and the eGFR level was lower for the patients treated with ADV. Finally, PKTD was diagnosed in 30.0% of the patients treated with ADV, but none of the patients without ADV treatment met the diagnostic criteria (P < 0.001). Moreover, three (10%) of the 30 ADV-treated patients were diagnosed with symptomatic osteomalacia (bone pain and gait difficulties) by radiological and serological testing[11,21,22].
| Patient without ADV | Patient with ADV | P value | |
| (n = 49) | (n = 30) | ||
| Age (yr) | |||
| At the initiation of NA | 51.1 ± 10.8 | 49.9 ± 10.0 | NS |
| At the evaluation of PKTD | 55.7 ± 11.1 | 58.9 ± 9.9 | NS |
| Male/female | 30/19 | 21/9 | NS |
| BMI (kg/m2) | 23.0 ± 2.9 | 22.6 ± 3.0 | NS |
| Current smokers | 4 (8.2) | 4 (13.3) | NS |
| Habitual drinkers | 5 (10.2) | 2 (6.7) | NS |
| Hypertension | 12 (24.5) | 12 (40.0) | NS |
| Diabetes | 1 (2.0) | 2 (6.7) | NS |
| Treatment duration of NA (mo) | 54.8 ± 38.8 | 107.5 ± 30.1 | < 0.001 |
| SLC22A6 (hOAT1) 4531 G/A, | |||
| G/G | 37 (75.5) | 17 (56.7) | NS |
| G/A | 11 (22.4) | 12 (40.0) | |
| A/A | 1 (2.0) | 1 (3.3) | |
| ABCC2 (MRP2) -24 C/T, rs717620 | |||
| C/C | 28 (57.1) | 17 (56.7) | NS |
| C/T | 19 (38.8) | 9 (30.0) | |
| T/T | 2 (4.1) | 4 (13.3) | |
| HBeAg-positive | 22.4 | 16.7 | NS |
| HBV DNA-positive | 63.3 | 30.0 | 0.004 |
| HBV genotype | |||
| B/C/undetected | 8/36/5 | 0/28/2 | 0.048 |
| Medicated | |||
| LAM | 19 (38.8) | 29 (96.7) | < 0.001 |
| ETV | 29 (59.2) | 1 (3.3) | < 0.001 |
| TDF | 1 (2.0) | 0 | NS |
| AST (U/L) | 29.4 ± 10.5 | 31.0 ± 10.8 | NS |
| ALT (U/L) | 28.5 ± 16.6 | 30.5 ± 19.6 | NS |
| ALP (U/L) | 242.9 ± 112.0 | 369.8 ± 293.8 | 0.03 |
| Serum calcium (mmol/L) | 2.3 ± 0.8 | 2.3 ± 0.1 | NS |
| Serum phosphate (mmol/L) | 1.1 ± 0.1 | 0.9 ± 0.2 | < 0.001 |
| Serum uric acid (umol/L) | 324.6 ± 71.9 | 266.1 ± 112.0 | 0.014 |
| UBCR (mg/gCre) | 117.1 (50.5-271.6) | 805.2 (61.8-10489.6) | < 0.001 |
| Phosphate diabetes | 0 | 9 (30.0) | < 0.001 |
| Renal hypouricemia | 0 | 10 (33.3) | < 0.001 |
| β2-microglobulinuria | 3 (6.1) | 15 (50.0) | < 0.001 |
| Nondiabetic glucosuria | 0 | 5 (16.7) | 0.003 |
| Metabolic acidosis | 0 | 2 (6.7) | NS |
| eGFR (mL/min per 1.73 m2) | |||
| At the initiation of NA | 82.6 ± 15.5 | 79.9 ± 14.9 | NS |
| At the evaluation of PKTD | 76.3 ± 18.2 | 63.0 ± 16.4 | 0.002 |
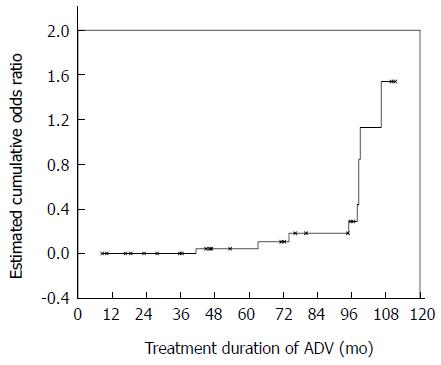
For the patients who were diagnosed with PKTD, β2-microglobulinuria (100%) and renal hypouricemia (100%) were the most prevalent abnormalities, followed by phosphate diabetes (66.7%), nondiabetic glucosuria (44.4%), and metabolic acidosis (22.2%). The correlations of factors related to the PKTD of patients treated with ADV are shown in Table 2. The patients with PKTD were older and had a lower eGFR level than those without PKTD at examination; however, these differences were not statistically significant in terms of the timing of the initiation of ADV. No significant difference was found between men and women. BMI was lower and the treatment duration of ADV was longer for the patients with PKTD. In addition, the incidence of PKTD dramatically increased after 96 mo from the start of ADV administration (Figure 1). The single-SNP analysis showed a higher, but non-significant, prevalence of PKTD in patients with the ABCC2 -24 genotype CC/CT (9/26) than in patients with genotype TT (0/4) (P = 0.160). The genotype at SLC22A6 was also not significantly related to the presence of PKTD. Logistic regression analysis adjusted for BMI extracted older age at the initiation of ADV and longer treatment duration of ADV as independent risks for PKTD (Table 3). Even after adjustment for the known covariates of BMI, sex, the eGFR level at the initiation of ADV, and genotype at ABCC2, both remained significantly associated with the presence of PKTD.
| PKTD (-) | PKTD (+) | P value | |
| (n = 21) | (n = 9) | ||
| Age (yr) | |||
| At the initiation of ADV | 52.1 ± 9.3 | 59.0 ± 9.7 | NS |
| At the evaluation of PKTD | 56.0 ± 9.1 | 65.7 ± 8.6 | 0.012 |
| Male/female | 16/5 | 5/4 | NS |
| BMI (kg/m2) | 23.3 ± 3.0 | 20.8 ± 2.5 | 0.038 |
| Current smokers | 4 (19.0) | 0 | NS |
| Habitual drinkers | 2 (9.5) | 0 | NS |
| Hypertension | 9 (42.9) | 3 (33.3) | NS |
| Diabetes | 1 (4.8) | 1 (11.1) | NS |
| Treatment duration of NA (mo) | 104.1 ± 32.1 | 115.1 ± 25.2 | NS |
| Treatment duration of ADV (mo) | 56.0 ± 33.5 | 86.0 ± 21.8 | 0.021 |
| SLC22A6 (hOAT1) 4531 G/A, | |||
| G/G | 13 (61.9) | 4 (44.4) | NS |
| G/A | 8 (38.1) | 4 (44.4) | |
| A/A | 0 | 1 (11.1) | |
| ABCC2 (MRP2) -24 C/T, rs717620 | |||
| C/C | 12 (57.1) | 5 (55.6) | NS |
| C/T | 5 (23.8) | 4 (44.4) | |
| T/T | 4 (19.0) | 0 | |
| eGFR (mL/min per 1.73 m2) | |||
| At the initiation of ADV | 81.4 ± 11.6 | 77.1 ± 17.0 | NS |
| At the evaluation of PKTD | 70.0 ± 10.9 | 46.9 ± 15.8 | < 0.001 |
Because of the different prevalence of PKTD between ABCC2 -24 genotype CC/CT and genotype TT, we additionally assessed the correlation of this SNP to the PKTD parameters of patients treated with ADV (Table 4). Although the prevalences of renal hypouricemia and β2-microglobulinuria were not significantly different, the FEUA and UBCR values were significantly higher for patients with genotype CC/CT than for those with genotype TT. On the contrary, all of these parameters were statistically similar for the genotype at SLC22A6 (data not shown).
| Genotype CC or CT | Genotype TT | P value | |
| (n = 26) | (n = 4) | ||
| Serum phosphate (mmol/L) | 0.86 ± 0.21 | 1.00 ± 0.25 | NS |
| TmP/GFR (mmol/L) | 0.73 ± 0.28 | 0.95 ± 0.42 | NS |
| Serum uric acid (umol/L) | 267.2 ± 120.3 | 258.7 ± 26.4 | NS |
| FEUA (%) | 16.6 ± 14.6 | 9.5 ± 1.6 | 0.025 |
| UBCR (mg/gCr) | 996.3 (68.2-14555.9) | 201.4 (81.5-497.7) | 0.038 |
| Phosphate diabetes | 8 (30.8) | 1 (25.0) | NS |
| Renal hypouricemia | 10 (38.5) | 0 | NS |
| β2-microglobulinuria | 14 (53.8) | 1 (25.0) | NS |
| Nondiabetic glucosuria | 5 (19.2) | 0 | NS |
| Metabolic acidosis | 2 (7.7) | 0 | NS |
| eGFR (mL/min per 1.73 m2) | 62.8 ± 17.6 | 64.4 ± 2.1 | NS |
The main finding of the present study was the high prevalence of PKTD found for chronic HBV infection patients treated long-term with a 10-mg daily dose of ADV. Furthermore, both age at the initiation of ADV and treatment duration of ADV were independently associated with the presence of PKTD. To the best of our knowledge, this is the first clinical research to evaluate ADV nephrotoxicity that focused on PKTD.
In this analysis, we clarified that the ADV nephrotoxicity of PKTD was duration-dependent. A daily dose of 60-120 mg ADV has been shown to be associated with PKTD in the treatment of HIV infection[23,24]. Moreover, the incidence of hypophosphatemia and serum creatinine elevation from a 10-mg dose of ADV was reported to be similar to that of a placebo and significantly lower than that from a 30-mg dose of ADV[10]. Based on these findings, 10 mg ADV is the currently approved dose for the treatment of chronic HBV infection. However, our results show that long-term treatment with this low-dose of ADV also causes PKTD, especially for elderly patients. Almost all of the patients with ADV were also receiving lamivudine in the present study. Although no patients with lamivudine monotherapy were diagnosed as having PKTD, it is possible that interactions between ADV and lamivudine would cause PKTD.
In the present study, PKTD was defined by the presence of at least two of five abnormalities: phosphate diabetes, renal hypouricemia, β2-microglobulinuria, nondiabetic glucosuria, or metabolic acidosis. Histological evaluation was not performed for all of our patients, but renal biopsy showed normal appearance or non-specific tubulointerstitial nephritis in cases of ADV-related PKTD with symptomatic osteomalacia[11]. The diagnosis of PKTD by renal biopsy is difficult.
Among patients with ADV, the prevalence of phosphate diabetes, which can cause osteomalacia, was 30.0% in this study. On the contrary, the incidence of hypophosphatemia was documented as 6.5%-35.3% of chronic HBV infection patients treated with 10 mg ADV in previous studies[10,25,26]. We believe that the different treatment duration of ADV may be responsible for these discrepancies. Therefore, together with the results of the present study, research suggests that kidney tubular function should be carefully evaluated, especially for patients receiving long-term ADV treatment. Although regular monitoring of serum creatinine and phosphate levels has been proposed for the evaluation of ADV nephrotoxicity[27,28], the prevalences of β2-microglobulinuria and renal hypouricemia were higher than that of phosphate diabetes in the patients who were diagnosed with PKTD in the present study. Thus, for the purpose of early detection of PKTD during ADV treatment, regular monitoring of urine β2-microglobulin, serum and urine uric acid, and urine creatinine would also be recommended.
Ha et al[29] previously reported that baseline eGFR was an independent predictor of renal dysfunction during ADV treatment, which is different from the results of the present study. This discrepancy could be due to the different definition of renal impairment: GFR reduction was used for ADV nephrotoxicity in the Ha study. Although we found that the patients with PKTD had a lower eGFR level than those without PKTD at examination, some cases of ADV-induced PKTD have been reported without elevation of serum creatinine[27,30]. Furthermore, Manolakopoulos et al[31] reported that baseline eGFR, not ADV treatment, was associated with GFR reduction during a mean of 37 mo of follow up of chronic HBV infection patients. Thus, it is possible that GFR reduction and PKTD do not occur simultaneously during ADV treatment.
In this study, we found that genotype CC/CT at position -24 seems to be related to PKTD; however, this relationship did not reach statistical significance, probably because of the small number of participants evaluated in the study. Among HIV patients, the correlation of SNPs in the ABCC2 gene to PKTD induced by tenofovir disoproxil fumarate has been reported[32,33]. The potential associated risk of polymorphisms in ABCC2 to ADV-induced PKTD should be elucidated in future studies.
The treatment of ADV-induced PKTD has not been standardized. The cessation of ADV and phosphorus supplementation were usually performed in previous cases of PKTD with osteomalacia[11]; however, dose reduction or the replacement of ADV with entecavir or tenofovir alafenamide fumarate would be suitable for the management of chronic HBV infection. Early detection of PKTD could help in the selection of the most appropriate therapy.
Limitations to this study are: (1) the sample size was too small, thus we could not discern the effect of the SNPs in SLC22A6 and ABCC2; (2) the HBV genotype was different between patients with/without ADV, and our evaluation of ADV-induced PKTD was limited to patients with HBV genotype C. The effect of HBV genotype on the incidence of PKTD should be further evaluated; (3) the definition of PKTD was based on a single examination, which could lead to misclassification; (4) because the study was retrospective, the treatment duration of patients with asymptomatic PKTD may be overestimated, therefore, the results presented here need to be tested in well-organized prospective studies; (5) we did not test HDV coinfection, because Japan is a non-endemic area of HDV; and (6) caution should be used in applying our results to other ethnic groups.
In conclusion, we found a high rate of ADV-induced PKTD and that both older age at the initiation of ADV and longer treatment duration with ADV are independently associated with its presence in chronic HBV infection patients. The results suggest that the tubular function of the kidney of older patients undergoing long-term ADV treatment should be carefully evaluated.
We are grateful to Drs. Kyoko Okada, Mosaburo Kainuma, Haru Mukae, Satoshi Hiramine, Kazuya Ura, Fujiko Mitsumoto, Kohji Takayama, Sakiko Hayasaki, Norihito Satoh, Yoshifumi Kato and Masaru Sakiyama from the Department of General Internal Medicine, Kyushu University Hospital for assistance in this study. We are also grateful to Mr. Yoshitaka Etoh for his excellent lab work on genotyping the SNPs of hOAT1 and MRP2.
Although adefovir dipivoxil (ADV), a nucleotide analogue of adenosine monophosphate, is effective in viral suppression for both treatment-naive and lamivudine-resistant chronic hepatitis B virus infection patients, some cases of proximal kidney tubular dysfunction (PKTD) associated with ADV treatment have been reported. There are few data on the prevalence and the predictors of PKTD by ADV.
Previous studies used serum creatinine or glomerular filtration rate to evaluate ADV nephrotoxicity. This is the first clinical research to evaluate ADV nephrotoxicity that focused on PKTD.
The present study showed that age at the initiation of ADV and treatment duration with ADV were independently associated with the presence of PKTD. The baseline estimated glomerular filtration rate was not an independent predictor of PKTD during ADV treatment.
The results suggest that the tubular function of the kidney of older patients undergoing long-term (over 96 mo) ADV treatment should be carefully evaluated.
The authors conducted a retrospective study with 79 patients and indicated that older age at initiation and longer treatment duration were significant factors associated with PKTD. The paper addresses an interesting and important issue for which many novel findings have been reported in the recent years. This paper appears thus valuable, it is however also challenging.
P- Reviewer: Bock CT, Kim sr, Makvandi M, Rodriguez-Frias F, Zhao HT, Zhou GX S- Editor: Ma YJ L- Editor: Logan S E- Editor: Liu XM
| 1. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 2. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] |
| 3. | Kashiwagi S, Hayashi J, Ikematsu H, Nomura H, Kusaba T, Shingu T, Hayashida K, Kaji M. An epidemiologic study of hepatitis B virus in Okinawa and Kyushu, Japan. Am J Epidemiol. 1983;118:787-794. [PubMed] |
| 4. | Furusyo N, Hayashi J, Sawayama Y, Kawakami Y, Kishihara Y, Kashiwagi S. The elimination of hepatitis B virus infection: changing seroepidemiology of hepatitis A and B virus infection in Okinawa, Japan over a 26-year period. Am J Trop Med Hyg. 1998;59:693-698. [PubMed] |
| 5. | Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther. 2006;11:669-679. [PubMed] |
| 6. | Furusyo N, Takeoka H, Toyoda K, Murata M, Tanabe Y, Kajiwara E, Shimono J, Masumoto A, Maruyama T, Nomura H. Long-term lamivudine treatment for chronic hepatitis B in Japanese patients: a project of Kyushu University Liver Disease Study. World J Gastroenterol. 2006;12:561-567. [PubMed] |
| 7. | Ogawa E, Furusyo N, Murata M, Ohnishi H, Toyoda K, Taniai H, Ihara T, Ikezaki H, Hayashi T, Kainuma M. Longitudinal assessment of liver stiffness by transient elastography for chronic hepatitis B patients treated with nucleoside analog. Hepatol Res. 2011;41:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Lim SG, Marcellin P, Tassopoulos N, Hadziyannis S, Chang TT, Tong M, Sievert W, Hu P, Arterburn S, Brosgart CL. Clinical trial: effects of adefovir dipivoxil therapy in Asian and Caucasian patients with chronic hepatitis B. Aliment Pharmacol Ther. 2007;26:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Akyildiz M, Gunsar F, Ersoz G, Karasu Z, Ilter T, Batur Y, Akarca U. Adefovir dipivoxil alone or in combination with lamivudine for three months in patients with lamivudine resistant compensated chronic hepatitis B. Dig Dis Sci. 2007;52:3444-3447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Izzedine H, Hulot JS, Launay-Vacher V, Marcellini P, Hadziyannis SJ, Currie G, Brosgart CL, Westland C, Arterbrun S, Deray G. Renal safety of adefovir dipivoxil in patients with chronic hepatitis B: two double-blind, randomized, placebo-controlled studies. Kidney Int. 2004;66:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Wu C, Zhang H, Qian Y, Wang L, Gu X, Dai Z. Hypophosphatemic osteomalacia and renal Fanconi syndrome induced by low-dose adefovir dipivoxil: a case report and literature review suggesting ethnic predisposition. J Clin Pharm Ther. 2013;38:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Miller DS. Nucleoside phosphonate interactions with multiple organic anion transporters in renal proximal tubule. J Pharmacol Exp Ther. 2001;299:567-574. [PubMed] |
| 13. | Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804-817. [PubMed] |
| 14. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5325] [Cited by in RCA: 5261] [Article Influence: 328.8] [Reference Citation Analysis (0)] |
| 15. | Barth JH, Jones RG, Payne RB. Calculation of renal tubular reabsorption of phosphate: the algorithm performs better than the nomogram. Ann Clin Biochem. 2000;37:79-81. [PubMed] |
| 16. | Dauchy FA, Lawson-Ayayi S, de La Faille R, Bonnet F, Rigothier C, Mehsen N, Miremont-Salamé G, Cazanave C, Greib C, Dabis F. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney Int. 2011;80:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Murata M, Furusyo N, Unno M, Ogawa E, Toyoda K, Taniai H, Ohnishi H, Hayashi J. Long-term effects of lamivudine treatment in Japanese chronic hepatitis B patients. World J Gastroenterol. 2011;17:2945-2952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Morris CJ, Hill M, de Medina M, Herman C, Cloherty GA, Martin P. Comparison of detection and quantification of HBV DNA in chronic HBeAg negative and positive patients by Abbott RealTime HBV and Roche Cobas TaqMan HBV assays. J Virol Methods. 2013;193:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Chan HL, Tsang SW, Liew CT, Tse CH, Wong ML, Ching JY, Leung NW, Tam JS, Sung JJ. Viral genotype and hepatitis B virus DNA levels are correlated with histological liver damage in HBeAg-negative chronic hepatitis B virus infection. Am J Gastroenterol. 2002;97:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Osiowy C, Giles E. Evaluation of the INNO-LiPA HBV genotyping assay for determination of hepatitis B virus genotype. J Clin Microbiol. 2003;41:5473-5477. [PubMed] |
| 21. | Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95:519-523. [PubMed] |
| 22. | Kim SH, Won KS, Song BI, Jo I, Zeon SK. Low-dose adefovir-induced hypophosphatemic osteomalacia on whole-body bone scintigraphy. Nucl Med Mol Imaging. 2013;47:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Kahn J, Lagakos S, Wulfsohn M, Cherng D, Miller M, Cherrington J, Hardy D, Beall G, Cooper R, Murphy R. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA. 1999;282:2305-2312. [PubMed] |
| 24. | Fisher EJ, Chaloner K, Cohn DL, Grant LB, Alston B, Brosgart CL, Schmetter B, El-Sadr WM, Sampson J. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS. 2001;15:1695-1700. [PubMed] |
| 25. | Tamori A, Enomoto M, Kobayashi S, Iwai S, Morikawa H, Sakaguchi H, Habu D, Shiomi S, Imanishi Y, Kawada N. Add-on combination therapy with adefovir dipivoxil induces renal impairment in patients with lamivudine-refractory hepatitis B virus. J Viral Hepat. 2010;17:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Shimizu Y, Hiraoka A, Yamago H, Shiraishi A, Imai Y, Tatsukawa H, Tanihira T, Miyata H, Ninomiya T, Tokumoto Y. Hypophosphatemia in patients with hepatitis B virus infection undergoing long-term adefovir dipivoxil therapy. Hepatol Res. 2014;44:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Law ST, Li KK, Ho YY. Nephrotoxicity, including acquired Fanconi’s syndrome, caused by adefovir dipivoxil - is there a safe dose? J Clin Pharm Ther. 2012;37:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Jung YK, Yeon JE, Choi JH, Kim CH, Jung ES, Kim JH, Park JJ, Kim JS, Bak YT, Byun KS. Fanconi’s Syndrome Associated with Prolonged Adefovir Dipivoxil Therapy in a Hepatitis B Virus Patient. Gut Liver. 2010;4:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Ha NB, Ha NB, Garcia RT, Trinh HN, Vu AA, Nguyen HA, Nguyen KK, Levitt BS, Nguyen MH. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology. 2009;50:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Tanaka M, Setoguchi T, Ishidou Y, Arishima Y, Hirotsu M, Saitoh Y, Nakamura S, Kakoi H, Nagano S, Yokouchi M. Pathological femoral fractures due to osteomalacia associated with adefovir dipivoxil treatment for hepatitis B: a case report. Diagn Pathol. 2012;7:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Manolakopoulos S, Striki A, Deutsch M, Mela M, Ketikoglou I, Tzourmakliotis D, Manesis EK, Papatheodoridis GV. Long-term adefovir plus lamivudine therapy does not decrease creatinine clearance in HBeAg-negative chronic hepatitis B patients. Liver Int. 2011;31:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Rodríguez-Nóvoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, Cuenca L, González-Pardo G, Khoo S, Back D. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48:e108-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Nishijima T, Komatsu H, Higasa K, Takano M, Tsuchiya K, Hayashida T, Oka S, Gatanaga H. Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: a pharmacogenetic study. Clin Infect Dis. 2012;55:1558-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |